OCCAMs study day 30012015 Data Relevance Dr Jan






- Slides: 38

OCCAMs study day - 30/01/2015 – Data Relevance Dr Jan Bornschein

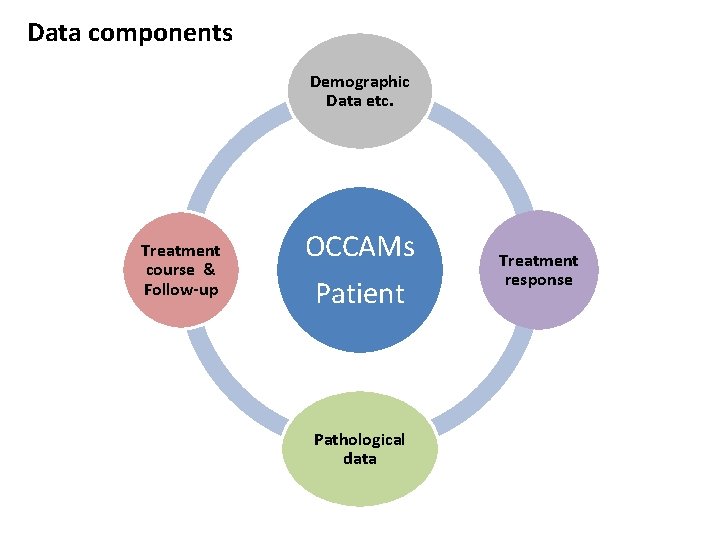
Data components Demographic Data etc. Treatment course & Follow-up OCCAMs Patient Pathological data Treatment response

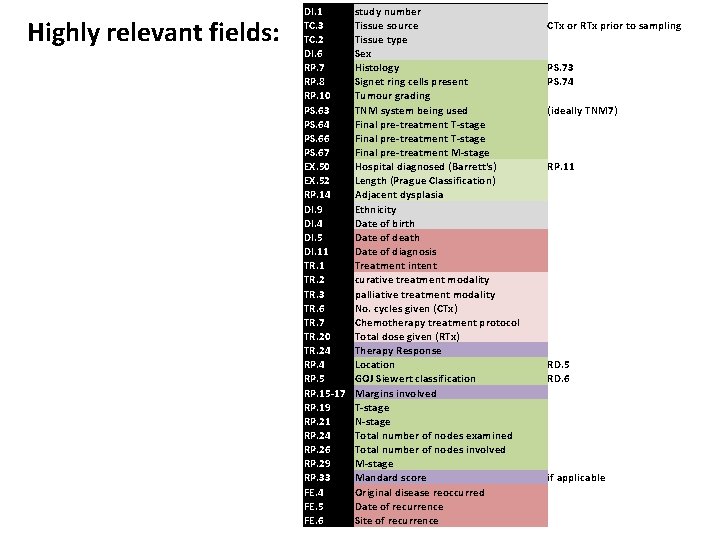
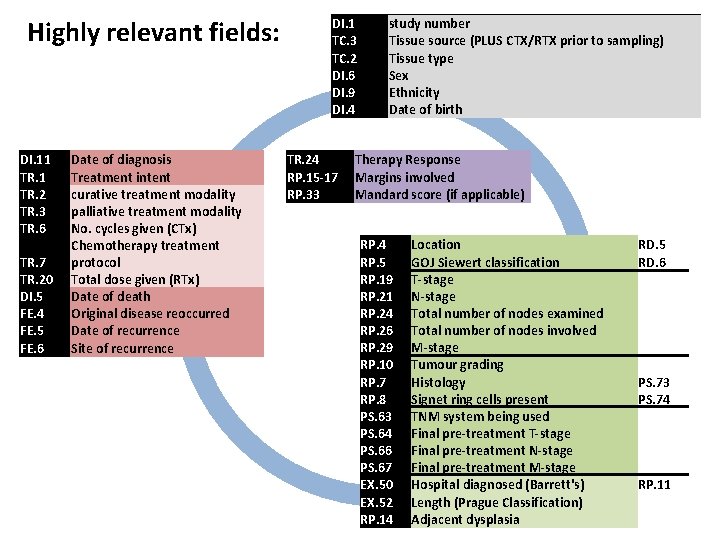
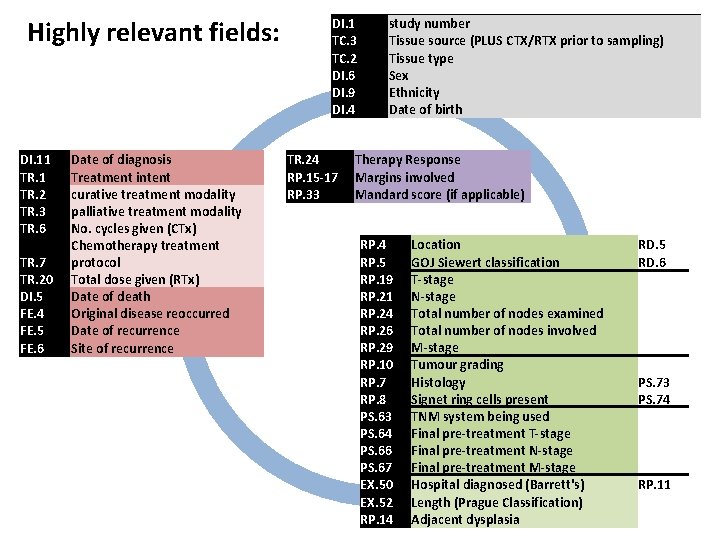
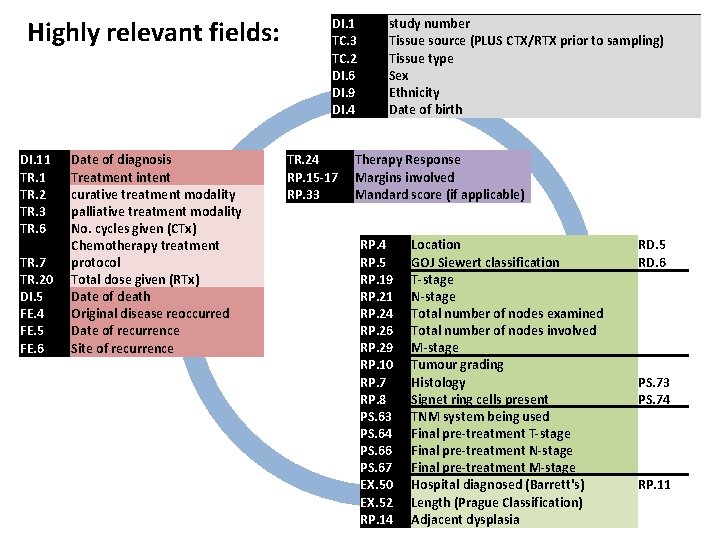
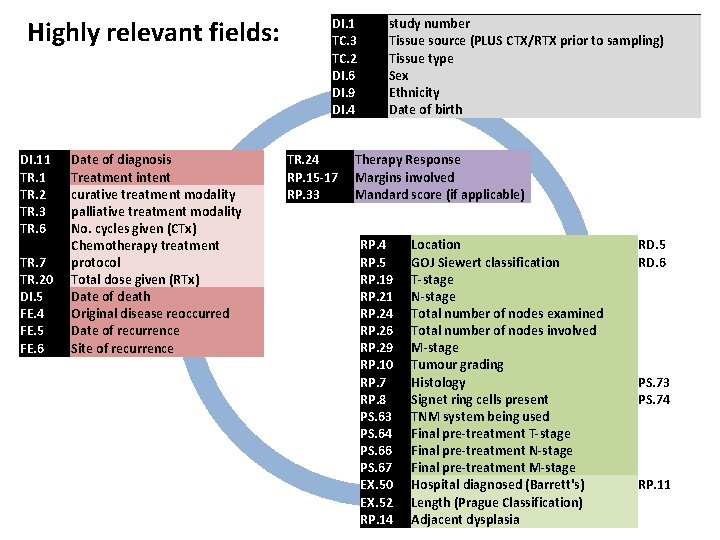
Highly relevant fields: DI. 1 TC. 3 TC. 2 DI. 6 RP. 7 RP. 8 RP. 10 PS. 63 PS. 64 PS. 66 PS. 67 EX. 50 EX. 52 RP. 14 DI. 9 DI. 4 DI. 5 DI. 11 TR. 2 TR. 3 TR. 6 TR. 7 TR. 20 TR. 24 RP. 5 RP. 15 -17 RP. 19 RP. 21 RP. 24 RP. 26 RP. 29 RP. 33 FE. 4 FE. 5 FE. 6 study number Tissue source Tissue type Sex Histology Signet ring cells present Tumour grading TNM system being used Final pre-treatment T-stage Final pre-treatment M-stage Hospital diagnosed (Barrett's) Length (Prague Classification) Adjacent dysplasia Ethnicity Date of birth Date of death Date of diagnosis Treatment intent curative treatment modality palliative treatment modality No. cycles given (CTx) Chemotherapy treatment protocol Total dose given (RTx) Therapy Response Location GOJ Siewert classification Margins involved T-stage N-stage Total number of nodes examined Total number of nodes involved M-stage Mandard score Original disease reoccurred Date of recurrence Site of recurrence CTx or RTx prior to sampling PS. 73 PS. 74 (ideally TNM 7) RP. 11 RD. 5 RD. 6 if applicable

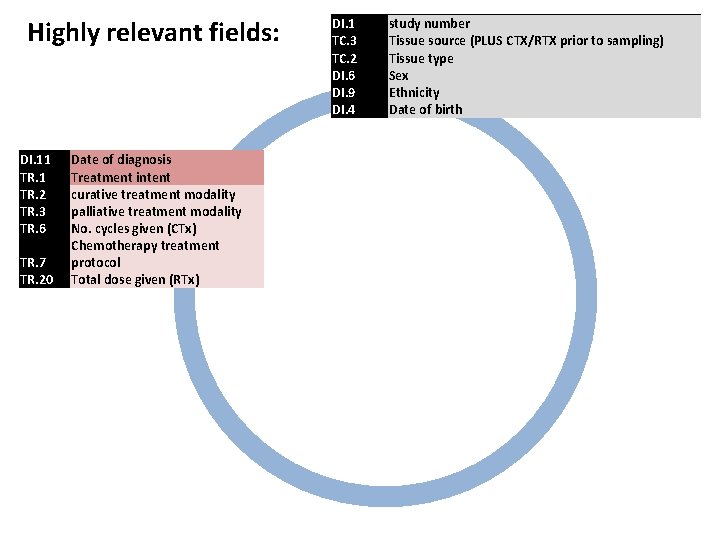
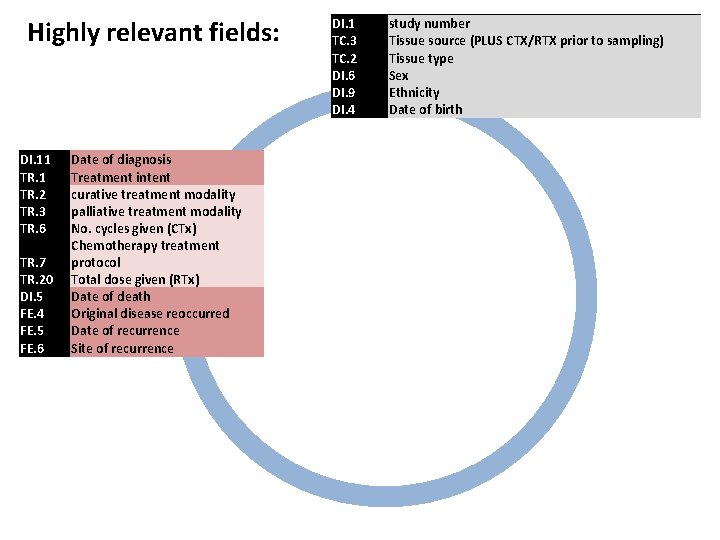
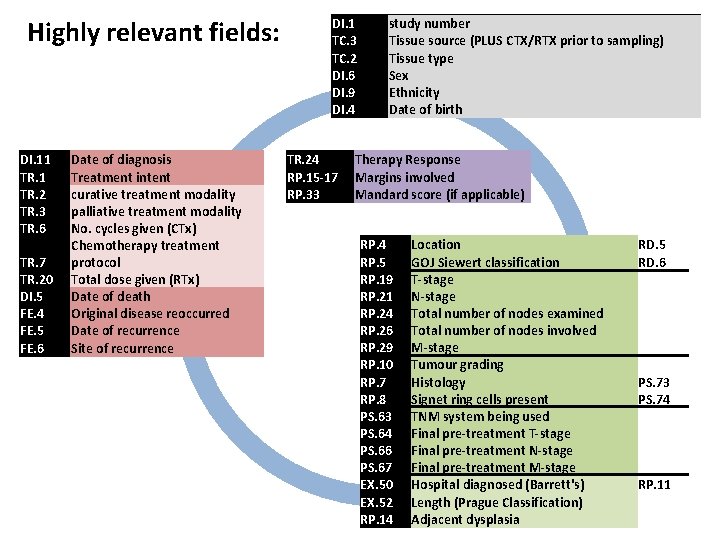
Highly relevant fields: DI. 11 TR. 2 TR. 3 TR. 6 TR. 7 TR. 20 Date of diagnosis Treatment intent curative treatment modality palliative treatment modality No. cycles given (CTx) Chemotherapy treatment protocol Total dose given (RTx) DI. 1 TC. 3 TC. 2 DI. 6 DI. 9 DI. 4 study number Tissue source (PLUS CTX/RTX prior to sampling) Tissue type Sex Ethnicity Date of birth

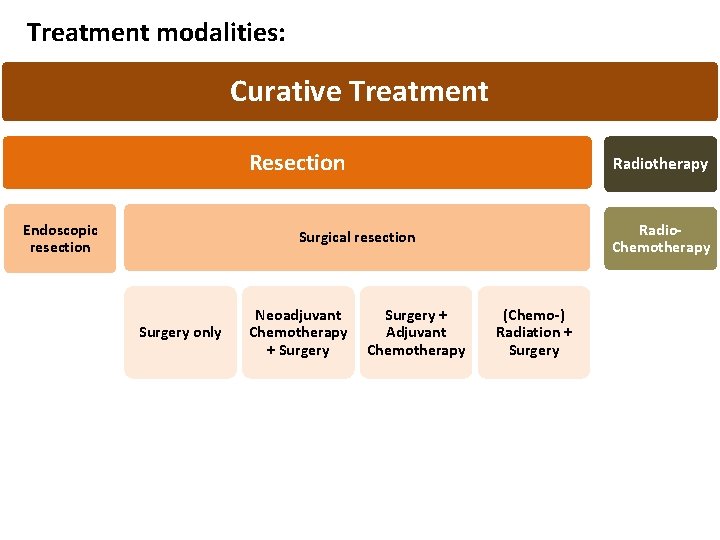
Treatment modalities: Curative Treatment Resection Endoscopic resection Radiotherapy Radio. Chemotherapy Surgical resection Surgery only Neoadjuvant Chemotherapy + Surgery + Adjuvant Chemotherapy (Chemo-) Radiation + Surgery

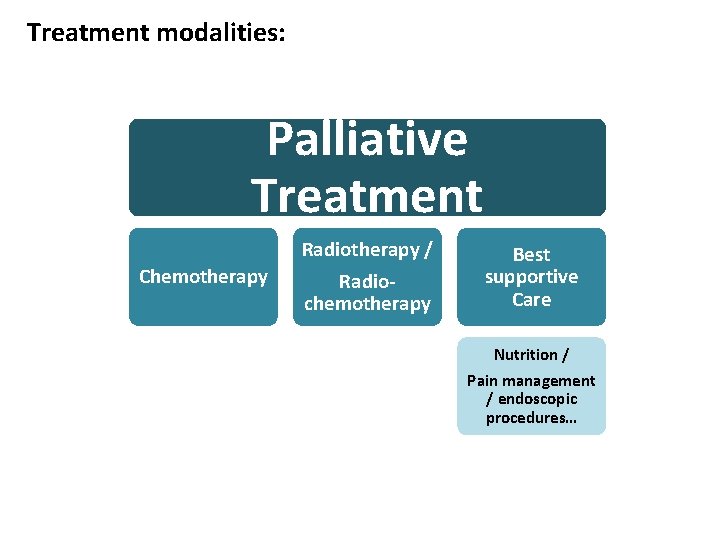
Treatment modalities: Palliative Treatment Chemotherapy Radiotherapy / Radiochemotherapy Best supportive Care Nutrition / Pain management / endoscopic procedures…

Highly relevant fields: DI. 11 TR. 2 TR. 3 TR. 6 TR. 7 TR. 20 DI. 5 FE. 4 FE. 5 FE. 6 Date of diagnosis Treatment intent curative treatment modality palliative treatment modality No. cycles given (CTx) Chemotherapy treatment protocol Total dose given (RTx) Date of death Original disease reoccurred Date of recurrence Site of recurrence DI. 1 TC. 3 TC. 2 DI. 6 DI. 9 DI. 4 study number Tissue source (PLUS CTX/RTX prior to sampling) Tissue type Sex Ethnicity Date of birth

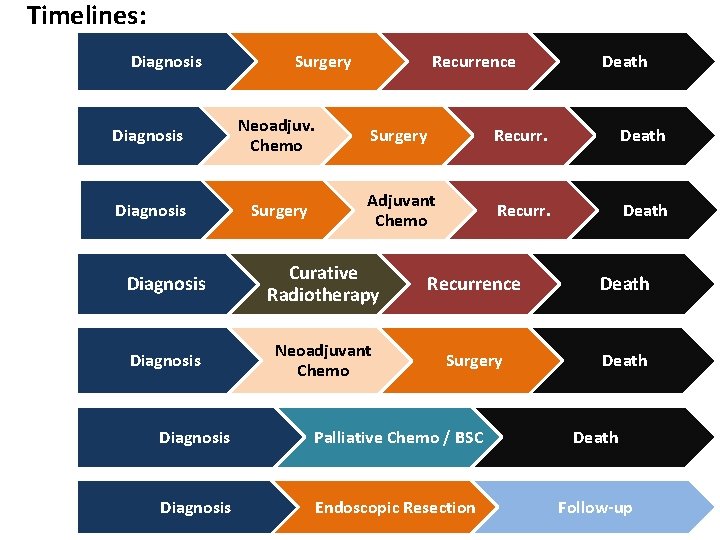
Timelines: Diagnosis Surgery Recurrence Death Diagnosis Neoadjuv. Chemo Surgery Recurr. Death Diagnosis Surgery Adjuvant Chemo Recurr. Death Diagnosis Curative Radiotherapy Recurrence Death Diagnosis Neoadjuvant Chemo Surgery Death Diagnosis Palliative Chemo / BSC Death Diagnosis Endoscopic Resection Follow-up

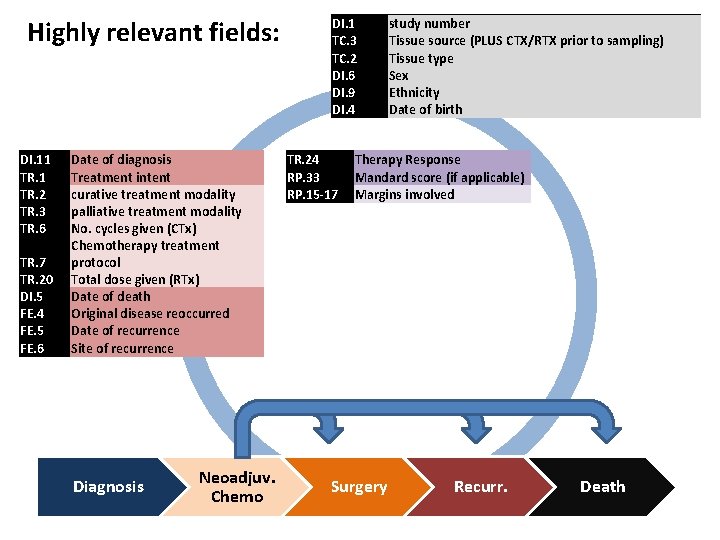
Highly relevant fields: DI. 11 TR. 2 TR. 3 TR. 6 TR. 7 TR. 20 DI. 5 FE. 4 FE. 5 FE. 6 Date of diagnosis Treatment intent curative treatment modality palliative treatment modality No. cycles given (CTx) Chemotherapy treatment protocol Total dose given (RTx) Date of death Original disease reoccurred Date of recurrence Site of recurrence Diagnosis Neoadjuv. Chemo DI. 1 TC. 3 TC. 2 DI. 6 DI. 9 DI. 4 TR. 24 RP. 33 RP. 15 -17 study number Tissue source (PLUS CTX/RTX prior to sampling) Tissue type Sex Ethnicity Date of birth Therapy Response Mandard score (if applicable) Margins involved Surgery Recurr. Death

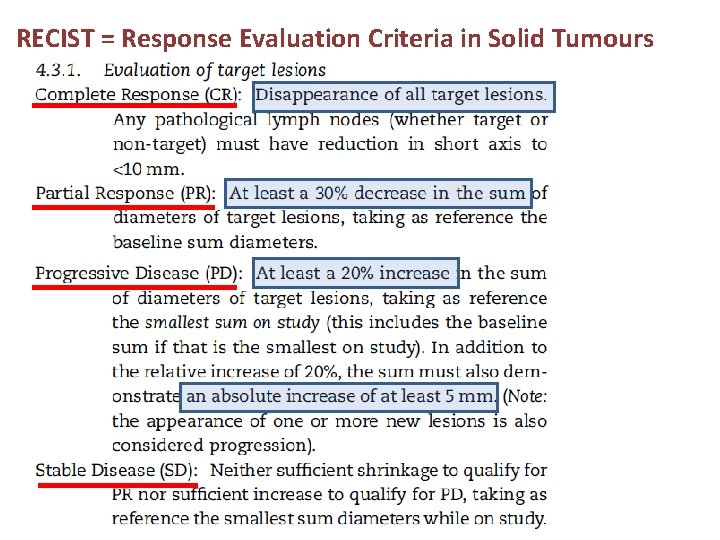
Treatment response (Radiology):

RECIST = Response Evaluation Criteria in Solid Tumours

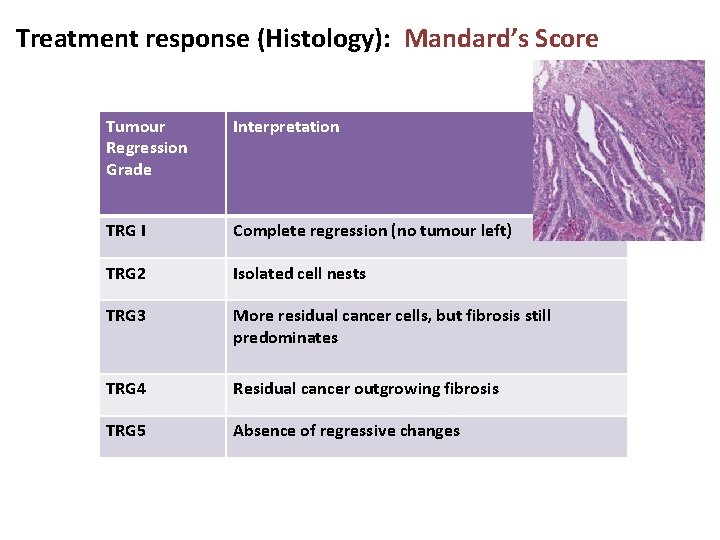
Treatment response (Histology): Mandard’s Score Tumour Regression Grade Interpretation TRG I Complete regression (no tumour left) TRG 2 Isolated cell nests TRG 3 More residual cancer cells, but fibrosis still predominates TRG 4 Residual cancer outgrowing fibrosis TRG 5 Absence of regressive changes

Highly relevant fields: DI. 11 TR. 2 TR. 3 TR. 6 TR. 7 TR. 20 DI. 5 FE. 4 FE. 5 FE. 6 Date of diagnosis Treatment intent curative treatment modality palliative treatment modality No. cycles given (CTx) Chemotherapy treatment protocol Total dose given (RTx) Date of death Original disease reoccurred Date of recurrence Site of recurrence DI. 1 TC. 3 TC. 2 DI. 6 DI. 9 DI. 4 TR. 24 RP. 15 -17 RP. 33 study number Tissue source (PLUS CTX/RTX prior to sampling) Tissue type Sex Ethnicity Date of birth Therapy Response Margins involved Mandard score (if applicable) RP. 4 RP. 5 RP. 19 RP. 21 RP. 24 RP. 26 RP. 29 RP. 10 RP. 7 RP. 8 PS. 63 PS. 64 PS. 66 PS. 67 EX. 50 EX. 52 RP. 14 Location GOJ Siewert classification T-stage N-stage Total number of nodes examined Total number of nodes involved M-stage Tumour grading Histology Signet ring cells present TNM system being used Final pre-treatment T-stage Final pre-treatment N-stage Final pre-treatment M-stage Hospital diagnosed (Barrett's) Length (Prague Classification) Adjacent dysplasia RD. 5 RD. 6 RP. 19 RP. 21 RP. 24 RP. 26 RP. 29 PS. 73 PS. 74 RP. 11

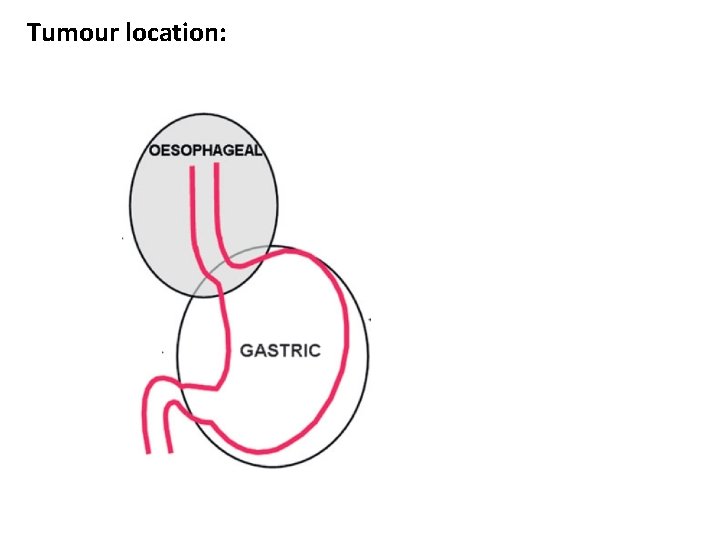
Tumour location:

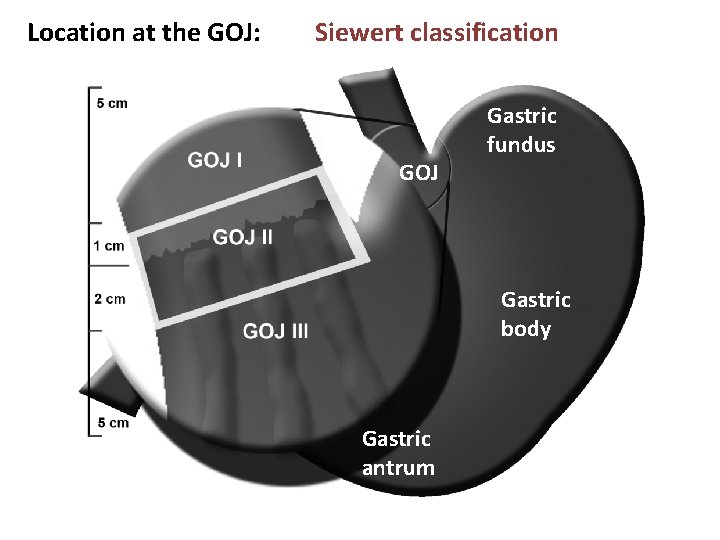
Location at the GOJ: Siewert classification GOJ Gastric fundus Gastric body Gastric antrum

Highly relevant fields: DI. 11 TR. 2 TR. 3 TR. 6 TR. 7 TR. 20 DI. 5 FE. 4 FE. 5 FE. 6 Date of diagnosis Treatment intent curative treatment modality palliative treatment modality No. cycles given (CTx) Chemotherapy treatment protocol Total dose given (RTx) Date of death Original disease reoccurred Date of recurrence Site of recurrence DI. 1 TC. 3 TC. 2 DI. 6 DI. 9 DI. 4 TR. 24 RP. 15 -17 RP. 33 study number Tissue source (PLUS CTX/RTX prior to sampling) Tissue type Sex Ethnicity Date of birth Therapy Response Margins involved Mandard score (if applicable) RP. 4 RP. 5 RP. 19 RP. 21 RP. 24 RP. 26 RP. 29 RP. 10 RP. 7 RP. 8 PS. 63 PS. 64 PS. 66 PS. 67 EX. 50 EX. 52 RP. 14 Location GOJ Siewert classification T-stage N-stage Total number of nodes examined Total number of nodes involved M-stage Tumour grading Histology Signet ring cells present TNM system being used Final pre-treatment T-stage Final pre-treatment N-stage Final pre-treatment M-stage Hospital diagnosed (Barrett's) Length (Prague Classification) Adjacent dysplasia RD. 5 RD. 6 RP. 19 RP. 21 RP. 24 RP. 26 RP. 29 PS. 73 PS. 74 RP. 11

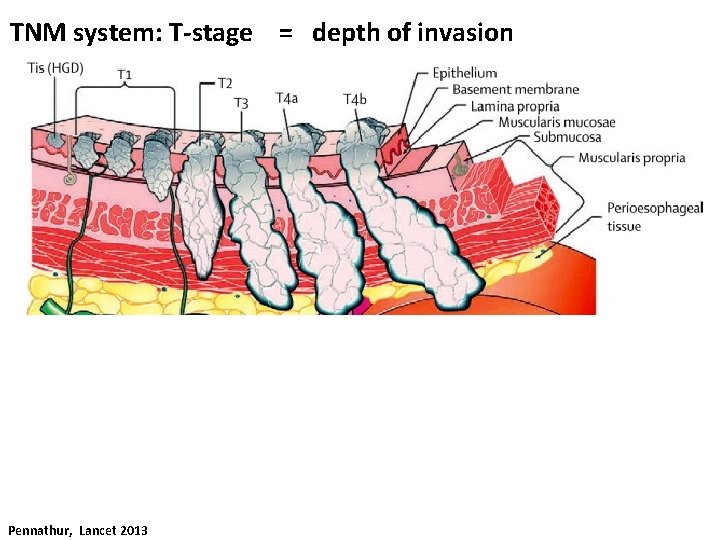
TNM system: T-stage = depth of invasion Pennathur, Lancet 2013

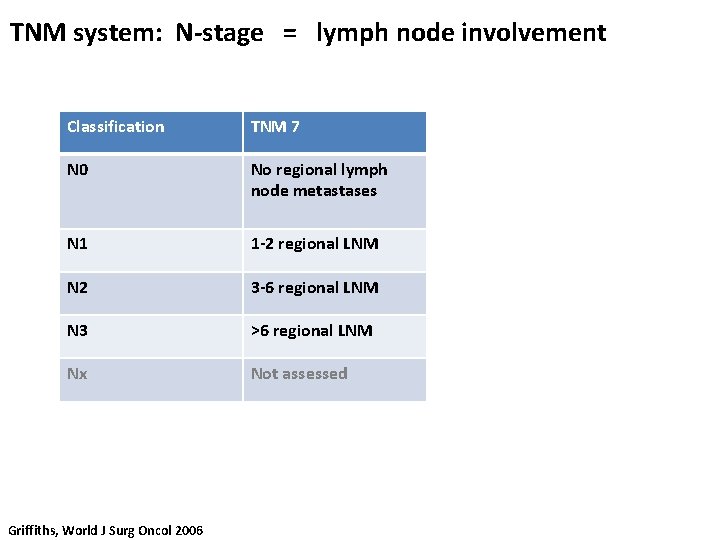
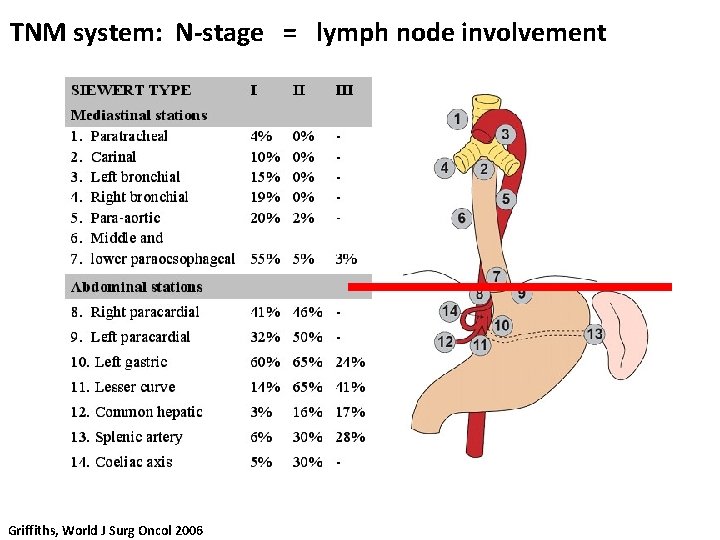
TNM system: N-stage = lymph node involvement Classification TNM 7 TNM 6 N 0 No regional lymph node metastases N 1 1 -2 regional LNM 1 -6 regional LNM N 2 3 -6 regional LNM 7 -15 regional LNM N 3 >6 regional LNM >15 regional LNM Nx Not assessed Griffiths, World J Surg Oncol 2006

TNM system: N-stage = lymph node involvement Griffiths, World J Surg Oncol 2006

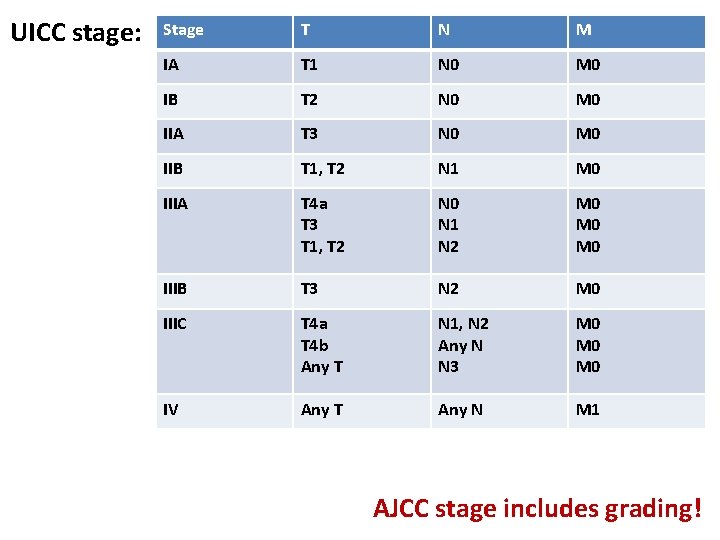
UICC stage: Stage T N M IA T 1 N 0 M 0 IB T 2 N 0 M 0 IIA T 3 N 0 M 0 IIB T 1, T 2 N 1 M 0 IIIA T 4 a T 3 T 1, T 2 N 0 N 1 N 2 M 0 M 0 IIIB T 3 N 2 M 0 IIIC T 4 a T 4 b Any T N 1, N 2 Any N N 3 M 0 M 0 IV Any T Any N M 1 AJCC stage includes grading!

Highly relevant fields: DI. 11 TR. 2 TR. 3 TR. 6 TR. 7 TR. 20 DI. 5 FE. 4 FE. 5 FE. 6 Date of diagnosis Treatment intent curative treatment modality palliative treatment modality No. cycles given (CTx) Chemotherapy treatment protocol Total dose given (RTx) Date of death Original disease reoccurred Date of recurrence Site of recurrence DI. 1 TC. 3 TC. 2 DI. 6 DI. 9 DI. 4 TR. 24 RP. 15 -17 RP. 33 study number Tissue source (PLUS CTX/RTX prior to sampling) Tissue type Sex Ethnicity Date of birth Therapy Response Margins involved Mandard score (if applicable) RP. 4 RP. 5 RP. 19 RP. 21 RP. 24 RP. 26 RP. 29 RP. 10 RP. 7 RP. 8 PS. 63 PS. 64 PS. 66 PS. 67 EX. 50 EX. 52 RP. 14 Location GOJ Siewert classification T-stage N-stage Total number of nodes examined Total number of nodes involved M-stage Tumour grading Histology Signet ring cells present TNM system being used Final pre-treatment T-stage Final pre-treatment N-stage Final pre-treatment M-stage Hospital diagnosed (Barrett's) Length (Prague Classification) Adjacent dysplasia RD. 5 RD. 6 RP. 19 RP. 21 RP. 24 RP. 26 RP. 29 PS. 73 PS. 74 RP. 11

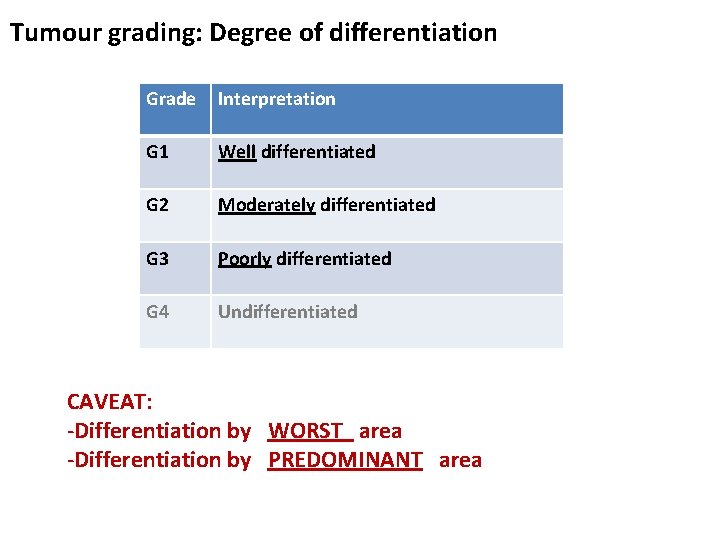
Tumour grading: Degree of differentiation Grade Interpretation G 1 Well differentiated G 2 Moderately differentiated G 3 Poorly differentiated G 4 Undifferentiated CAVEAT: -Differentiation by WORST area -Differentiation by PREDOMINANT area

Highly relevant fields: DI. 11 TR. 2 TR. 3 TR. 6 TR. 7 TR. 20 DI. 5 FE. 4 FE. 5 FE. 6 Date of diagnosis Treatment intent curative treatment modality palliative treatment modality No. cycles given (CTx) Chemotherapy treatment protocol Total dose given (RTx) Date of death Original disease reoccurred Date of recurrence Site of recurrence DI. 1 TC. 3 TC. 2 DI. 6 DI. 9 DI. 4 TR. 24 RP. 15 -17 RP. 33 study number Tissue source (PLUS CTX/RTX prior to sampling) Tissue type Sex Ethnicity Date of birth Therapy Response Margins involved Mandard score (if applicable) RP. 4 RP. 5 RP. 19 RP. 21 RP. 24 RP. 26 RP. 29 RP. 10 RP. 7 RP. 8 PS. 63 PS. 64 PS. 66 PS. 67 EX. 50 EX. 52 RP. 14 Location GOJ Siewert classification T-stage N-stage Total number of nodes examined Total number of nodes involved M-stage Tumour grading Histology Signet ring cells present TNM system being used Final pre-treatment T-stage Final pre-treatment N-stage Final pre-treatment M-stage Hospital diagnosed (Barrett's) Length (Prague Classification) Adjacent dysplasia RD. 5 RD. 6 RP. 19 RP. 21 RP. 24 RP. 26 RP. 29 PS. 73 PS. 74 RP. 11

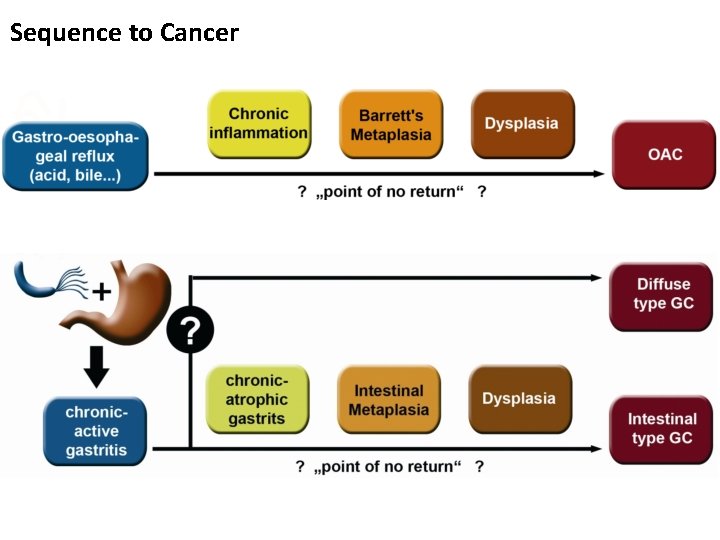
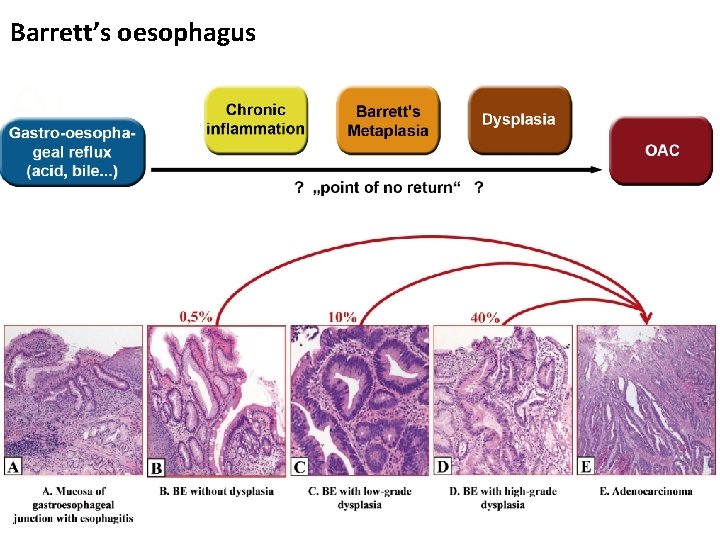
Sequence to Cancer

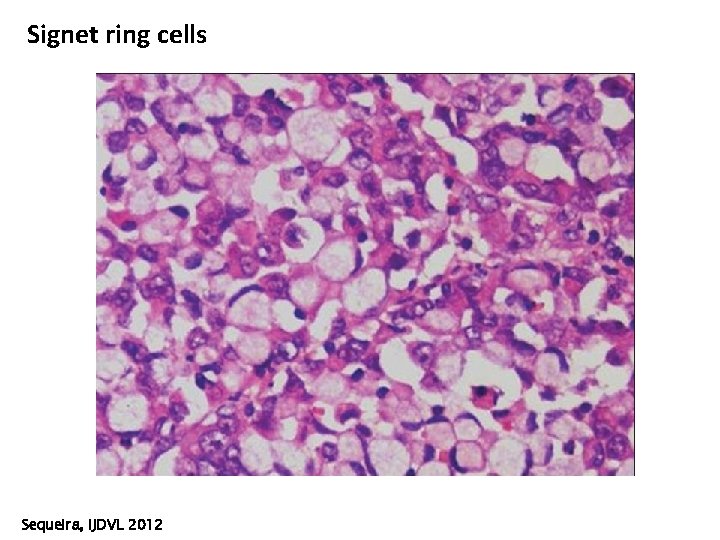
Signet ring cells Sequeira, IJDVL 2012

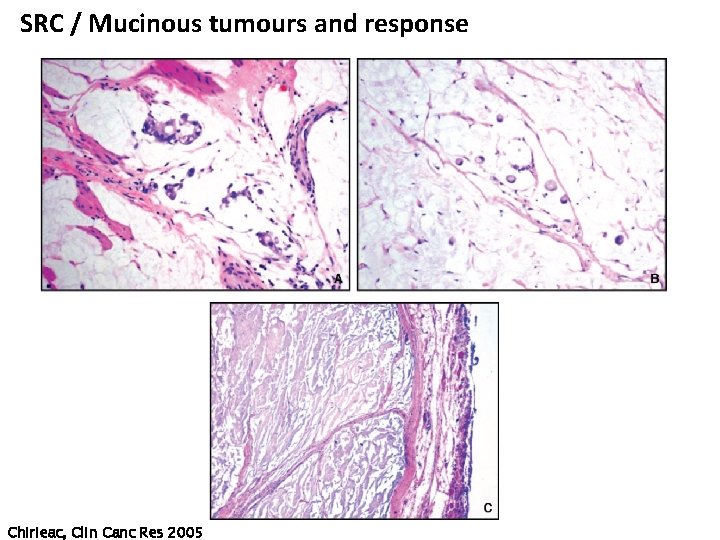
SRC / Mucinous tumours and response Chirieac, Clin Canc Res 2005


Highly relevant fields: DI. 11 TR. 2 TR. 3 TR. 6 TR. 7 TR. 20 DI. 5 FE. 4 FE. 5 FE. 6 Date of diagnosis Treatment intent curative treatment modality palliative treatment modality No. cycles given (CTx) Chemotherapy treatment protocol Total dose given (RTx) Date of death Original disease reoccurred Date of recurrence Site of recurrence DI. 1 TC. 3 TC. 2 DI. 6 DI. 9 DI. 4 TR. 24 RP. 15 -17 RP. 33 study number Tissue source (PLUS CTX/RTX prior to sampling) Tissue type Sex Ethnicity Date of birth Therapy Response Margins involved Mandard score (if applicable) RP. 4 RP. 5 RP. 19 RP. 21 RP. 24 RP. 26 RP. 29 RP. 10 RP. 7 RP. 8 PS. 63 PS. 64 PS. 66 PS. 67 EX. 50 EX. 52 RP. 14 Location GOJ Siewert classification T-stage N-stage Total number of nodes examined Total number of nodes involved M-stage Tumour grading Histology Signet ring cells present TNM system being used Final pre-treatment T-stage Final pre-treatment N-stage Final pre-treatment M-stage Hospital diagnosed (Barrett's) Length (Prague Classification) Adjacent dysplasia RD. 5 RD. 6 RP. 19 RP. 21 RP. 24 RP. 26 RP. 29 PS. 73 PS. 74 RP. 11

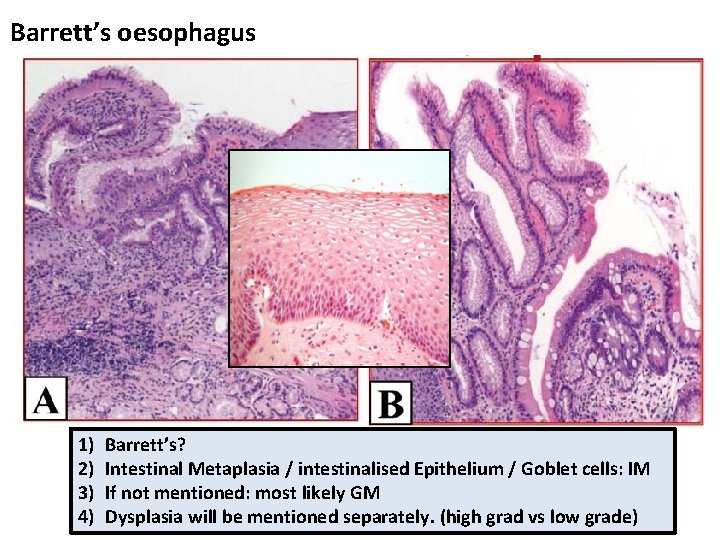
Barrett’s oesophagus

Barrett’s oesophagus 1) 2) 3) 4) Barrett’s? Intestinal Metaplasia / intestinalised Epithelium / Goblet cells: IM If not mentioned: most likely GM Dysplasia will be mentioned separately. (high grad vs low grade)

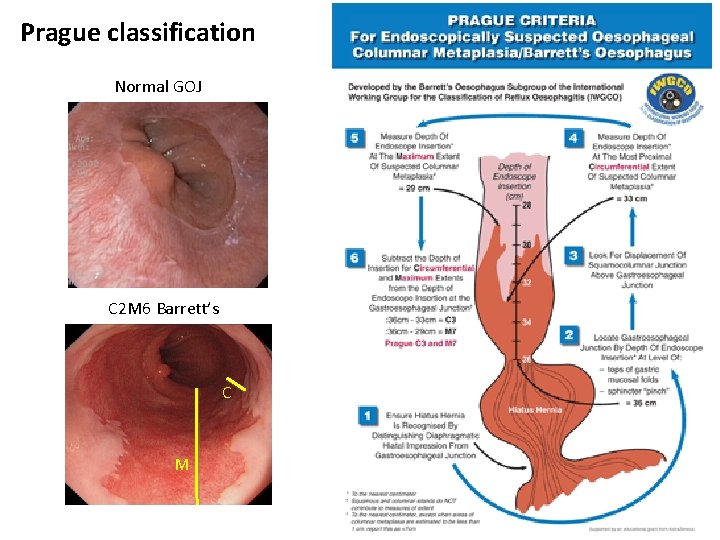
Prague classification Normal GOJ C 2 M 6 Barrett’s C M

Highly relevant fields: DI. 11 TR. 2 TR. 3 TR. 6 TR. 7 TR. 20 DI. 5 FE. 4 FE. 5 FE. 6 Date of diagnosis Treatment intent curative treatment modality palliative treatment modality No. cycles given (CTx) Chemotherapy treatment protocol Total dose given (RTx) Date of death Original disease reoccurred Date of recurrence Site of recurrence DI. 1 TC. 3 TC. 2 DI. 6 DI. 9 DI. 4 TR. 24 RP. 15 -17 RP. 33 study number Tissue source (PLUS CTX/RTX prior to sampling) Tissue type Sex Ethnicity Date of birth Therapy Response Margins involved Mandard score (if applicable) RP. 4 RP. 5 RP. 19 RP. 21 RP. 24 RP. 26 RP. 29 RP. 10 RP. 7 RP. 8 PS. 63 PS. 64 PS. 66 PS. 67 EX. 50 EX. 52 RP. 14 Location GOJ Siewert classification T-stage N-stage Total number of nodes examined Total number of nodes involved M-stage Tumour grading Histology Signet ring cells present TNM system being used Final pre-treatment T-stage Final pre-treatment N-stage Final pre-treatment M-stage Hospital diagnosed (Barrett's) Length (Prague Classification) Adjacent dysplasia RD. 5 RD. 6 RP. 19 RP. 21 RP. 24 RP. 26 RP. 29 PS. 73 PS. 74 RP. 11

Examples

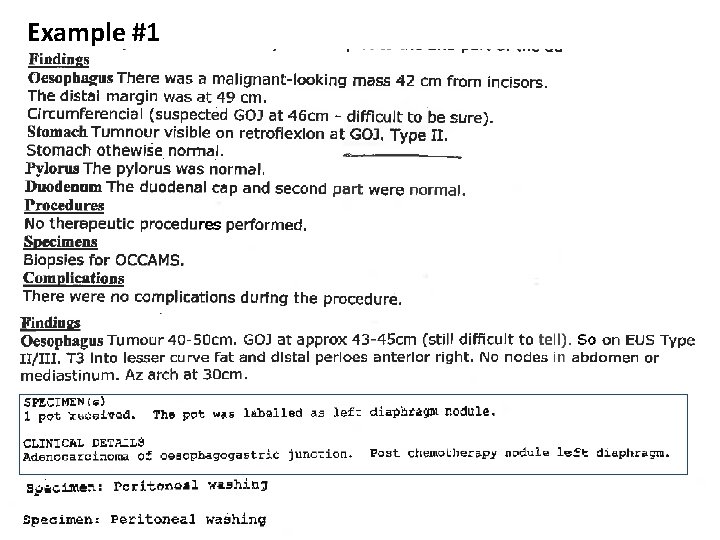
Example #1

Example #2

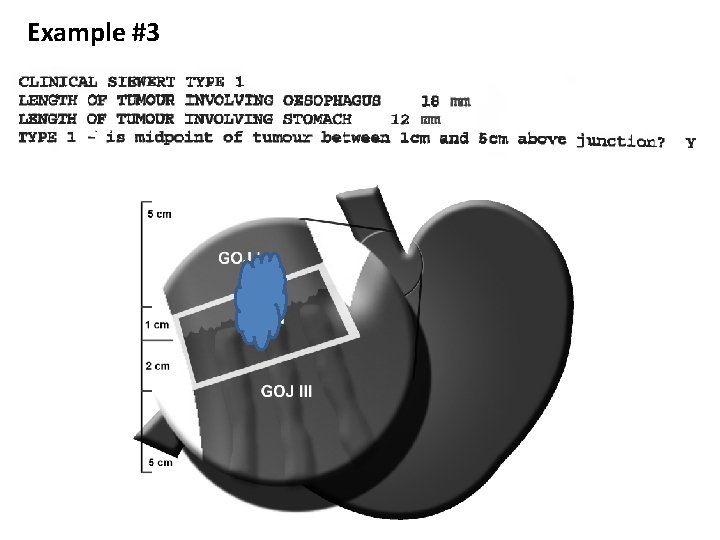
Example #3

Example #4

Thank you for your attention Questions?