Oceans Properties of Ocean Water Why are Oceans























- Slides: 37

Oceans Properties of Ocean Water

Why are Oceans Important? • Generate 70% of our oxygen • Absorb CO 2 from atmosphere • Drive climate and weather • Stabilize temperatures • Shape Earth’s chemistry • Economy – fish, salt, pleasure • https: //www. ck 12. org/c/earth-science/importance-of-the-oceans/lecture/Exploring. Oceans/? referrer=concept_details

Global Ocean • 71% of Earth’s surface is covered by liquid water - most of it is in oceans. • There are five main oceans on Earth. As they are all connected, scientists often refer to them as the global ocean. • How many can you name?

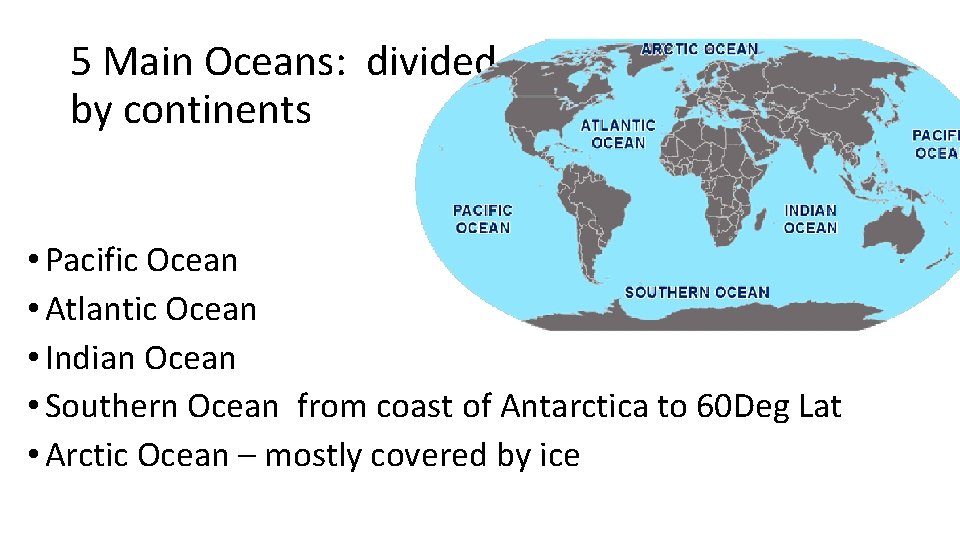
5 Main Oceans: divided by continents • Pacific Ocean • Atlantic Ocean • Indian Ocean • Southern Ocean from coast of Antarctica to 60 Deg Lat • Arctic Ocean – mostly covered by ice

How did oceans form? 1. Ash, dust, and gases, water vapor from volcanoes formed the atmosphere. 2. Earth slowly cooled. 3. When cool enough, water vapor in the atmosphere condensed. 4. The liquid water fell as rain, filled the basins in Earth’s surface, forming the oceans.

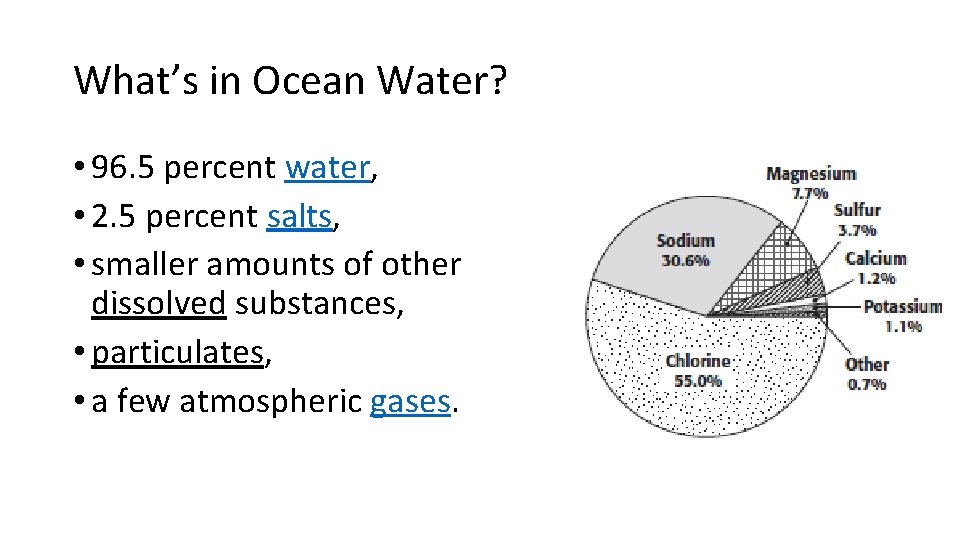
What’s in Ocean Water? • 96. 5 percent water, • 2. 5 percent salts, • smaller amounts of other dissolved substances, • particulates, • a few atmospheric gases.

What else? • Much of the world’s magnesium is recovered from seawater. • And large quantities of bromine. • In certain parts of the world, sodium chloride (table salt) is still obtained by evaporating seawater. • Bromine used in a variety of things: agricultural chemicals, dyes, flame retardants, water purification

How do these particles get into the ocean? • Rivers dissolve minerals and carry them to the ocean. • Wind blows particles in (possibly thousands of km from their source. • Hydrothermal vents billow particles from below earth’s crust. • Organisms in ocean convert dissolved materials to solids (shells), which eventually settle to greater oceanic depths. • Chemical reactions within the ocean.

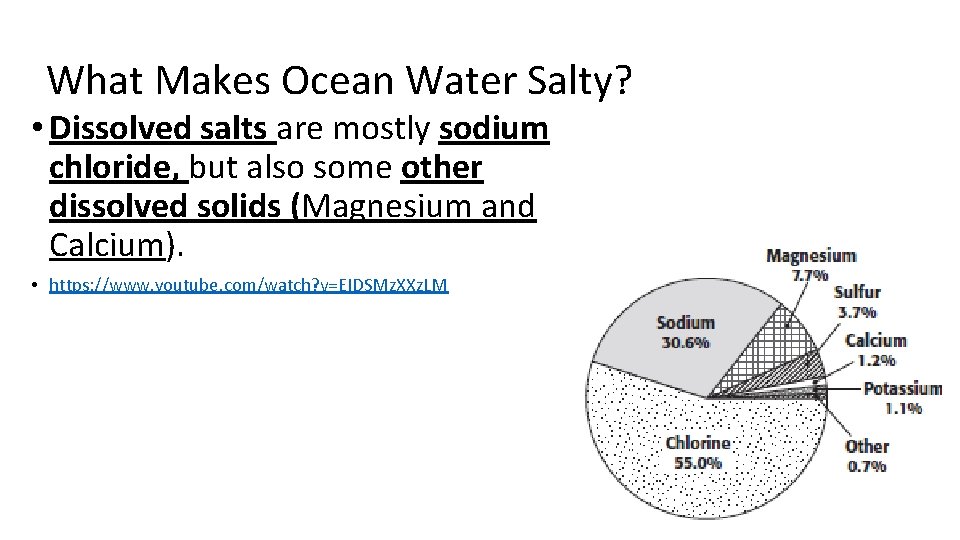
What Makes Ocean Water Salty? • Dissolved salts are mostly sodium chloride, but also some other dissolved solids (Magnesium and Calcium). • https: //www. youtube. com/watch? v=EIDSMz. XXz. LM

What is Salinity? • Salinity is a measure of the amount of dissolved salts in a certain amount of liquid. • Average salinity is approximately 3. 5%, or 35 parts per thousand. • This means that for every 1 litre (1000 m. L) of seawater there are 35 grams of salts (mostly, but not entirely, sodium chloride) dissolved in it. • 35 g dissolved solids/kg of dissolved solids • If you evaporated 1 kg of ocean water, 35 g of solids would remain.

How do we Measure Salinity? • Scientists measure electrical conductivity of water. • Salt dissolved in sea water makes it possible for an electrical current to pass through. • The greater the quantity of dissolved salts, the more easily current flows. • Conductivity increases as salinity increases

Important Properties of Ocean Water • Salinity • Density • Temperature • These are related and have an affect on each other

Processes that Affect Salinity

Processes that Decrease Salinity: • Precipitation • Sea Ice melting

Processes that Increase Salinity: • Evaporation • Formation of Sea Ice (mostly fresh water)

Location also affects Salinity • Oceans in hot, dry climates have high salinities. • (the hot weather causes water to evaporate quickly). • Salt is left behind. For example, the Red Sea in the Middle East is very salty. The climate there is very hot and dry. • Some parts of the ocean are less salty than others: • Near coastlines, fresh water from streams and rivers runs into the ocean. As fresh water mixes with ocean water, the salinity of the ocean water decreases.

Why is Salinity Important? • Salinity affects density. • Density is a mass per unit volume. (g/ml, kg/l). • Why is density important? • Density affects deep ocean currents

Why is knowing Density of oceans Important? • Density affects ocean currents. • Knowing density helps us understand how water masses move through the ocean and mix together (ocean currents) • Ocean currents have major impact on climate.

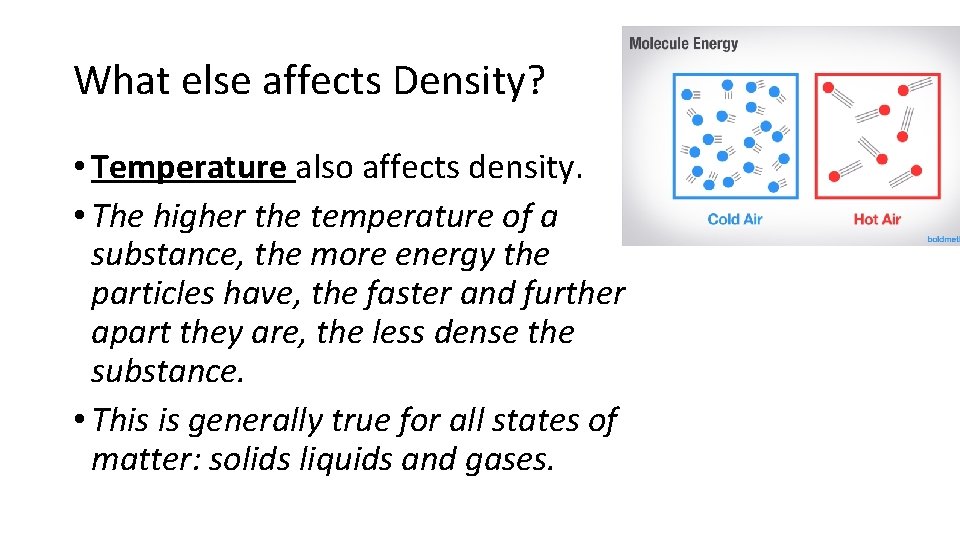
What else affects Density? • Temperature also affects density. • The higher the temperature of a substance, the more energy the particles have, the faster and further apart they are, the less dense the substance. • This is generally true for all states of matter: solids liquids and gases.

What affects Ocean Temperature? • Ocean’s temperature varies with amount of solar radiation received (heat from the sun) • Depends on Latitude.

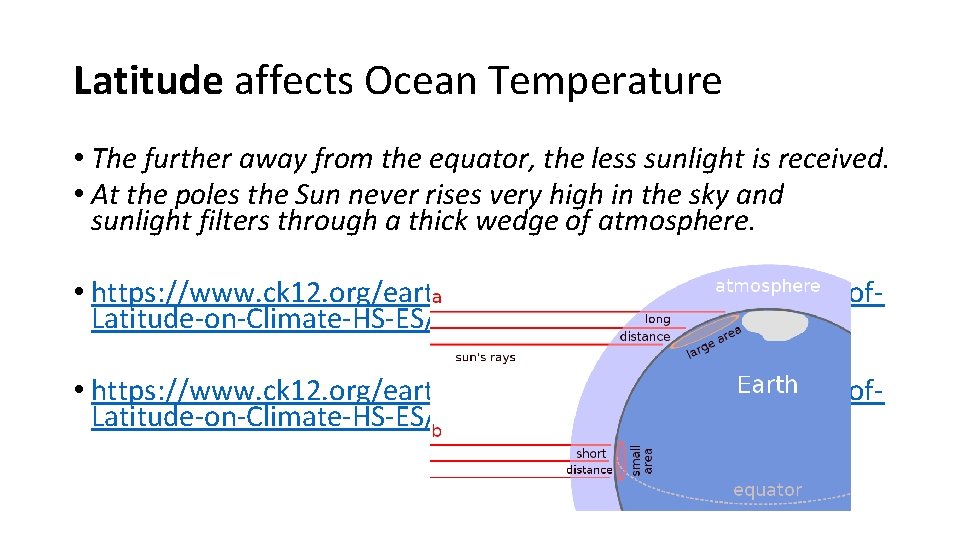
Latitude affects Ocean Temperature • The further away from the equator, the less sunlight is received. • At the poles the Sun never rises very high in the sky and sunlight filters through a thick wedge of atmosphere. • https: //www. ck 12. org/earth-science/latitude/lesson/Effect-of. Latitude-on-Climate-HS-ES/

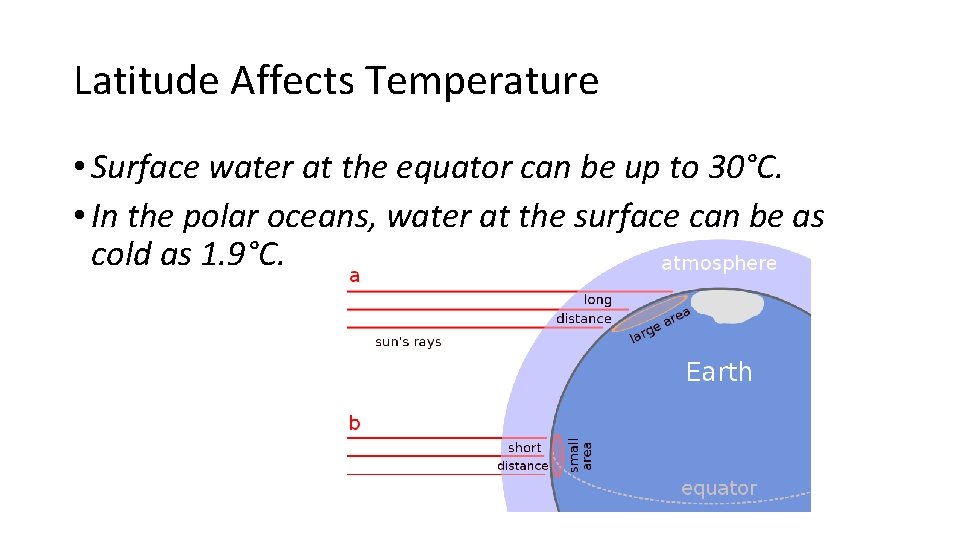
Latitude Affects Temperature • Surface water at the equator can be up to 30°C. • In the polar oceans, water at the surface can be as cold as 1. 9°C.

Time of year can affect Ocean temperature • In areas that receive more sunlight in summer than winter, the surface water in the oceans is warmer.

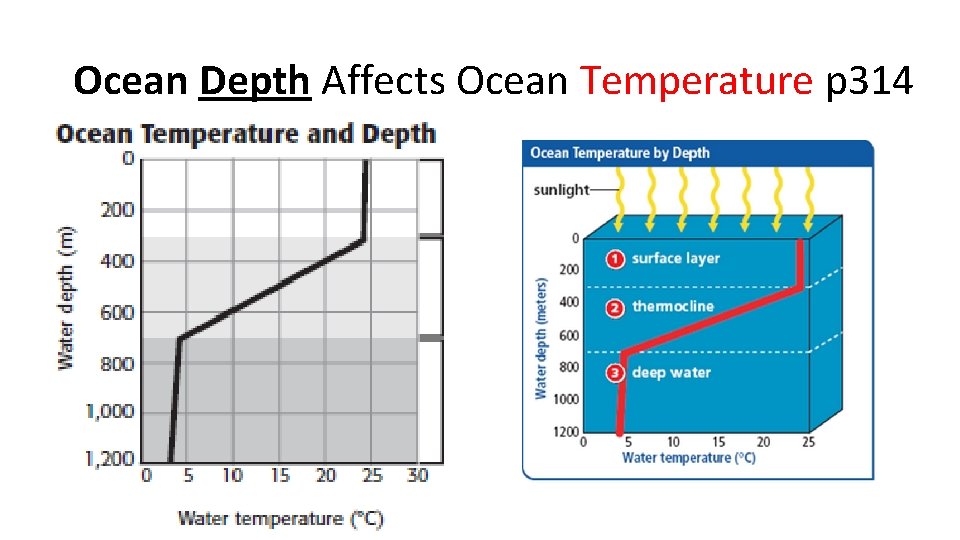
Ocean Depth Affects Ocean Temperature p 314

Check your Understanding 1. 2. 3. 4. 5. 6. 7. 8. 9. Why are oceans important? Name the 5 main oceans. How did our oceans form? What is salinity? What processes affect salinity? What properties affect salinity? Why is density important? What affect does temperature have on density? What happens to ocean temperature the deeper you go?

Practice: Mind Map • Create a mind map demonstrating how properties of oceans are important and how they are related.

Extras

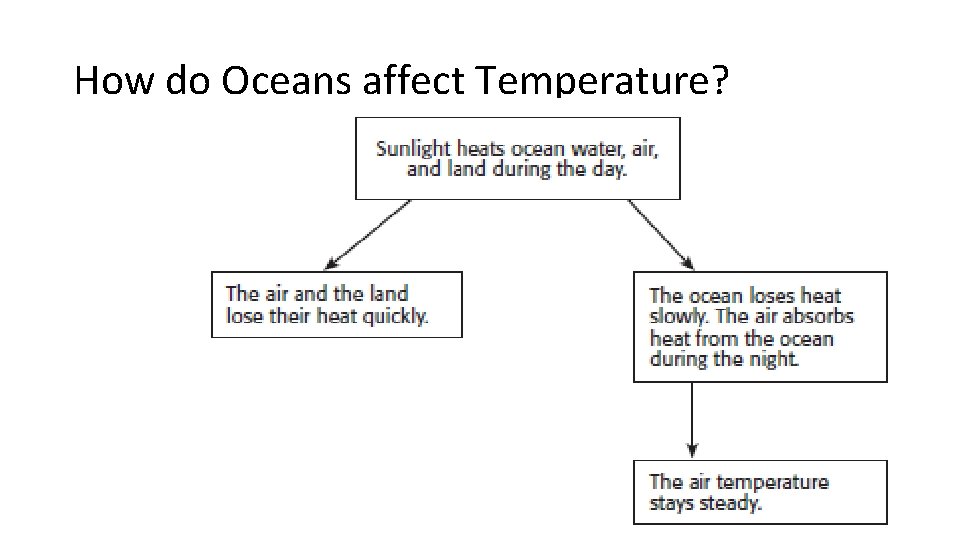
How do Oceans affect Temperature?

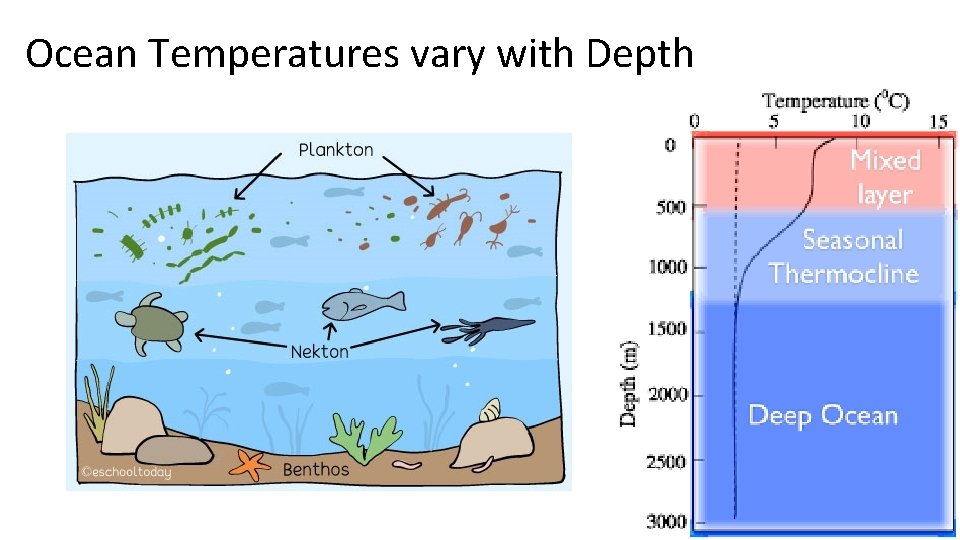
Ocean Temperatures vary with Depth

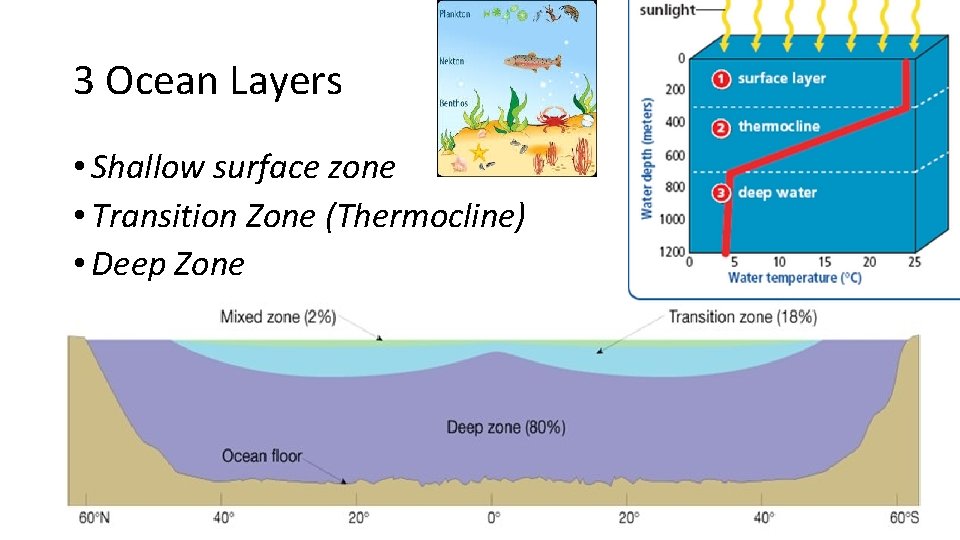
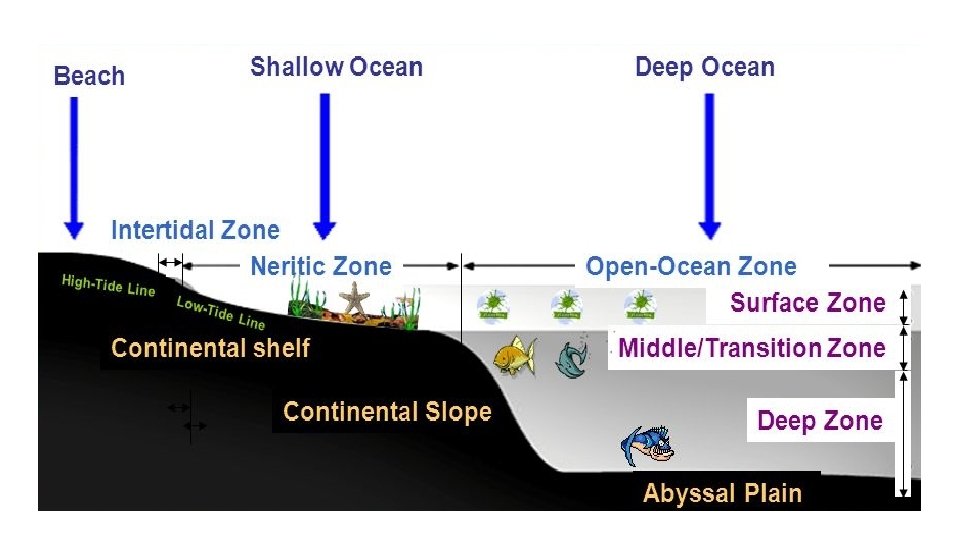
3 Ocean Layers • Shallow surface zone • Transition Zone (Thermocline) • Deep Zone

Surface Zone – top layer • Top layer • Shallow (300 -450 m) • Warmed by sun • As the ocean water is heated, it becomes less dense and rises above denser, cooler water. • Convection currents form as the water moves. • temperature within the surface zone is fairly uniform. • Plankton live here.


Transition Zone - Thermocline • just beneath the surface zone. • Rapid temperature drop • Bottom is very cold, even at the equator • warm water of the surface zone do not mix easily with the water below. • Variable depth: 100 m to almost 1, 000 m below the surface of the ocean. • Nekton live here

Deep Zone • No Sunlight • very cold (2°C), very dense. • It moves slowly across the ocean floor and forms the deep ocean currents. • Bottom dwellers live here

Practice • Ocean WS • Or p 313 #4 -7: salinity • P 315 #8 -10: describing ocean layers • P 321 Critical Thinking 1 -3: Interpreting a density graph • Ocean WS • p 112 -113 prentice Hall WB

Water is a polar molecule • Ted ed • https: //ed. ted. com/lessons/how-polarity-makeswater-behave-strangely-christina-kleinberg
Activity: Water Olympics