Oceanography Introduction Ocean Chemistry Oceanography The study of










- Slides: 14

Oceanography Introduction Ocean Chemistry

Oceanography The study of the physical, chemical, biological, and geological processes that interact in the world’s oceans.

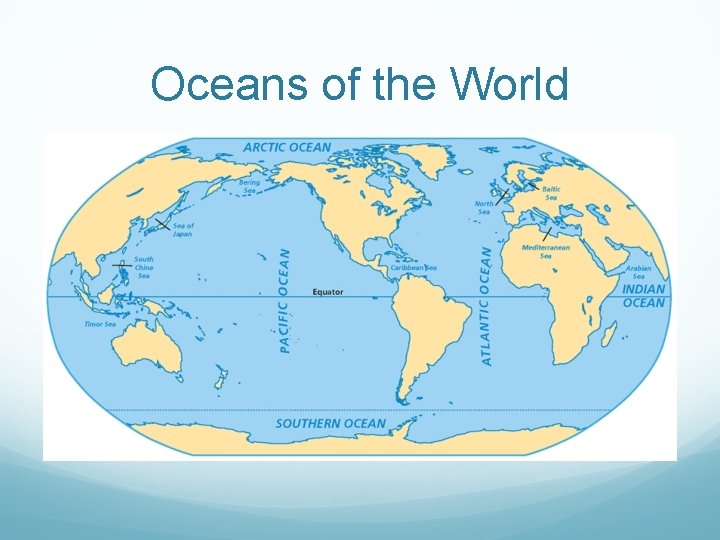
Name the 5 Major Oceans Atlantic Pacific Indian Southern Arctic

Oceans of the World

Chemical Properties of Ocean Water: Dissolved Gasses High amounts of dissolved gasses such as carbon dioxide and oxygen. More dissolved gasses in cold water than warm water. Where would most animals like to live? Cold or Warm water? Constant exchange of gasses between ocean and atmosphere as temperatures change.

Oceans are Carbon Sinks Carbon Sink: Any body of water or land that absorbs carbon (specifically carbon dioxide). Oceans contain 60 times more carbon than the atmosphere does and holds carbon for much longer. Helps regulate earth’s climate!

What’s in that water? 96. 5% pure H 2 O. 3. 5% Dissolved solids (Salts). Common salts include: chlorine, sodium, magnesium, sulfur, calcium, and potassium. Na. Cl (Sodium Chloride/Table Salt) Makes up 85% of all salts in the ocean.

Water, Water Everywhere but not a drop to drink. Question: How did the ocean get salty? Answer: The salts came from weathering and erosion of the continents, volcanic eruptions, and chemical reactions between sea water and newly formed volcanic rocks.

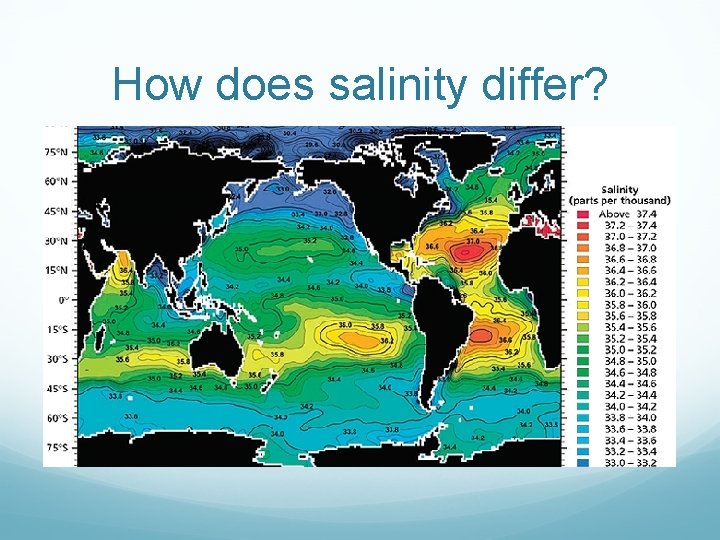
How salty is salty? Ocean water has a salinity of 35 parts per thousand. (same as 3. 5%). Compare that to an estuary like the Chesapeake Bay. How salty is the bay? Answer: About 15 -25 parts per thousand. How about fresh water? 0 -1 Parts per thousand

How does salinity differ?

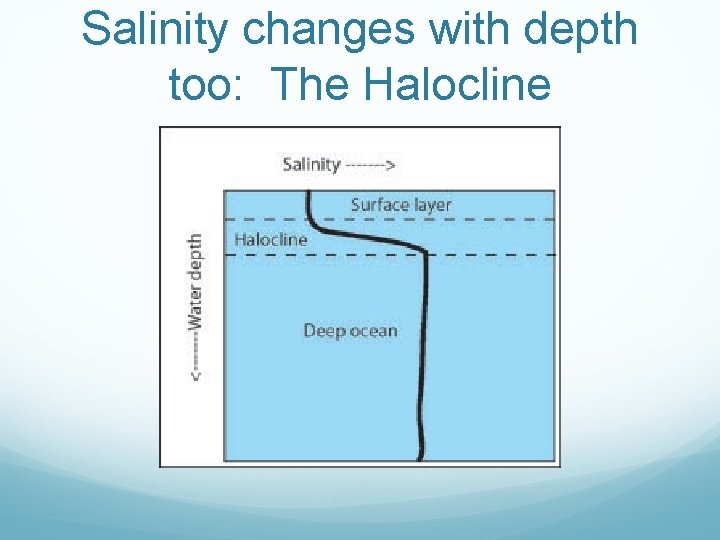
Salinity changes with depth too: The Halocline

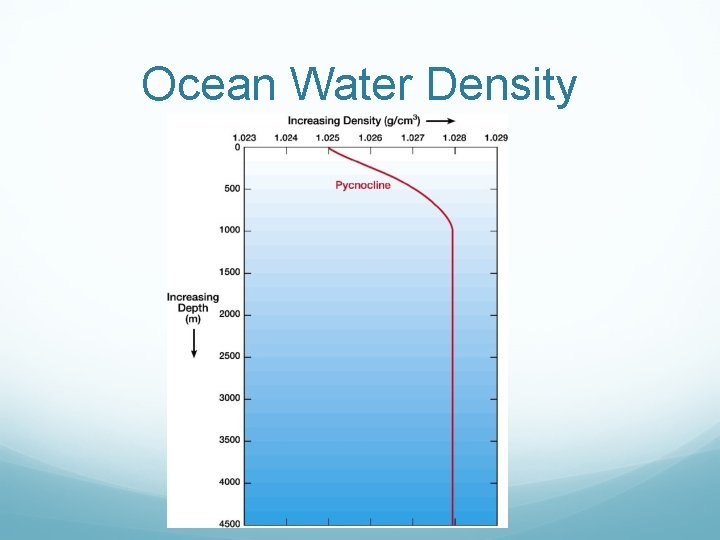
Ocean Water Density

When is ocean water the most dense? Least Dense? Most dense right before freezing (about 4 degrees celcius). Least dense when evaporated as a gas.

Key Ideas The earth has 5 major oceans. The ocean’s chemistry is determined by biologic, geologic, and atmospheric processes. Weathering and erosion contribute most of the salt to the oceans. Oceans act as carbon sinks and regulate earth’s climate. Salinity changes with temperature and depth. Density changes with salinity and temperature.