ODAC Temozolomide Oncology Drug Advisory Committee March 13






- Slides: 21

ODAC Temozolomide Oncology Drug Advisory Committee March 13, 2003 Craig L. Tendler, M. D. Vice President, Oncology Clinical Research 1 SCHERING-PLOUGH RESEARCH INSTITUTE

ODAC Agenda • Basis for the accelerated approval of Temozolomide in refractory Anaplastic Astrocytoma (94 -123) • Post-approval commitment study (98 -13) – Design – Key study milestones – Current status • Ongoing challenges associated with post-approval commitment study • Initiatives to expedite completion of post approval commitment • Schering-Plough development programs with temozolomide in primary brain tumors 2 SCHERING-PLOUGH RESEARCH INSTITUTE

ODAC Temozolomide NDA Submission for Recurrent Glioma (at first relapse) • Recurrent Glioblastoma Multiforme – Phase 2 randomized study temozolomide vs procarbazine (94 -091) – Phase 2 single arm study (94 -122) • Recurrent Anaplastic Astrocytoma – Phase 2 single arm study Anaplastic Astrocytoma 123) 3 (94 - SCHERING-PLOUGH RESEARCH INSTITUTE

ODAC Temozolomide Indication (August 1999) Adult patients with refractory anaplastic astrocytoma, i. e. patients at first relapse who have experienced disease progression on a regimen containing a nitrosourea and procarbazine 4 SCHERING-PLOUGH RESEARCH INSTITUTE

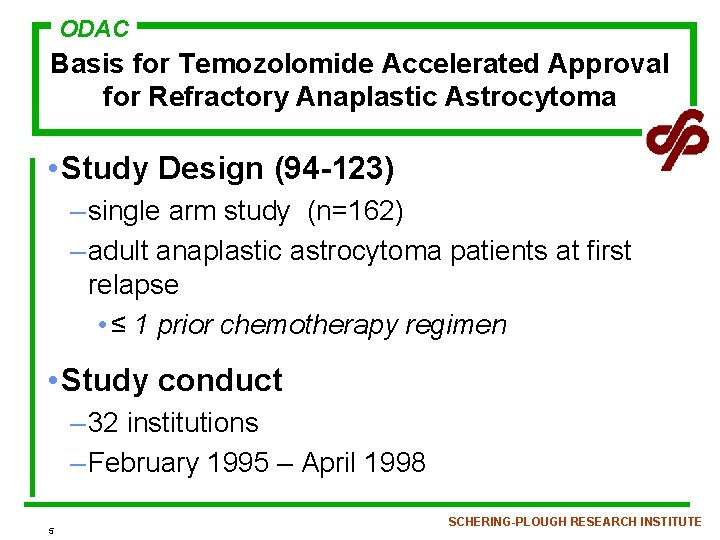
ODAC Basis for Temozolomide Accelerated Approval for Refractory Anaplastic Astrocytoma • Study Design (94 -123) – single arm study (n=162) – adult anaplastic astrocytoma patients at first relapse • ≤ 1 prior chemotherapy regimen • Study conduct – 32 institutions – February 1995 – April 1998 5 SCHERING-PLOUGH RESEARCH INSTITUTE

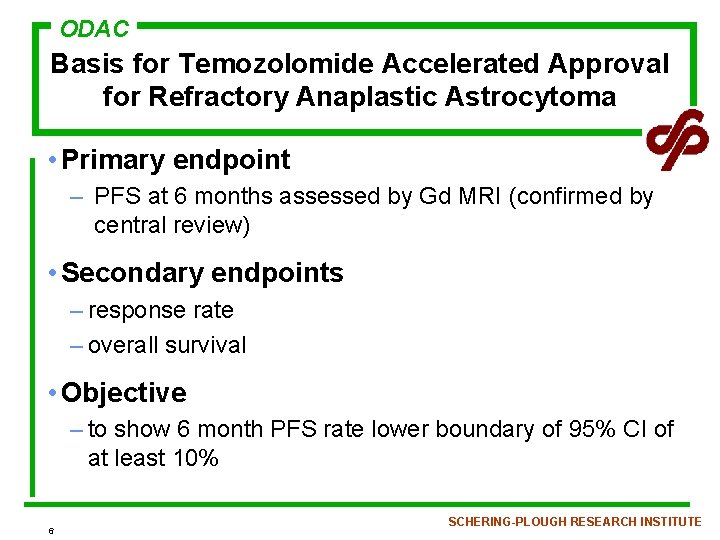
ODAC Basis for Temozolomide Accelerated Approval for Refractory Anaplastic Astrocytoma • Primary endpoint – PFS at 6 months assessed by Gd MRI (confirmed by central review) • Secondary endpoints – response rate – overall survival • Objective – to show 6 month PFS rate lower boundary of 95% CI of at least 10% 6 SCHERING-PLOUGH RESEARCH INSTITUTE

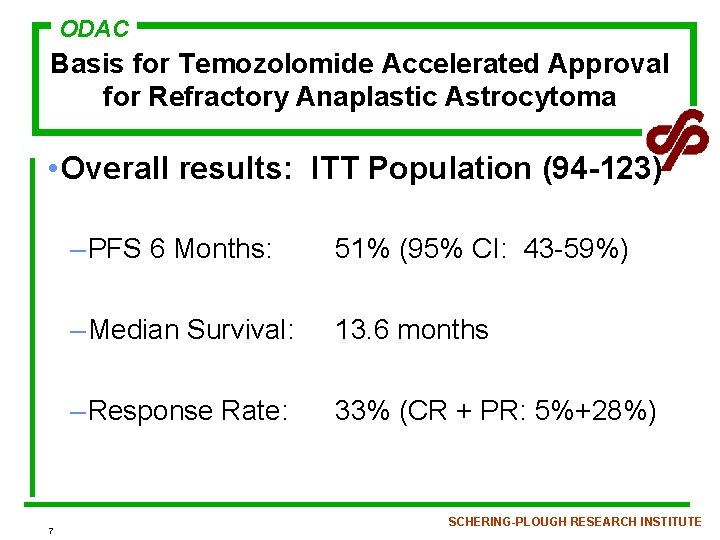
ODAC Basis for Temozolomide Accelerated Approval for Refractory Anaplastic Astrocytoma • Overall results: ITT Population (94 -123) 7 – PFS 6 Months: 51% (95% CI: 43 -59%) – Median Survival: 13. 6 months – Response Rate: 33% (CR + PR: 5%+28%) SCHERING-PLOUGH RESEARCH INSTITUTE

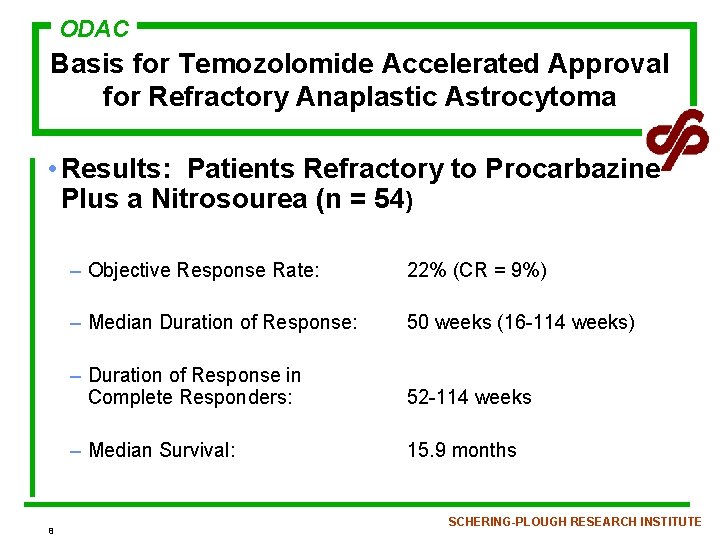
ODAC Basis for Temozolomide Accelerated Approval for Refractory Anaplastic Astrocytoma • Results: Patients Refractory to Procarbazine Plus a Nitrosourea (n = 54) 8 – Objective Response Rate: 22% (CR = 9%) – Median Duration of Response: 50 weeks (16 -114 weeks) – Duration of Response in Complete Responders: 52 -114 weeks – Median Survival: 15. 9 months SCHERING-PLOUGH RESEARCH INSTITUTE

ODAC Temozolomide Safety in Malignant Glioma • Safety database: 1, 017 temozolomide treated patients (400 GBM & AA) • Temozolomide was administered with few dose reductions or dose delays • Most adverse events were mild to moderate in severity • Study treatment discontinuation due to adverse events was infrequent • Grade 3/4 myelosuppression was also infrequent and non-cumulative 9 SCHERING-PLOUGH RESEARCH INSTITUTE

ODAC Unanimous ODAC Opinion • Patients with relapsed anaplastic astrocytoma after procarbazine and a nitrosourea are considered unresponsive to otherapies – Unmet medical need • Objective response in this setting could be an adequate surrogate for clinical benefit if well defined and of sufficient magnitude 10 SCHERING-PLOUGH RESEARCH INSTITUTE

ODAC Unanimous ODAC Opinion • Efficacy: – Temozolomide is effective for the treatment of anaplastic astrocytoma in patients previously treated with a nitrosourea and procarbazine • Safety: – Safety of Temozolomide is acceptable for this indication 11 SCHERING-PLOUGH RESEARCH INSTITUTE

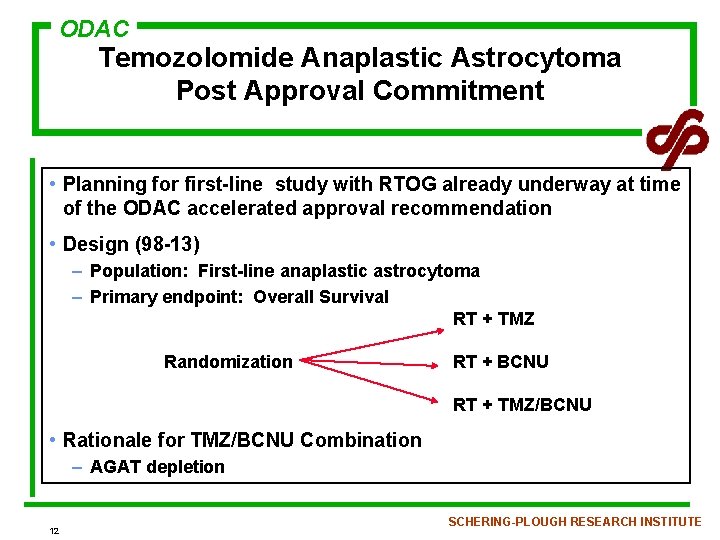
ODAC Temozolomide Anaplastic Astrocytoma Post Approval Commitment • Planning for first-line study with RTOG already underway at time of the ODAC accelerated approval recommendation • Design (98 -13) – Population: First-line anaplastic astrocytoma – Primary endpoint: Overall Survival RT + TMZ Randomization RT + BCNU RT + TMZ/BCNU • Rationale for TMZ/BCNU Combination – AGAT depletion 12 SCHERING-PLOUGH RESEARCH INSTITUTE

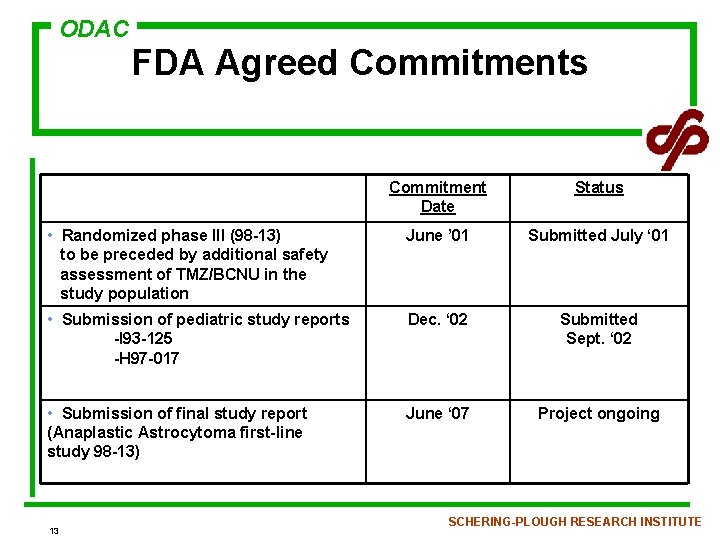
ODAC FDA Agreed Commitments Commitment Date Status • Randomized phase III (98 -13) to be preceded by additional safety assessment of TMZ/BCNU in the study population June ’ 01 Submitted July ‘ 01 • Submission of pediatric study reports -I 93 -125 -H 97 -017 Dec. ‘ 02 Submitted Sept. ‘ 02 • Submission of final study report (Anaplastic Astrocytoma first-line study 98 -13) June ‘ 07 Project ongoing 13 SCHERING-PLOUGH RESEARCH INSTITUTE

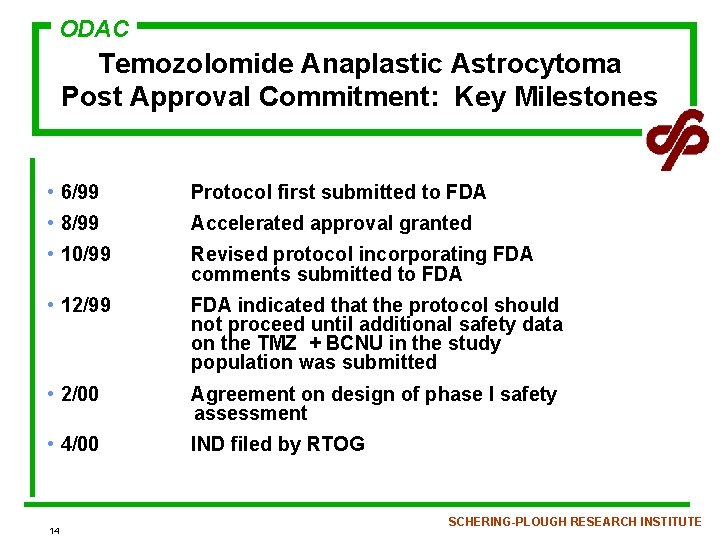
ODAC Temozolomide Anaplastic Astrocytoma Post Approval Commitment: Key Milestones • 6/99 Protocol first submitted to FDA • 8/99 Accelerated approval granted • 10/99 Revised protocol incorporating FDA comments submitted to FDA • 12/99 FDA indicated that the protocol should not proceed until additional safety data on the TMZ + BCNU in the study population was submitted • 2/00 Agreement on design of phase I safety assessment • 4/00 IND filed by RTOG 14 SCHERING-PLOUGH RESEARCH INSTITUTE

ODAC Key Milestones - Continued • 6/00 Initiation of Phase I safety assessment with TMZ/BCNU • 3/01 Completion of enrollment for safety assessment • 7/01 Submission of safety data to FDA • 9/01 Enrollment initiated for second safety cohort • 1/02 Completion of enrollment for 2 nd safety cohort • 6/02 Combination arm discontinued due to safety profile • 1/03 Randomized Phase 3 opened 15 SCHERING-PLOUGH RESEARCH INSTITUTE

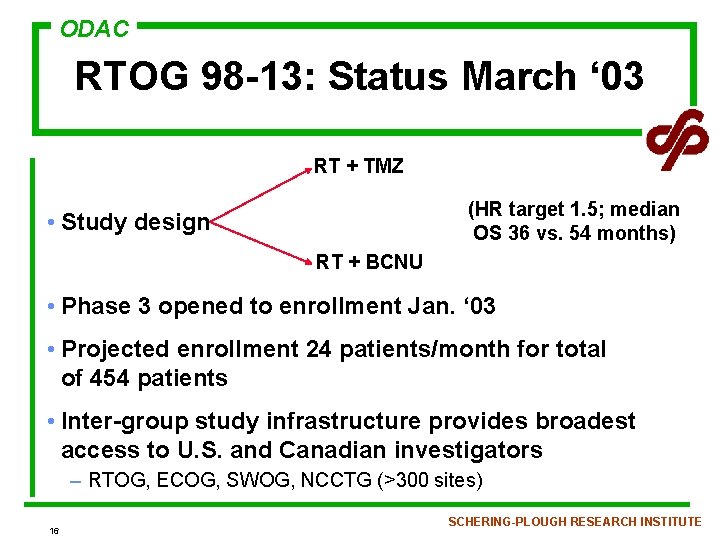
ODAC RTOG 98 -13: Status March ‘ 03 RT + TMZ (HR target 1. 5; median OS 36 vs. 54 months) • Study design RT + BCNU • Phase 3 opened to enrollment Jan. ‘ 03 • Projected enrollment 24 patients/month for total of 454 patients • Inter-group study infrastructure provides broadest access to U. S. and Canadian investigators – RTOG, ECOG, SWOG, NCCTG (>300 sites) 16 SCHERING-PLOUGH RESEARCH INSTITUTE

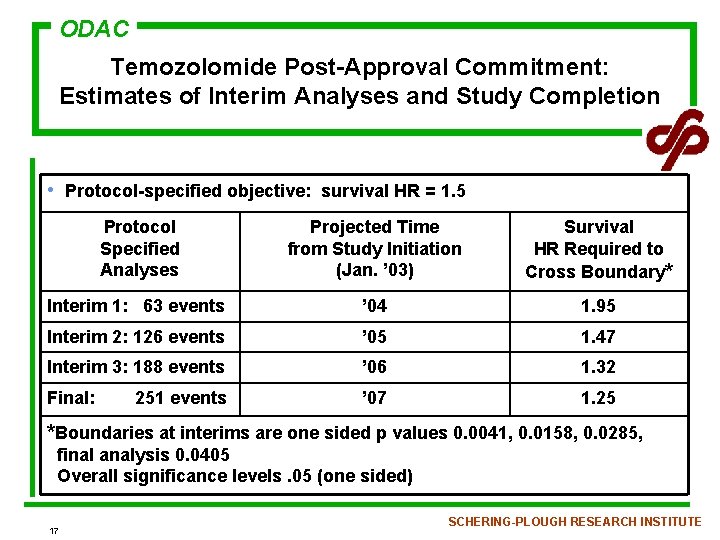
ODAC Temozolomide Post-Approval Commitment: Estimates of Interim Analyses and Study Completion • Protocol-specified objective: survival HR = 1. 5 Protocol Specified Analyses Projected Time from Study Initiation (Jan. ’ 03) Survival HR Required to Cross Boundary* Interim 1: 63 events ’ 04 1. 95 Interim 2: 126 events ’ 05 1. 47 Interim 3: 188 events ’ 06 1. 32 Final: ’ 07 1. 25 251 events *Boundaries at interims are one sided p values 0. 0041, 0. 0158, 0. 0285, final analysis 0. 0405 Overall significance levels. 05 (one sided) 17 SCHERING-PLOUGH RESEARCH INSTITUTE

ODAC Challenges of Survival Trial in First. Line Anaplastic Astrocytoma • Low and declining annual incidence (~3, 000 newly diagnosed U. S. patients/year) • Long median survival (3 -4 years) 18 SCHERING-PLOUGH RESEARCH INSTITUTE

ODAC Schering-Plough and RTOG Initiatives to Expedite Study Completion • Communications: – Investigators: investigator meetings, target neuro-surgeons, monthly teleconference with the lead investigators at each cooperative group, cooperative group newsletters – Patients: internet listing, patient brochures, patient brain tumor support groups (National Brain Tumor Foundation and American Brain Tumor Foundation) • Project Management: – RTOG HQ staff – Monthly progress reviews (SPRI / Inter-group PIs) – Institutional data management support • International Sites 19 SCHERING-PLOUGH RESEARCH INSTITUTE

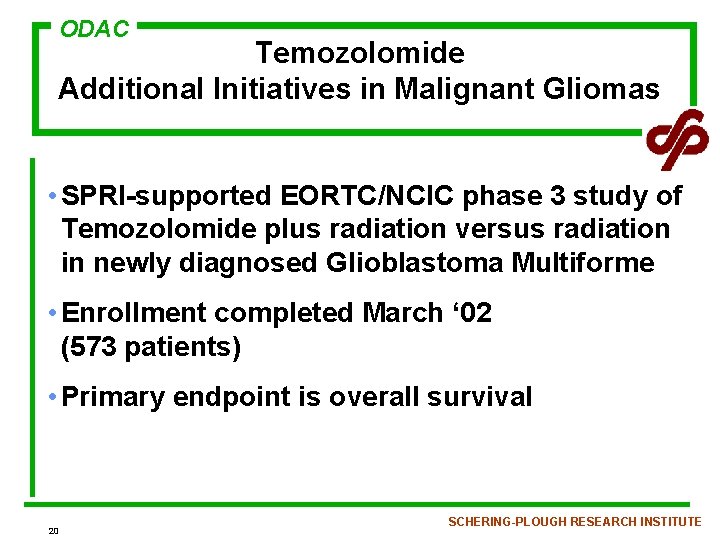
ODAC Temozolomide Additional Initiatives in Malignant Gliomas • SPRI-supported EORTC/NCIC phase 3 study of Temozolomide plus radiation versus radiation in newly diagnosed Glioblastoma Multiforme • Enrollment completed March ‘ 02 (573 patients) • Primary endpoint is overall survival 20 SCHERING-PLOUGH RESEARCH INSTITUTE

ODAC Temozolomide Development Programs in Primary Brain Cancer • Phase 3 Newly diagnosed Anaplastic Astrocytoma (98 -13) • Phase 3 Newly diagnosed Glioblastoma Multiforme • Phase 2 Anaplastic Oligodendroglioma-RTOG • Phase 3 Low Grade Glioma-EORTC • Phase 1/2 studies in recurrent pediatric CNS tumors 21 SCHERING-PLOUGH RESEARCH INSTITUTE
 Odac oncology
Odac oncology March march dabrowski
March march dabrowski Mousai msd 192 dac
Mousai msd 192 dac Odac 64bit 설치
Odac 64bit 설치 C# 오라클 db 연결
C# 오라클 db 연결 National advisory committee 1993
National advisory committee 1993 Trade union advisory committee
Trade union advisory committee Robert kerzner
Robert kerzner Nasa astrophysics advisory committee
Nasa astrophysics advisory committee Aviation rulemaking advisory committee
Aviation rulemaking advisory committee History of pharmaceutical legislation in india
History of pharmaceutical legislation in india Deliberate adulteration examples
Deliberate adulteration examples Rad onc job market
Rad onc job market Ukons triage tool
Ukons triage tool Bdl pharma
Bdl pharma Oncological emergencies wikipedia
Oncological emergencies wikipedia Lurbinectedin posologie
Lurbinectedin posologie Nrg oncology meeting 2018
Nrg oncology meeting 2018 Pico question
Pico question Impact of nf1pn
Impact of nf1pn Testicular cancer nursing interventions
Testicular cancer nursing interventions Chapter 2 body structure color and oncology
Chapter 2 body structure color and oncology