Oceans Average depth of the oceans is 3730





- Slides: 11

Oceans • Average depth of the oceans is 3730 meters, but we consider the top part somewhat separately- why? ? • Thermocline – T gradient change • Pycnocline – Density gradient change • Chemocline – Chemical gradient change

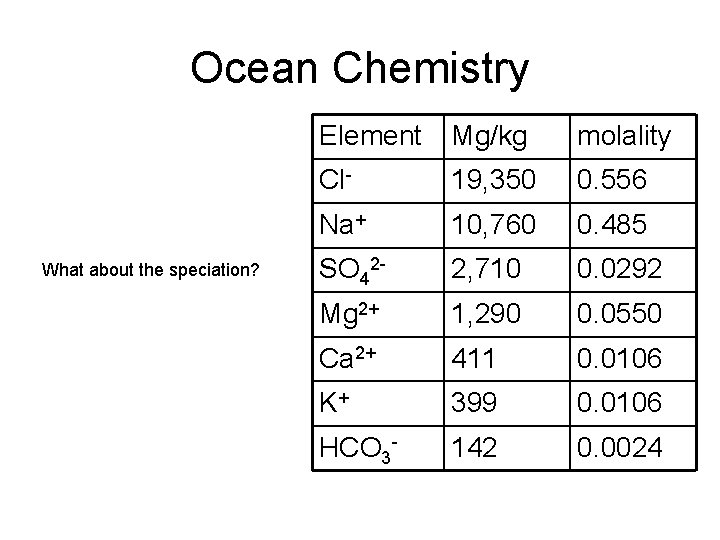
Ocean Chemistry What about the speciation? Element Mg/kg molality Cl- 19, 350 0. 556 Na+ 10, 760 0. 485 SO 42 - 2, 710 0. 0292 Mg 2+ 1, 290 0. 0550 Ca 2+ 411 0. 0106 K+ 399 0. 0106 HCO 3 - 142 0. 0024

Elements in the Oceans • Split elemental abundances in the ocean into 3 classes: – Conservative - constant – Recycled – used by organisms in photic zone – Scavenged – taken out by precipitation of small particles – don’t dissolve, settle out… • Where do major elements come from? – Table 7 -9…

Oxygen in the Ocean • Oxygen is supersaturated in surface water – WHY? ? • Oxygen becomes depleted from consumption by organisms, goes back up some at the bottom (? ? ? )

Ocean Chemistry reservoirs • INPUT: Precipitation, river drainage (dissolved and particulate), atmosphere (gases and particles) – anything else? ?

Ocean p. H buffering • What buffers p. H in ocean waters? ? • Are there any solids that can buffer p. H?

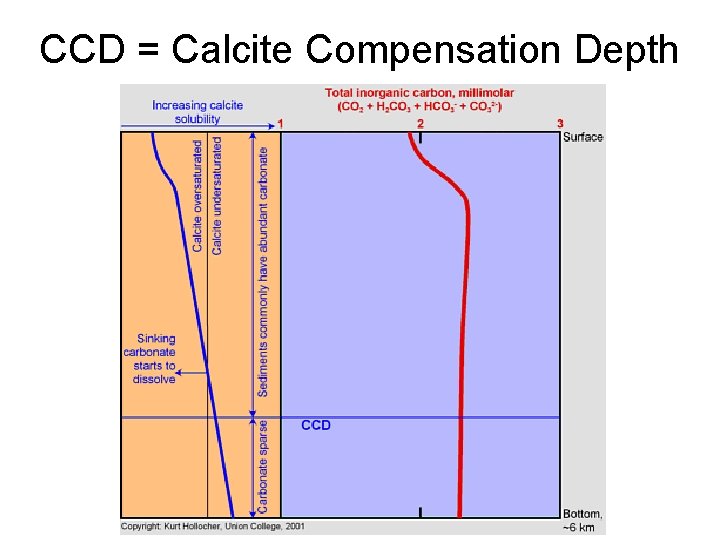
CCD = Calcite Compensation Depth

Evaporation • Minerals that are very soluble only precipitate as more dilute waters evaporate, leaving behind an increasingly concentrated solution • Ca 2+ + SO 42 - + 2 H 2 O Ca. SO 4*2 H 2 O

Seafloor hydrothermal systems • Spreading centers heat source drives convection cells, leach materials from country rock, spews out at fractures…

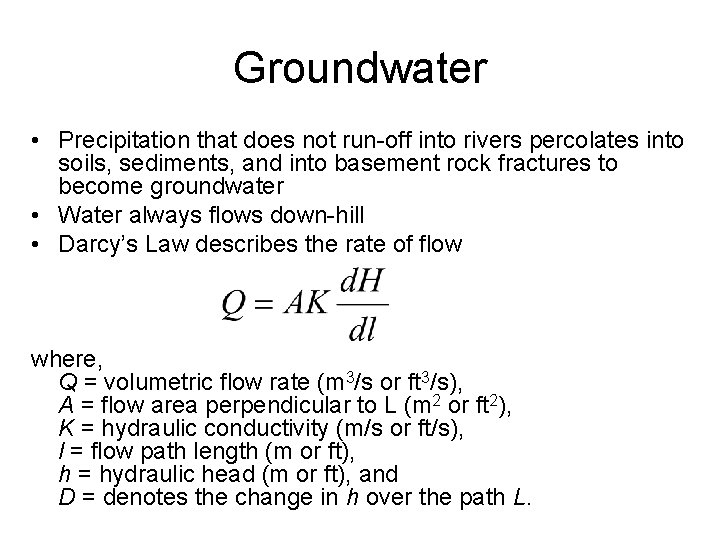
Groundwater • Precipitation that does not run-off into rivers percolates into soils, sediments, and into basement rock fractures to become groundwater • Water always flows down-hill • Darcy’s Law describes the rate of flow where, Q = volumetric flow rate (m 3/s or ft 3/s), A = flow area perpendicular to L (m 2 or ft 2), K = hydraulic conductivity (m/s or ft/s), l = flow path length (m or ft), h = hydraulic head (m or ft), and D = denotes the change in h over the path L.

Groundwater Chemistry • Just like other waters, encounters minerals, gases, etc. • Some key differences from other waters: – PCO 2 variable – respiration! – Segregation of flowpaths in different units