Ocean chemistry and corrosion This material was adapted

























- Slides: 43

Ocean chemistry and corrosion This material was adapted from ‘Shipwrecks, corrosion and conservation’ produced by Learning Materials Production, OTEN.

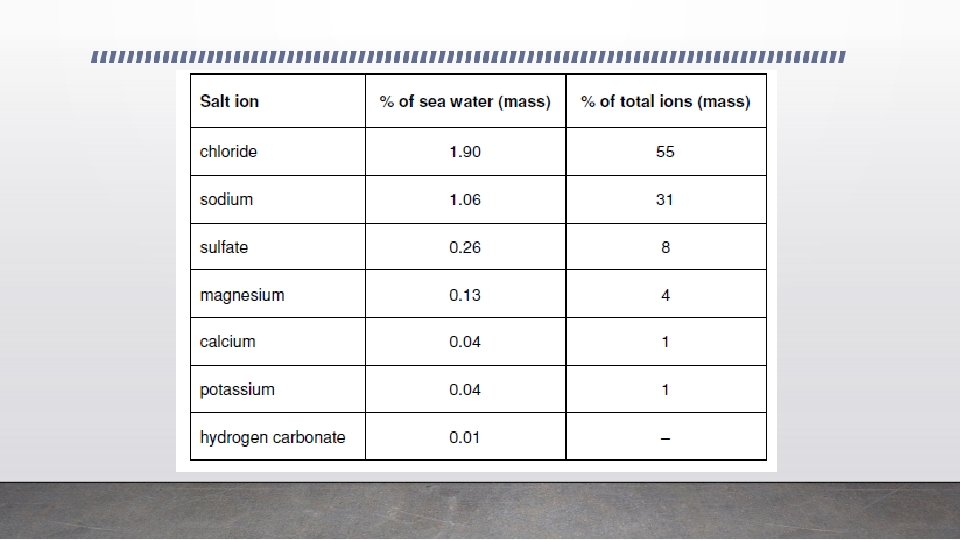
Oceans and Electrolytes • About 3. 5% of the mass of ocean water is dissolved substances, mostly positive and negative ions from salts. • The ions are free to move in this aqueous solution. So, ocean water is an electrolyte, able to conduct electricity. • This enables oxidation-reduction (redox) reactions to occur involving dissolved gases, water and metal parts of shipwrecks. These reactions involve electron transfer from the place where oxidation occurs, usually a metal, to the place where reduction occurs – a non-metal, non-metal compound or metal of lower activity. • Although called electron transfer reactions the movement of charge in solution is carried out by movement of ions (cations and anions). Electrons do not actually move through water by themselves.


Introduction to Redox Reactions • Electron transfer reactions are oxidation-reduction or redox reactions. • Redox reactions result in the generation of an electric current (electricity) or can be forced to occur by imposing an electric current. • OXIDATION = Loss of electrons, species will get less negative or more positive • REDUCTION = Gain of electrons, species will become more negative or less positive • REDOX = When reduction and oxidation take place

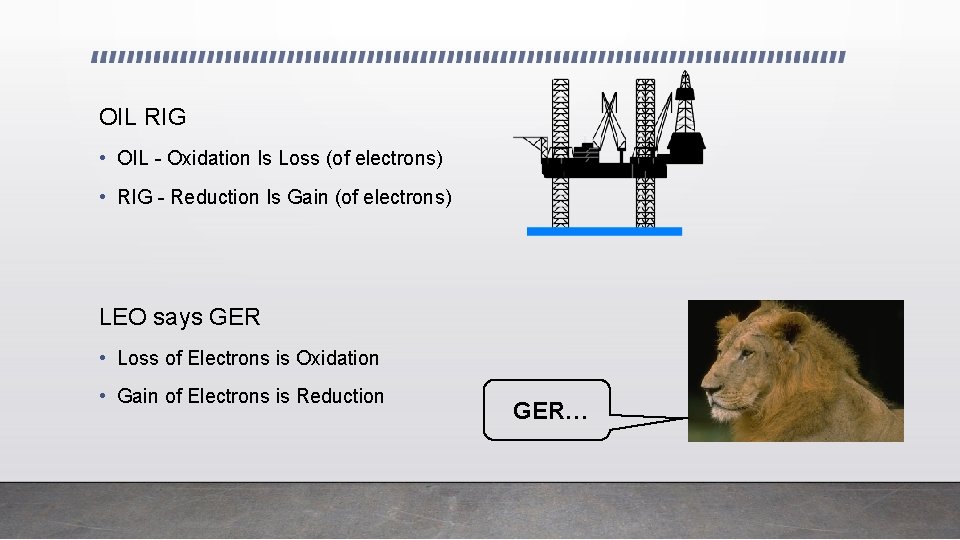
OIL RIG • OIL - Oxidation Is Loss (of electrons) • RIG - Reduction Is Gain (of electrons) LEO says GER • Loss of Electrons is Oxidation • Gain of Electrons is Reduction GER…

• Example: 2 Mg + O 2 2 Mg. O • Can be written as two half-equations: • Oxidation: 2 Mg 2+ + 4 e- (loss of e-) • Reduction: O 2 + 2 e- O 2 - (gain of e-) • Complete these half equations and determine whether it represents reduction or oxidation: • Fe 2+ Fe 3+ • I 2 2 I¯ • Write the oxidation and reduction half equations for this redox reactions: • Ag. NO 3 + Cu Ag + Cu(NO 3)2

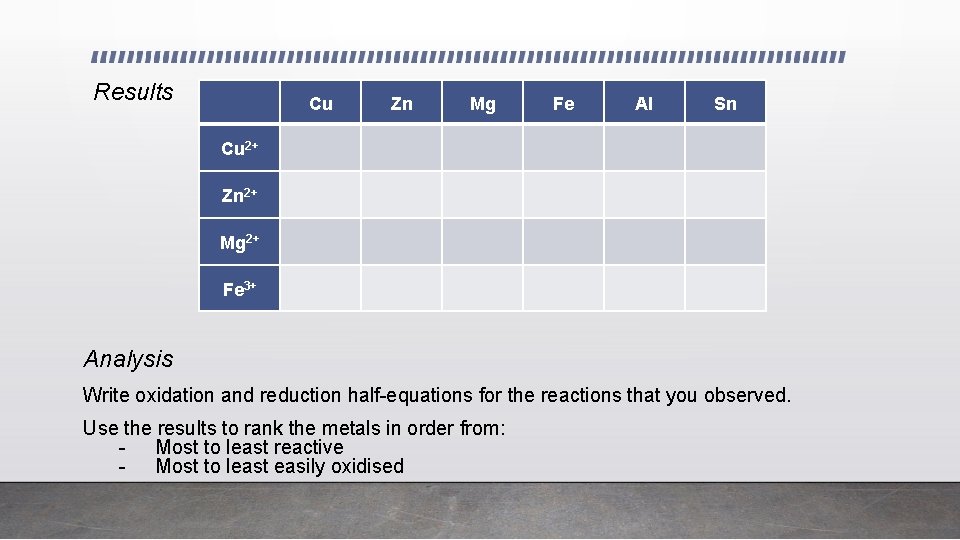
Experiment – Reactivity and Redox Reactions • Some metals are more reactive than others. In this experiment, a strip of metal is added to a solution of a compound of another metal. A more reactive metal displaces (pushes out) a less reactive metal from its compound. In carrying out the experiment, you will investigate competition reactions of metals and arrive at a reactivity series of the metals used.

Results Cu Zn Mg Fe Al Sn Cu 2+ Zn 2+ Mg 2+ Fe 3+ Analysis Write oxidation and reduction half-equations for the reactions that you observed. Use the results to rank the metals in order from: - Most to least reactive - Most to least easily oxidised

Interpretation of reactivity experiment • When a metal reacts with another metal ion solution, this means that • The metal is more reactive than the metal ion in solution • The metal is more easily oxidised than the metal ion solution • And vice versa • The metal ion in solution is less reactive than the metal • The metal ion in solution is more easily reduced than the metal

More terminology • OXIDIZING AGENT = the species that is reduced, by causing the other species to oxidise. Or, the electron acceptor. • REDUCING AGENT = the species that is oxidised, by causing the other species to reduce. Or, the electron donor. Identify the oxidizing and reducing agents in each of the reactions in the experiment.

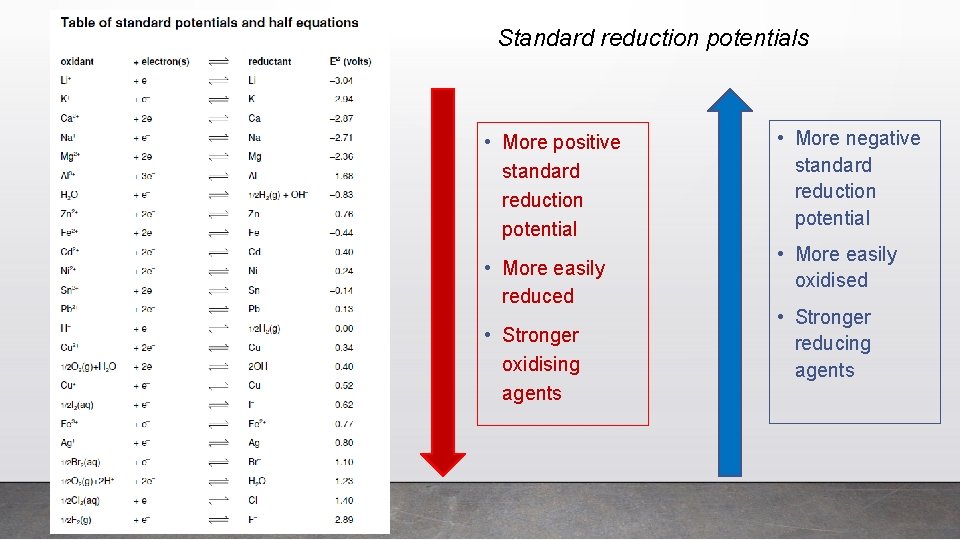
Standard reduction potentials • More positive standard reduction potential • More easily reduced • Stronger oxidising agents • More negative standard reduction potential • More easily oxidised • Stronger reducing agents

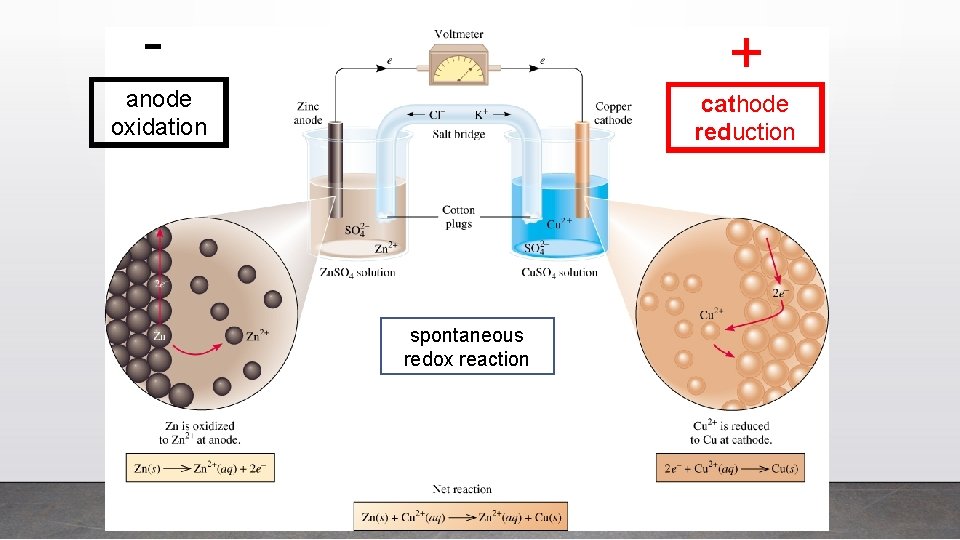
Galvanic Cells • Galvanic cells make use of the spontaneous redox reaction that occurs between metals and metal ions in solutions. • Galvanic cells produce electric current by separating the oxidizing and reducing agents in such a way that forces electron transfer through an external wire.

- + anode oxidation cathode reduction spontaneous redox reaction

In galvanic cells, • Oxidation always occurs at the anode, and the anode is negative. • Reduction always occurs at the cathode, and the cathode is positive. AN OIL RIG CAt OR AN OX & RED CAt Oxes are bad Cats are good (negative) (positive)

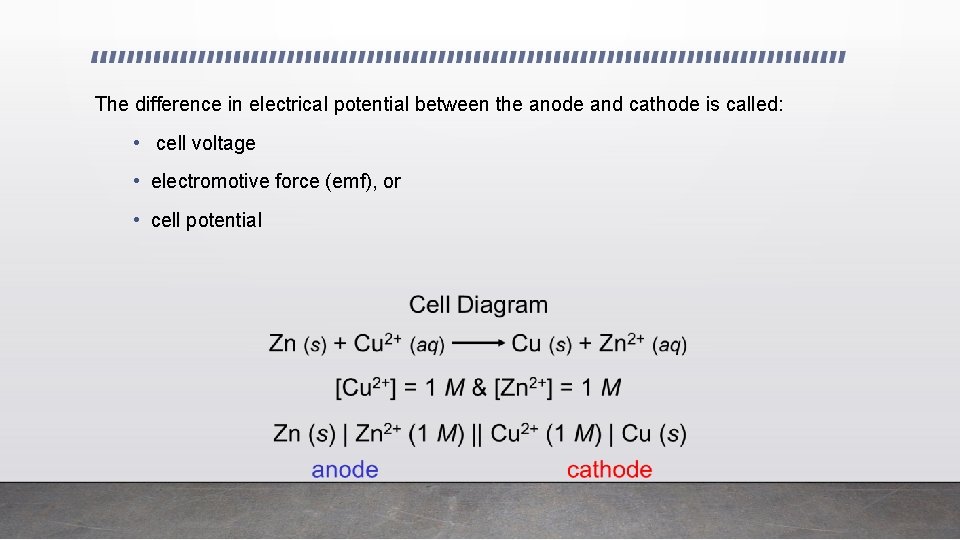
The difference in electrical potential between the anode and cathode is called: • cell voltage • electromotive force (emf), or • cell potential

• Note that when the shorthand notation Zn | Zn 2+ || Cu 2+ | Cu is used: • || represents a salt bridge • | represents a phase change from solid to solution • The anode (oxidation) is always written on the left, the cathode (reduction on the right)

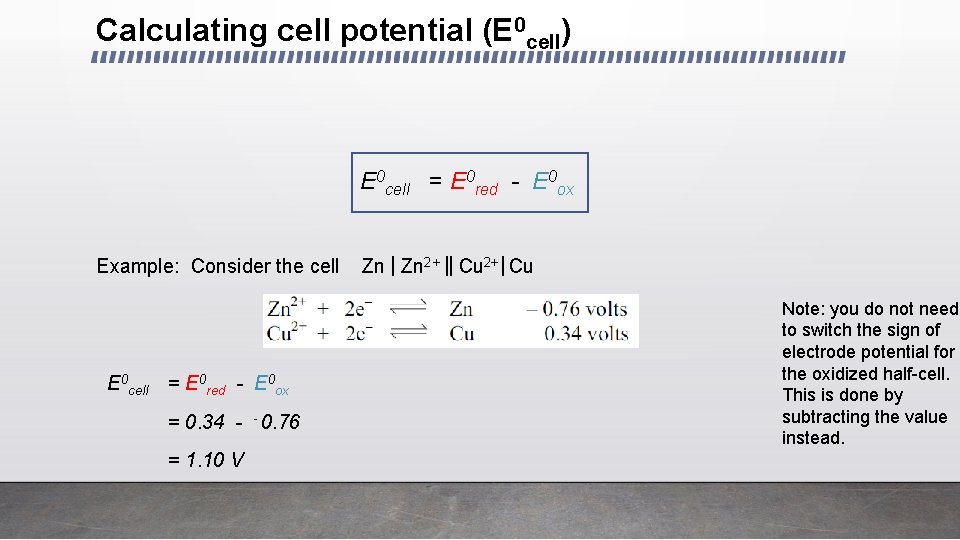
Calculating cell potential (E 0 cell) E 0 cell = E 0 red - E 0 ox Example: Consider the cell E 0 cell = E 0 red - E 0 ox = 0. 34 - - 0. 76 = 1. 10 V Zn | Zn 2+ || Cu 2+ | Cu Note: you do not need to switch the sign of electrode potential for the oxidized half-cell. This is done by subtracting the value instead.

Practice Questions 1. Draw labelled diagrams for the following galvanic cells. 2. Calculate the cell potential for each.

• Voltaic Cell Simulations http: //content. blackgold. ca/ict/Division 4/Science/Div. %204/Voltaic%20 Cells/Voltaic. htm • Experiment – Investigating and Daniell cell • Experiment – Reduction Potentials

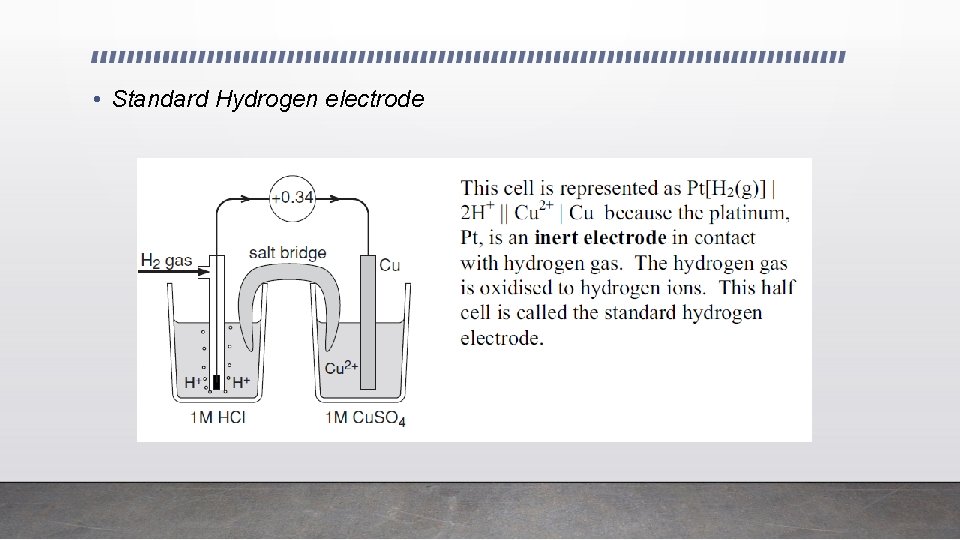
• Standard Hydrogen electrode

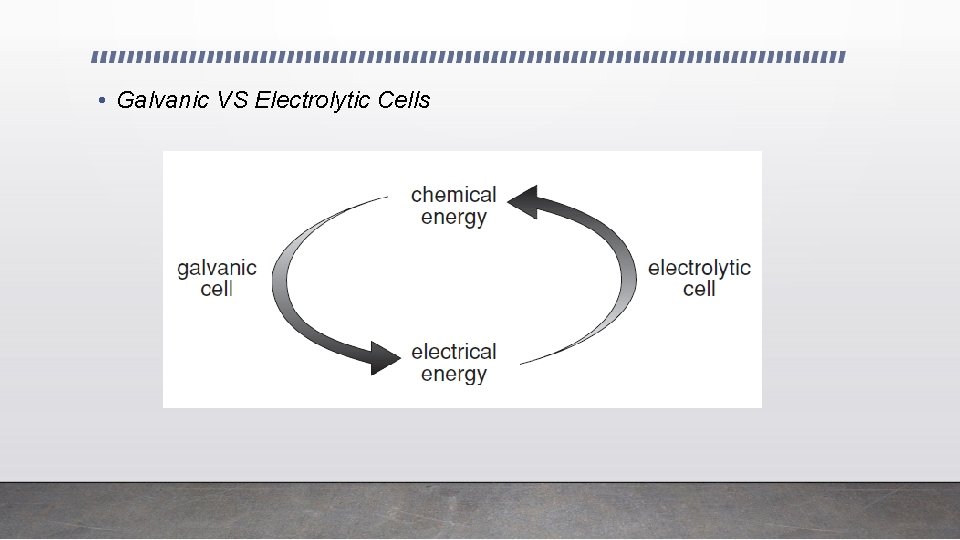
• Galvanic VS Electrolytic Cells

Corrosion • Water soluble minerals such as sodium chloride provide charged ions that can move easily through sea water. • Corrosion reactions are redox reactions that involve electron transfer from metal. • These reactions require movement of ions in the electrolyte between the anode and cathode, and so salty seawater facilitates corrosion.

• The metal commonly used in oceangoing vessels is iron in the form of a steel alloy. • Experiment – Percentage of iron in steel wool • Experiment – Investigating Corrosion

The process of rusting • Corrosion of a metal involves oxidation of metal atoms to metal ions so that the metal structure is broken down. • Rusting of iron on the surface of iron or steel is the most common type of corrosion. The iron atoms become part of a layer of hydrated iron(III) oxide. • The formula for rust is Fe 2 O 3. x. H 2 O where x can be from 0. 5 to 2 and colour from orange-brown to black colour.

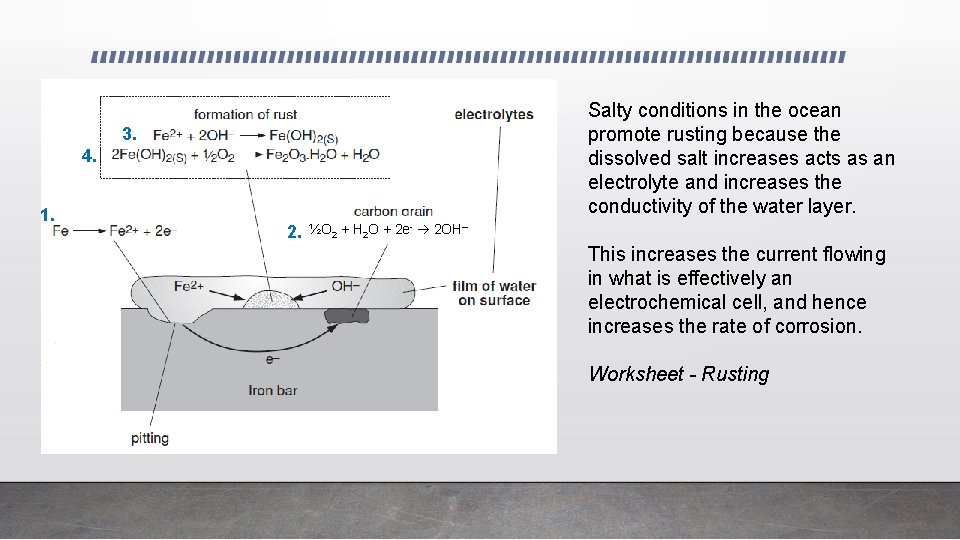
1. The oxidation of iron occurs first to iron(II) ions. Fe 2+ + 2 e. This causes pitting on the surface, as the soluble iron(II) ions can move away in any moisture on the surface of the iron. The part of the iron surface where pitting occurs is called the anode (since oxidation is occurring here) 2. At another part of the iron, non-metal impurities such as carbon can act as a cathode. Electrons released from the oxidation of iron atoms in the anode region can pass through the metal (a good conductor) to the non-metal cathode. At the cathode, oxygen gas and water are reduced by these electrons to form hydroxide ions. ½O 2 + H 2 O + 2 e- 2 OH–

3. The hydroxide ions formed can move through any moisture on the surface of the iron. When iron(II) ions and hydroxide ions combine in the moisture layer they form insoluble light green iron(II) hydroxide. Fe 2+ + 2 OH- Fe(OH)2(s) 4. Oxygen easily oxidises the iron(II) ions in Fe(OH)2 to iron(III) ions forming rust. 2 Fe(OH)2 + ½O 2 Fe 2 O 3. H 2 O(s) + H 2 O rust The rust can form some distance away from the pitting or erosion of iron. This is because electrons released at the pitting site can be conducted through the metal and iron(II) ions can diffuse through the surface water layer to wherever oxygen is available.

Salty conditions in the ocean promote rusting because the dissolved salt increases acts as an electrolyte and increases the conductivity of the water layer. 3. 4. 1. 2. ½O 2 + H 2 O + 2 e- 2 OH– This increases the current flowing in what is effectively an electrochemical cell, and hence increases the rate of corrosion. Worksheet - Rusting

Active and passivating metals • Use the standard reduction potential table to order these metals in order from most reactive at top (most easily oxidised) to lease reactive at the bottom. • Copper, tin, zinc, magnesium, chromium, iron, lead, cadmium, aluminium, nickel • Compare the reactivity of the two metals used in largest amounts in ship construction - aluminium and iron. • Much more effort goes into protecting the less active metal iron from corrosion.

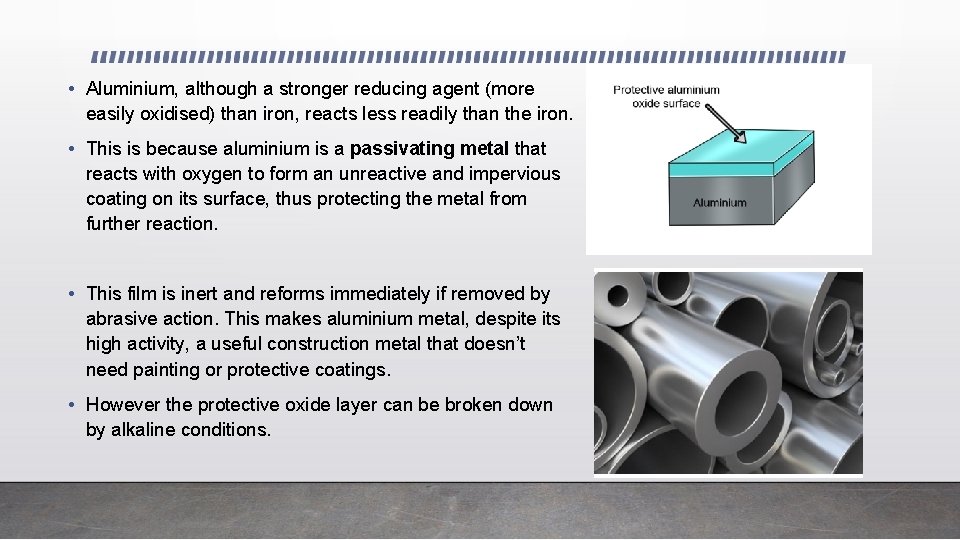
• Aluminium, although a stronger reducing agent (more easily oxidised) than iron, reacts less readily than the iron. • This is because aluminium is a passivating metal that reacts with oxygen to form an unreactive and impervious coating on its surface, thus protecting the metal from further reaction. • This film is inert and reforms immediately if removed by abrasive action. This makes aluminium metal, despite its high activity, a useful construction metal that doesn’t need painting or protective coatings. • However the protective oxide layer can be broken down by alkaline conditions.

• The chromium used in stainless steel and tin coating of tin cans has thin passivating layers which maintain their shiny metallic appearance. • On the other hand the oxide layer that forms more slowly on iron, i. e. rust, flakes off easily. The outer rust layer is porous allowing oxygen and water to penetrate and convert the iron layer below the surface to rust. • Iron is an active metal. Active metals are reactive metals that forms a nonadherent and porous oxide layer. The oxide layer of active metals can flake off, allowing continued corrosion. Most metals are active metals and exist in nature as compounds.


Predicting corrosion • Galvanic corrosion is caused by the formation of a galvanic cell, when two metals are in contact with an electrolyte solution. • The further apart the reduction potentials of two metals are, the greater the possibility of a galvanic corrosion cell forming between them. • Question - Predict which metal will be the anode and which will be the cathode in the following metal pairs. • • • zinc and iron copper and zinc iron and magnesium copper and iron and tin. Note: The E 0 values in the table of reduction potentials are for one molar solutions, gases at atmospheric pressure and a temperature of 25°C. If conditions are different from any of these conditions then predictions made, based upon these conditions, may not be fulfilled.

• Other factors which can affect the extent of corrosion: • impurities in the metal, • build up of gas layers on the surface of an electrode, • distance between the anode and cathode, • cathode/anode area ratios. • Corrosion can also occur on the one metal if conditions are different at different locations. 1. If a piece of metal has different oxygen concentrations at different locations, this differential aeration cause a galvanic cell to exist between these two locations. For example, in metal supports of wharves where the top of the support is in air and other parts are in the tidal zone or submersed in seawater. Anode (oxidation): The iron that has least exposure to oxygen will corrode. Cathode (reduction): The iron which is most exposed to oxygen.

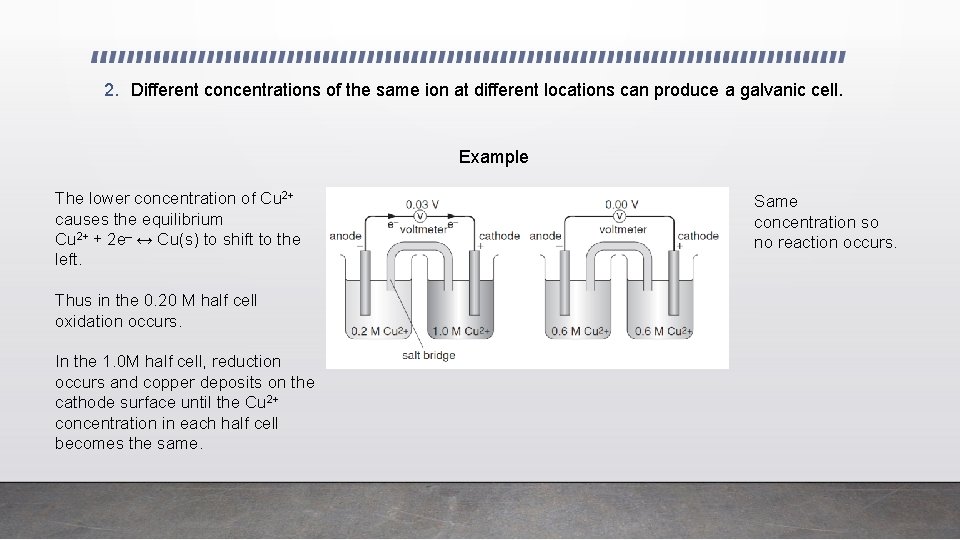
2. Different concentrations of the same ion at different locations can produce a galvanic cell. Example The lower concentration of Cu 2+ causes the equilibrium Cu 2+ + 2 e– ↔ Cu(s) to shift to the left. Thus in the 0. 20 M half cell oxidation occurs. In the 1. 0 M half cell, reduction occurs and copper deposits on the cathode surface until the Cu 2+ concentration in each half cell becomes the same. Same concentration so no reaction occurs.

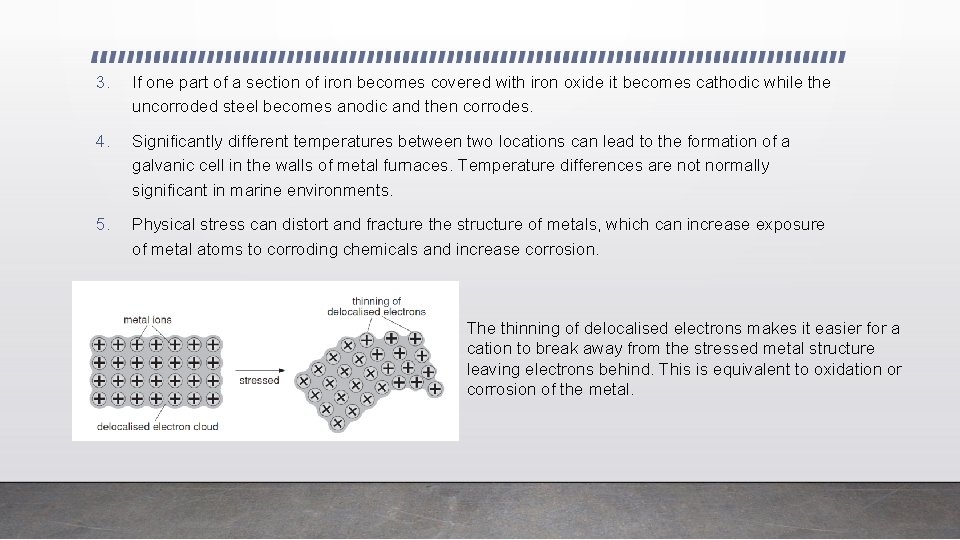
3. If one part of a section of iron becomes covered with iron oxide it becomes cathodic while the uncorroded steel becomes anodic and then corrodes. 4. Significantly different temperatures between two locations can lead to the formation of a galvanic cell in the walls of metal furnaces. Temperature differences are not normally significant in marine environments. 5. Physical stress can distort and fracture the structure of metals, which can increase exposure of metal atoms to corroding chemicals and increase corrosion. The thinning of delocalised electrons makes it easier for a cation to break away from the stressed metal structure leaving electrons behind. This is equivalent to oxidation or corrosion of the metal.

Corrosion Protection Metal coatings to protect steel • Tin plating - a physical barrier, eg. tin cans are made from steel with a very thin coating of tin. • Galvanising with zinc – a chemical barrier Galvanising is covering a metal with a zinc layer which itself becomes covered with a layer of dull white zinc hydroxide/carbonate that protects it from further corrosion. When the surface of zinc is scratched through, exposing the steel, a galvanic cell is formed involving zinc and iron when moisture is present: anode: Zn 2+ + 2 ecathode: 2 H 2 O + O 2 + 4 e– 4 OH–

The zinc ions and hydroxide ions form zinc hydroxide Zn 2+ + 2 OH– Zn(OH)2 which loses water to form a protective coating of zinc oxide over exposed steel. Zn(OH)2 Zn. O + H 2 O Zinc carbonate can also form to further protect the exposed steel Zn. O + CO 2 Zn. CO 3 Experiment – Corrosion of iron in feroxyl gel

Metal hull protection • Older methods: • lead and copper sheeting over timber • coal tar, asphalt and other petroleum based coatings • varnishes made from plant resins and oils. • More recent methods: • using corrosion resistant metals and alloys, e. g. surface alloys • new paints which: – prevent metal contact with electrolytes or oxygen or water e. g. Rustmaster Pro® – contain more active metals such as zinc which corrode first – release biocides such as copper or tin which inhibit marine organism growth (but cause environmental concerns)

Passivating metal and alloys • Aluminium, titanium and stainless steels have durable oxide layers on their surface. The oxide layer does not conduct electricity so the metal cannot form a galvanic cell with other metals and therefore resists corrosion. • If placed in a low oxygen environment (such as the reducing conditions in sea water or sediment) the oxide layer can be removed or damaged and corrosion occurs. • Alkaline conditions can also dissolve the protective oxide layer.

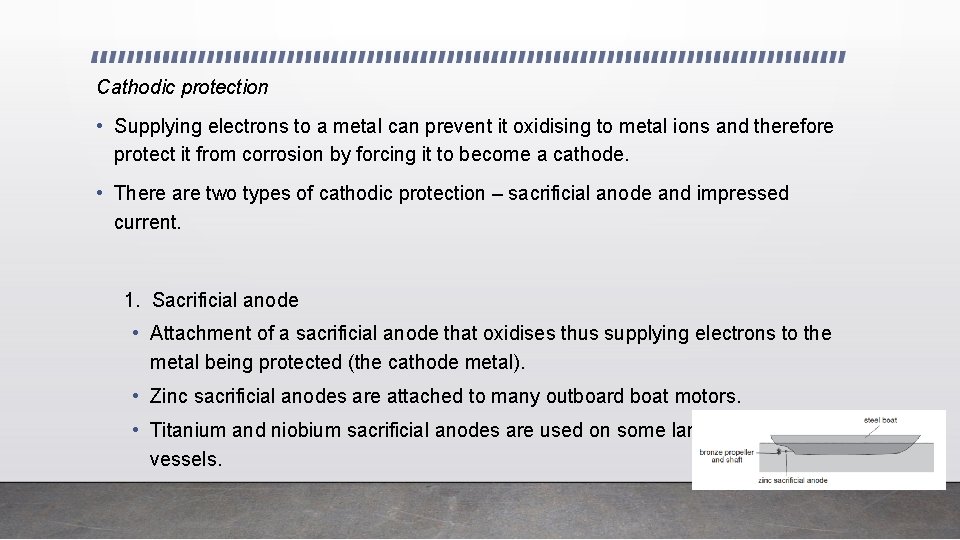
Cathodic protection • Supplying electrons to a metal can prevent it oxidising to metal ions and therefore protect it from corrosion by forcing it to become a cathode. • There are two types of cathodic protection – sacrificial anode and impressed current. 1. Sacrificial anode • Attachment of a sacrificial anode that oxidises thus supplying electrons to the metal being protected (the cathode metal). • Zinc sacrificial anodes are attached to many outboard boat motors. • Titanium and niobium sacrificial anodes are used on some large oceangoing vessels.

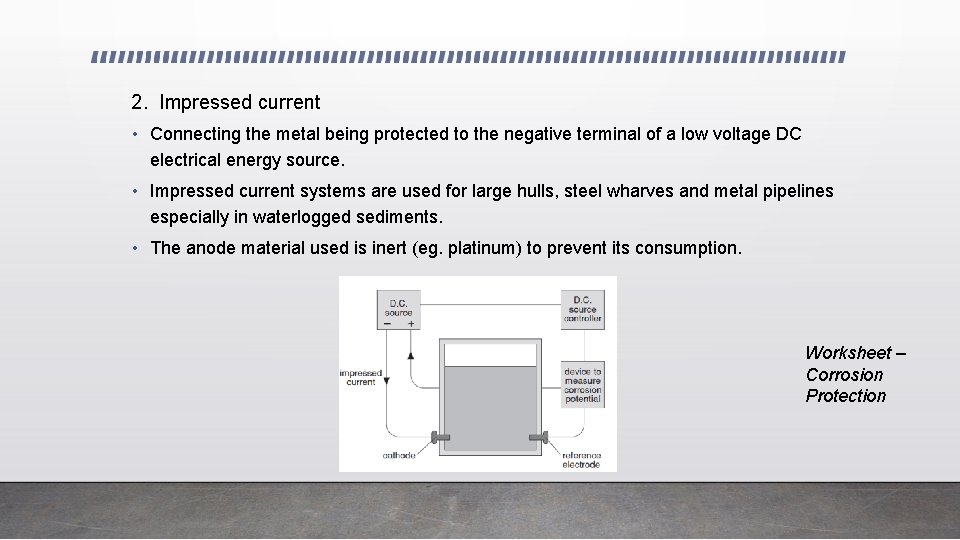
2. Impressed current • Connecting the metal being protected to the negative terminal of a low voltage DC electrical energy source. • Impressed current systems are used for large hulls, steel wharves and metal pipelines especially in waterlogged sediments. • The anode material used is inert (eg. platinum) to prevent its consumption. Worksheet – Corrosion Protection

Rates of Corrosion • The rate of corrosion of a wreck will depend on its final depth, which will determine the temperature and pressure of dissolved gases and the concentration of dissolved salts. Temperature, pressure and solubility • At greater depths, there will be an increase in pressure and a decrease in temperature. • Decreasing the temperature will increase the concentration of dissolved gases including oxygen. • Increasing the pressure will increase the concentration of dissolved gases. • The greater the pressure of gas above a liquid the more particles there are colliding with the surface and the greater the solubility. • The solubility of salts will not be affected by pressures, but generally decreases with a decrease in temperature (depending on the solubility equilibrium)

Rate of metal corrosion • The environmental factors that are most important are listed below. • Oxygen concentration. The higher the oxygen concentration, the greater the rate of oxidation. • Salinity. The higher the salinity, the greater the conductivity and the greater the rate galvanic corrosion. • Temperature. The lower the temperature, the slower the rate of reaction. • p. H. The more acidic the p. H, the greater the rate of corrosion for most metals. Worksheet – Solubility at Depths, Case Study – The Titanic of