Ocean Acidification Todays Talk on Ocean Acidification The






















- Slides: 39

Ocean Acidification

Today’s Talk on Ocean Acidification • The Consequences: What does ocean acidification mean for natural ecosystems and humans? • The Science: Understand why ocean acidification spells trouble for shell-building organisms. • The Solutions: What can we do about this problem?

How big is the ocean “carbon pool” relative to land atmosphere? 1. Much smaller. 2. About the same. 3. Much bigger.

According to the May 2008 Seattle Times article, ocean acidification is not confined to the deep ocean due to: 1. Increased alkalinity 2. Natural upwelling 3. Colder waters 4. Dead plankton

Organisms that building their shells from calcium carbonate are negatively impacted by ocean acidification due to a decrease in: 1. Methane dissolution 2. Nitrogen and phosphorous 3. Carbonate ions 4. General happiness

The Consequences The shells of marine organisms will dissolve.

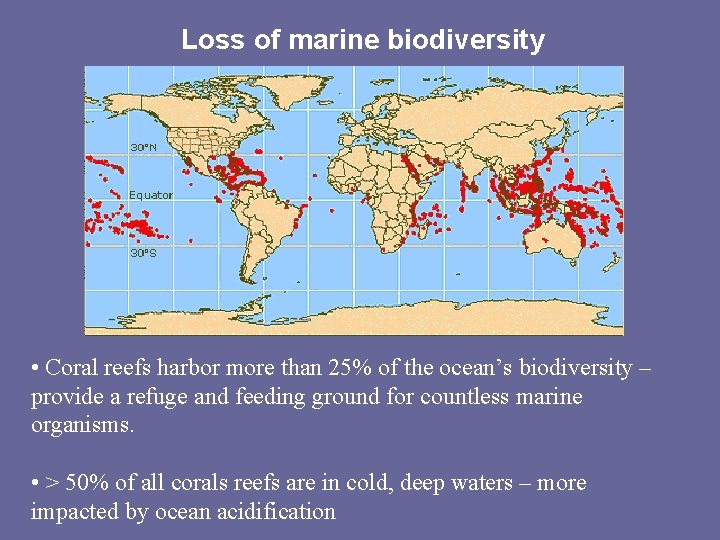
Loss of marine biodiversity • Coral reefs harbor more than 25% of the ocean’s biodiversity – provide a refuge and feeding ground for countless marine organisms. • > 50% of all corals reefs are in cold, deep waters – more impacted by ocean acidification

Loss of food sources (fish, shellfish, etc) for subsistence food gathering

Loss of sources of income for local communities, often in developing countries Fishing Ecotourism

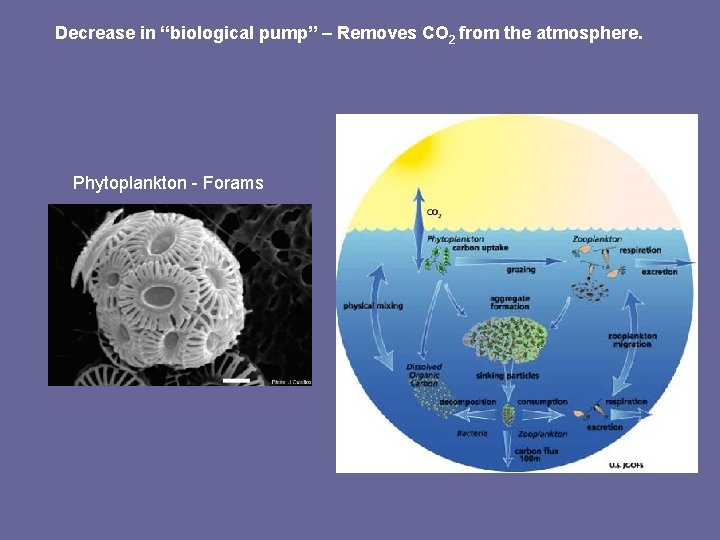
Decrease in “biological pump” – Removes CO 2 from the atmosphere. Phytoplankton - Forams

The Science Why ocean acidification is occurring Why it harms marine organisms

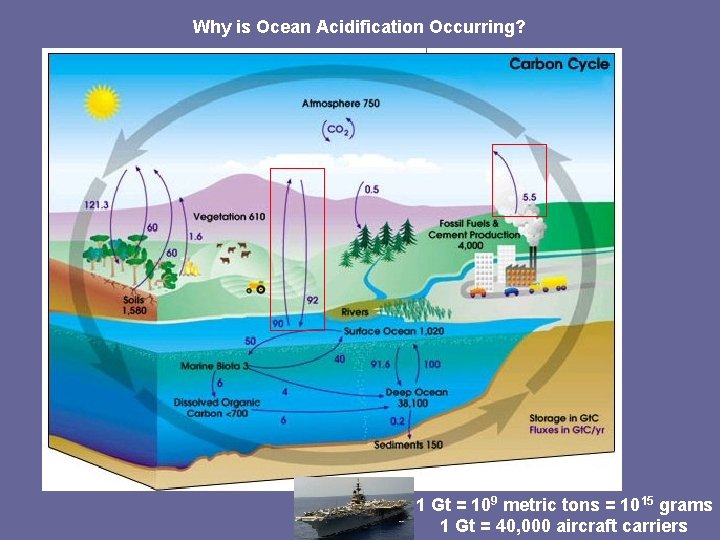
Why is Ocean Acidification Occurring? 1 Gt = 109 metric tons = 1015 grams 1 Gt = 40, 000 aircraft carriers

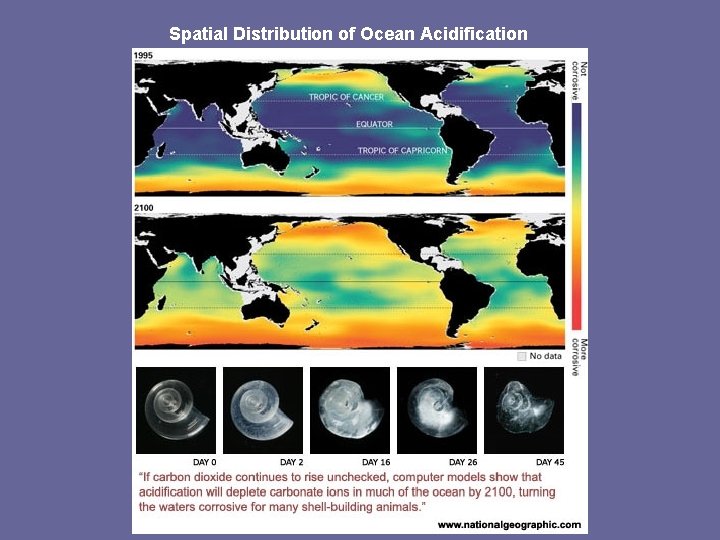
Spatial Distribution of Ocean Acidification

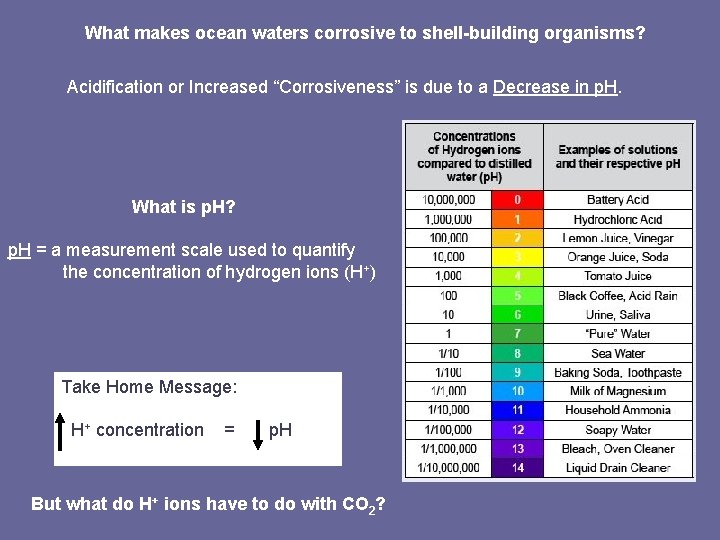
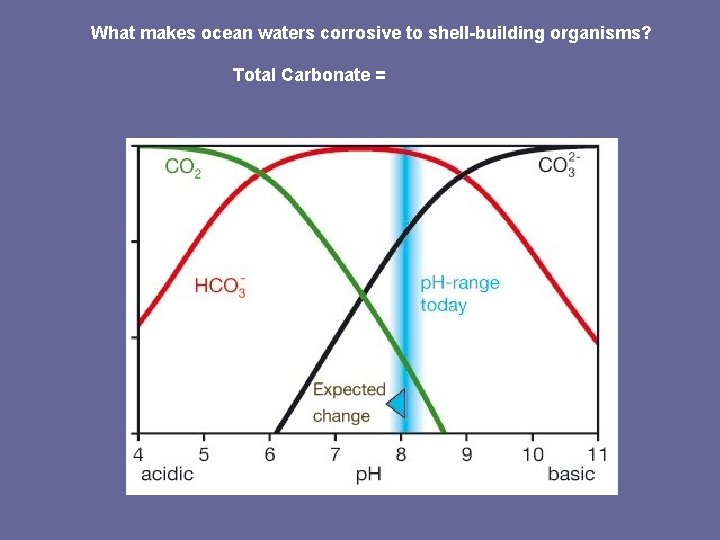
What makes ocean waters corrosive to shell-building organisms? Acidification or Increased “Corrosiveness” is due to a Decrease in p. H. What is p. H? p. H = a measurement scale used to quantify the concentration of hydrogen ions (H+) Take Home Message: H+ concentration = p. H But what do H+ ions have to do with CO 2?

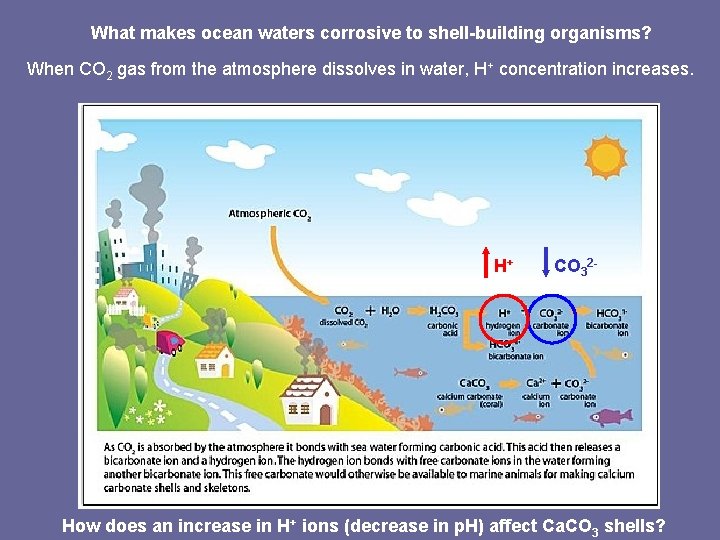
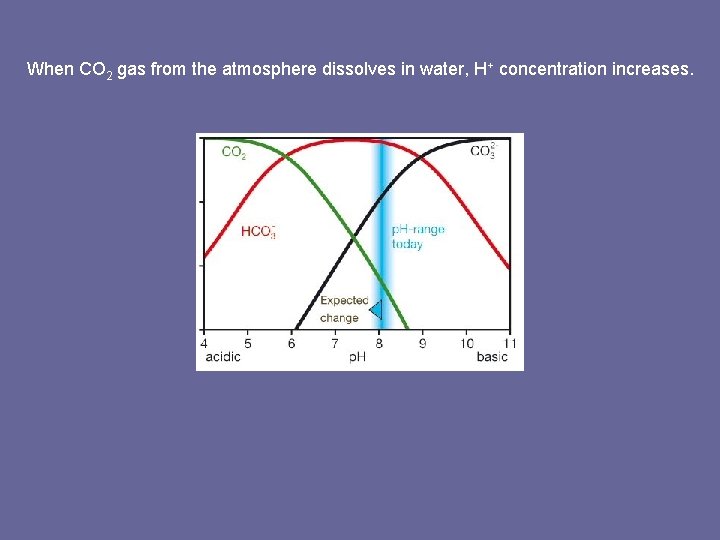
What makes ocean waters corrosive to shell-building organisms? When CO 2 gas from the atmosphere dissolves in water, H+ concentration increases. H+ CO 32 - How does an increase in H+ ions (decrease in p. H) affect Ca. CO 3 shells?

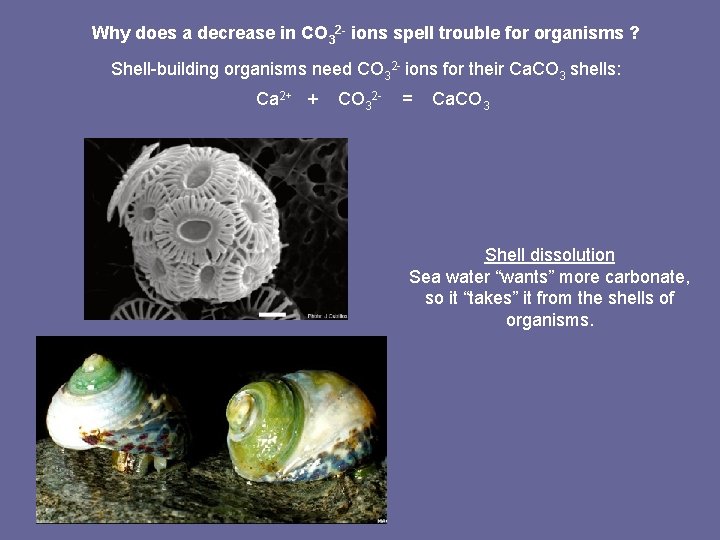
Why does a decrease in CO 32 - ions spell trouble for organisms ? Shell-building organisms need CO 32 - ions for their Ca. CO 3 shells: Ca 2+ + CO 32 - = Ca. CO 3 Shell dissolution Sea water “wants” more carbonate, so it “takes” it from the shells of organisms.

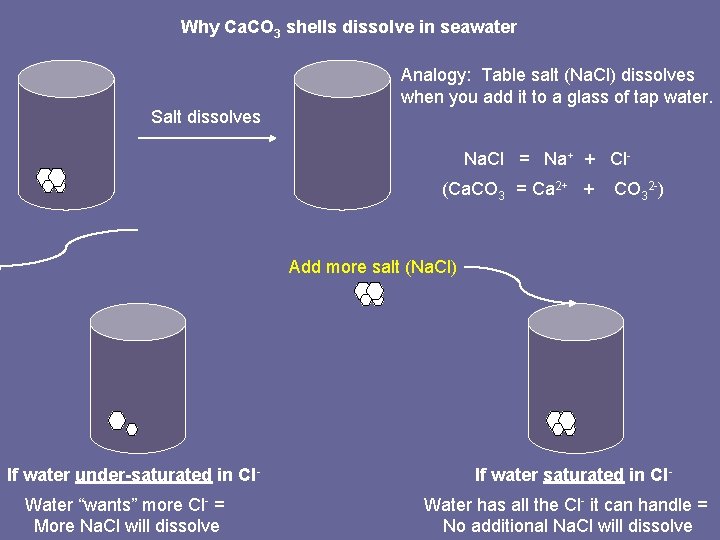
Why Ca. CO 3 shells dissolve in seawater Analogy: Table salt (Na. Cl) dissolves when you add it to a glass of tap water. Salt dissolves Na. Cl = Na+ + Cl(Ca. CO 3 = Ca 2+ + CO 32 -) Add more salt (Na. Cl) If water under-saturated in Cl. Water “wants” more Cl- = More Na. Cl will dissolve If water saturated in Cl. Water has all the Cl- it can handle = No additional Na. Cl will dissolve

Back to the ocean: Why do Ca. CO 3 shells dissolve in seawater? 2+ 2 Shells 3 2 Shells are made of of Ca. CO 33 = = Ca Ca 2+ ++ CO CO 3 H+ CO 32 - The pressure generated by CO 2 gas dissolved in the water causes the Ca. CO 3 shells to explode. The decrease in the p. H of ocean water due to the input of atmospheric CO 2 results in and ocean that is saturated in CO 32 -. The ocean is made more acidic when CO 2 from the atmosphere results in an increase in the H+ ion concentration and an under-saturation of CO 32 - in the ocean.

Why do Ca. CO 3 shells dissolve in seawater? 1. Pressure generated by CO 2 2. Decreased p. H 3. Increase in H+ and undersaturation of CO 32 -

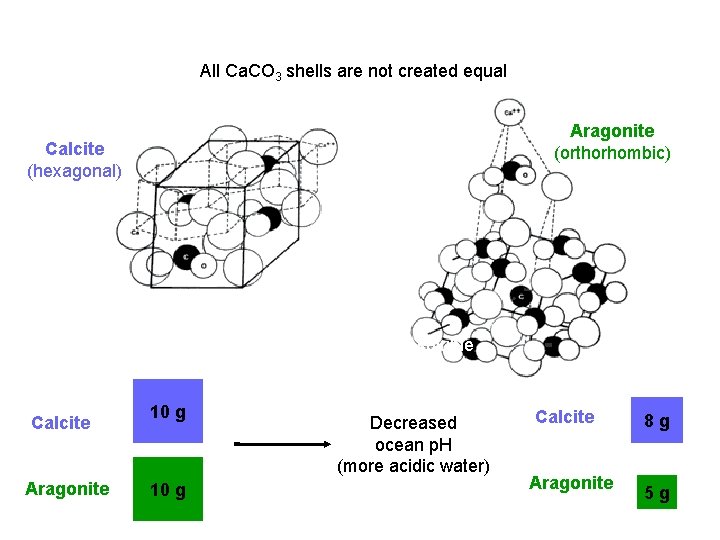
All Ca. CO 3 shells are not created equal Aragonite (orthorhombic) Calcite (hexagonal) Aragonite is more soluble Calcite Aragonite 10 g Decreased ocean p. H (more acidic water) Calcite Aragonite 8 g 5 g

All Ca. CO 3 shells are not created equal Organism Form of Ca. CO 3 Foraminifera Calcite Coccolithophores Calcite Macroalgae Aragonite or Calcite Corals: Aragonite warm water cold water Pteropod molluscs Aragonite Crustaceans Calcite Echinoderms (sea urchin) Calcite

The Solutions What can we do about ocean acidification?

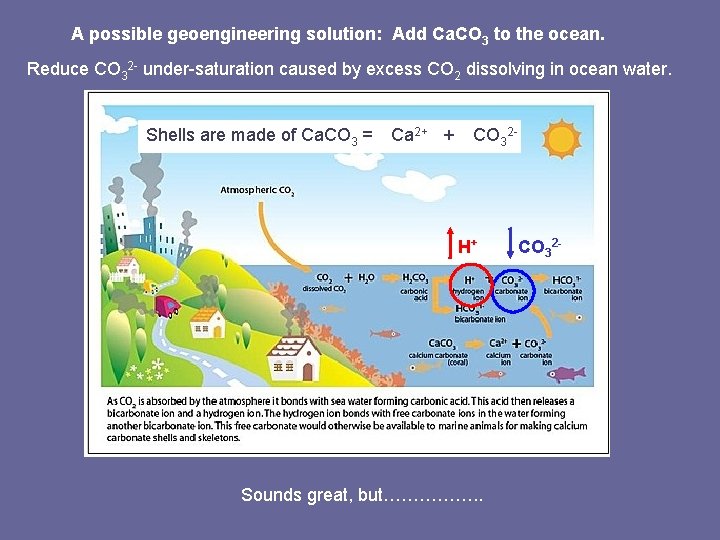
A possible geoengineering solution: Add Ca. CO 3 to the ocean. Reduce CO 32 - under-saturation caused by excess CO 2 dissolving in ocean water. 2+ 2 Shells 3 2 Shells are made of of Ca. CO 33 = = Ca Ca 2+ ++ CO CO 3 H+ Sounds great, but……………. . CO 32 -

To counteract 2 Gt C/yr input of CO 2, would need 20 Gt Ca. CO 3/yr. White Cliffs of Dover would be rapidly consumed. Limestone Rock (Ca. CO 3) • Limestone mining would be expensive and would cause ecological damage. • All the energy needed to move massive amounts of rock into the ocean would likely add more CO 2 to the atmosphere.

Stop adding CO 2 to the atmosphere

Questions?




What makes ocean waters corrosive to shell-building organisms? Total Carbonate =

The p. H change is small: What’s the big deal? • Seattle Times article: p. H changed from 8. 1 to 7. 6 along Pacific Coast of the US • Turley February 2008 article: Average p. H of entire ocean has changed by 0. 1 p. H units What is p. H? p. H = a measurement scale used to quantify the concentration of hydrogen ions (H+) p. H = - log (H+) Take Home Message: Small changes in p. H represent large changes in H+ concentration. p. H H+ 1 10000 2 10000000 3 1000000 4 100000 5 10000 6 1000 7 100 8 10 9 1 10 0. 1 11 0. 01 12 0. 001 13 0. 0001 14 0. 00001

When CO 2 gas from the atmosphere dissolves in water, H+ concentration increases.

All Ca. CO 3 is not equal – Corals made of aragonite will be more affected Calcite (shellfish, forams) and aragonite (corals) are both Ca. CO 3 minerals. Same chemical composition: Ca. CO 3

What can society do about Ocean Acidification? 1) Stop adding CO 2 to the atmosphere 1) Geoengineering (a) Fe fertilization – removes CO 2 from the atmosphere, but may have decreased effectiveness due to damage to phytoplankton that use calcium carbonate to build shells (b) Add alkalinity to the ocean – economic and ecological costs of this would be enormous

What is alkalinity?

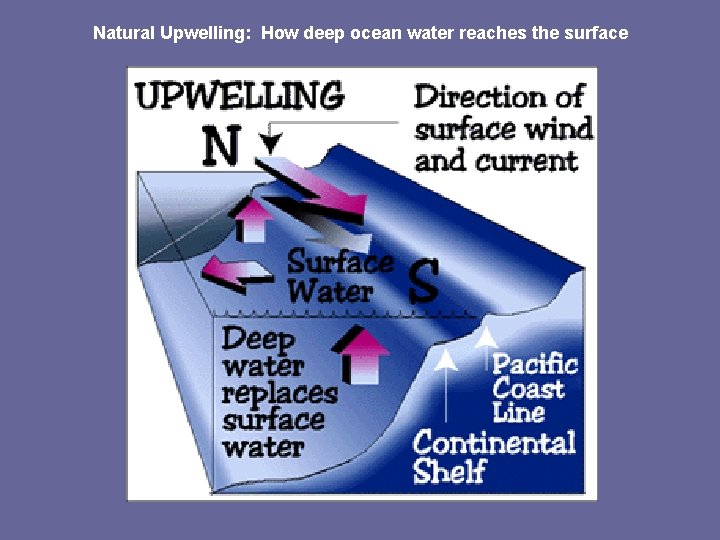
Natural Upwelling: How deep ocean water reaches the surface


