OBTURATING MATERIALS CLASSIFICATION OF ROOT CANAL FILLING MATERIALS

OBTURATING MATERIALS

CLASSIFICATION OF ROOT CANAL FILLING MATERIALS BY GROSSMAN SOLID CORE MATERIALS § Metals § Plastics § Cements /pastes SEALERS § Plastics § Cements § Pastes

SOLID – CORE MATERIALS METAL SILVER STAINLESS STEEL FILES AMALGAM PLASTICS GUTTA PERCHA § § § § § Source Chemistry Phases Composition Properties Advantages Disadvantages Forms of guta percha Medicated gutta percha RESILON

Cement/paste Fills Hydron Endocal 10 (Biocalex 6. 9) Resorcinol – Formaldehyde resin Mineral trioxide aggregate Calcium phosphate

►INSTRUMENTS USED FOR OBTURATION §Endodontic pliers §Paper points §Spreaders §Pluggers §Device for cutting gutta percha §Heat carriers §Spiral paste fillers

SOLID-CORE MATERIALS METAL CORE MATERIALS

SILVER CONE Introduced by Elmer A. Jasper in 1933 Pure silver molded in a conical shape Canal preparation Tapered converging walls Advantage Stiffer than gutta-percha Easier to insert in very narrow/ fine tortuous canals

Disadvantages Poor lateral seal - cannot conform to the pulp space Cannot independently seal root canal – cementing medium required Corrosion of silver cones due to Presence of small amounts of other trace metals (e. g. 0. 1% to 0. 2% of copper and nickel) Loss of integrity of coronal restoration and exposure to saliva Canal irrigants Corrode by oxidation – surface compound –silver amine sulfate amide hydrate (SELTZERETAL) -Sulfur –blood , saliva, bone, cementum. Corrosion products Toxic Localized argyria/ tattoo Higher failure rates -overuse -degree of enlargement- not reached -necrotic, pulpal debris not removed Difficulty in retrieving cones in case of retreatment

INDICATIONS Mature teeth with small or well calcified round tapered canals Maxillary first premolar with 2 or 3 canals Buccal roots of maxillary molars Mesial roots of mandibular molars NOT INDICATED Youngsters Anterior teeth Single canal premolars Large single canals in molars

STAINLESS STEEL FILES Originally suggested by Sampeck in 1961 Used to fill Fine, tortuous canals Heavily calcified dilacerated narrow canals Used instead of silver cones Advantages More rigid than silver cones Inserted into a canal with greater ease Less susceptible to corrosion

Disadvantages Cannot independently seal the root canal, needs a cementing medium Excess sealer collects in the flutes of the instrument rather than being forced against canal walls Technique Cementing one file Handle cut off with a high-speed hand piece, 3 -4 mm below occlusal surface

OTHER METAL CORE MATERIALS Gold (by Grove) Iridioplatinum Tantalum Titanium (by Messing) Amalgam

DISADVANTAGES OF METAL CORE MATERIALS Require an absolutely circular canal preparation Often bind in one or two places of the root canal wall, giving a false sense of fit Radiographically are deceptive because they give a dense appearance to the root canal fill Corrode when in contact with either periradicular tissue fluids or oral fluids, the corrosions products are highly cytotoxic Cannot obturate the canal system three dimensionally, requires a sealer

SOLID-CORE MATERIALS PLASTIC CORE MATERIALS

GUTTA PERCHA The word ‘Gutta Percha’ is an English derived word from the Malay origin “Getah Pertja” meaning ‘strings of sticky plant juices’ Getah – gum Pertja – name of the tree in Malay language

SOURCE Malays call it ‘TABAN’ English call it ‘MAZER WOOD TREE’ Also called ‘ISONANDRA GUTTA TREE’ Scientifically called ‘PALAQUIUM GUTTA BAIL’
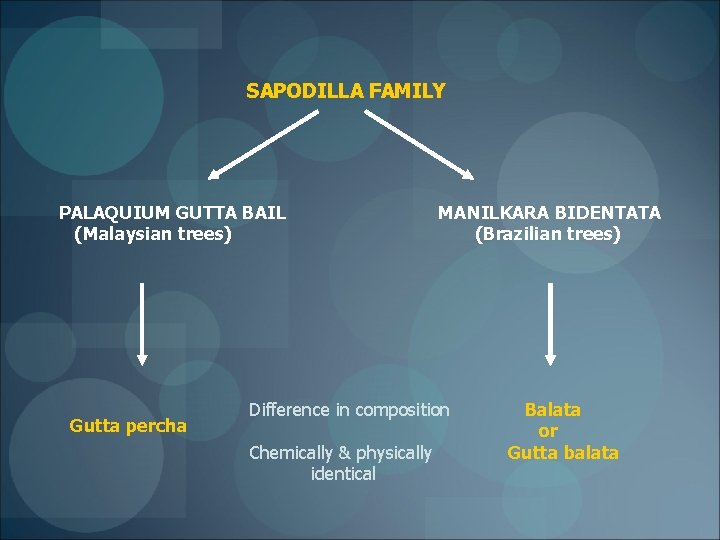
SAPODILLA FAMILY PALAQUIUM GUTTA BAIL (Malaysian trees) Gutta percha MANILKARA BIDENTATA (Brazilian trees) Difference in composition Chemically & physically identical Balata or Gutta balata
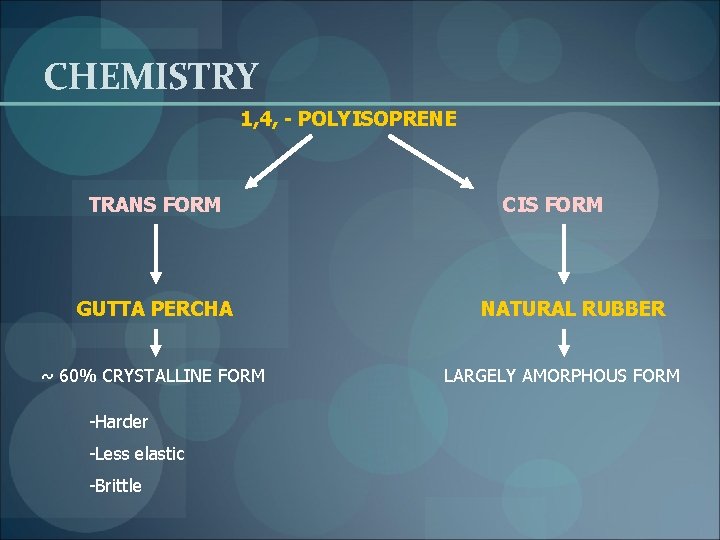
CHEMISTRY 1, 4, - POLYISOPRENE TRANS FORM GUTTA PERCHA ~ 60% CRYSTALLINE FORM -Harder -Less elastic -Brittle CIS FORM NATURAL RUBBER LARGELY AMORPHOUS FORM

Phases of Gutta -Percha Bunn 1942 Chemicall pure gp – two different crystalline forms -alpha (directly from trees) - beta (commercial gp) -converted into each other ►Fisher D. 1953 gamma –unstable form - amorphous in nature
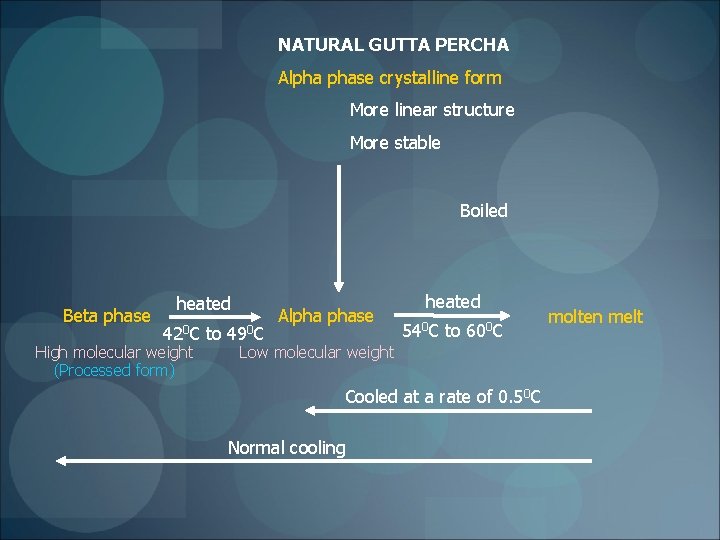
NATURAL GUTTA PERCHA Alpha phase crystalline form More linear structure More stable Boiled Beta phase heated 420 C High molecular weight (Processed form) to 490 C Alpha phase heated 540 C to 600 C Low molecular weight Cooled at a rate of 0. 50 C Normal cooling molten melt
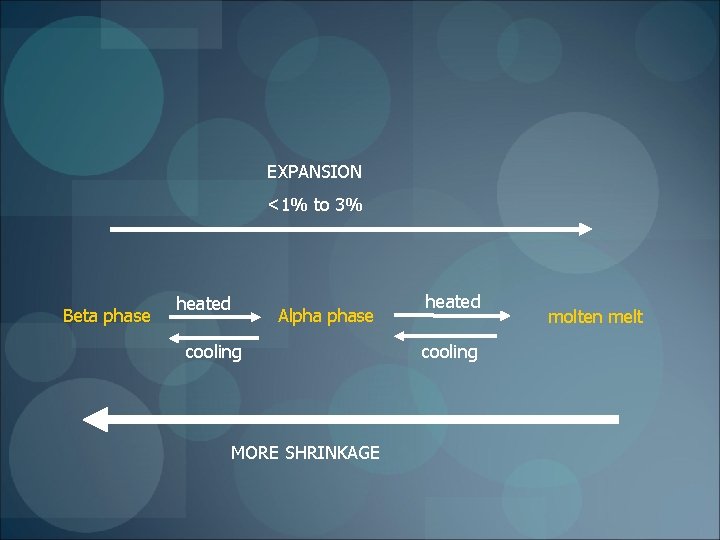
EXPANSION <1% to 3% Beta phase heated Alpha phase cooling MORE SHRINKAGE heated cooling molten melt

Greater shrinkage when More the phase changes Higher the temperature Compensated Compaction of gutta percha

PHASES OF GUTTA PERCHA ALPHA PHASE BETA PHASE Natural tree product Processed form Low molecular weight polymer High molecular weight polymer Lower melting point Higher melting point Low viscosity Higher viscosity Increased stickiness Reduced stickiness Less shrinkage (2. 2%) More shrinkage (2. 6%) Newer products Thermafil Micro. Seal Most commercial forms
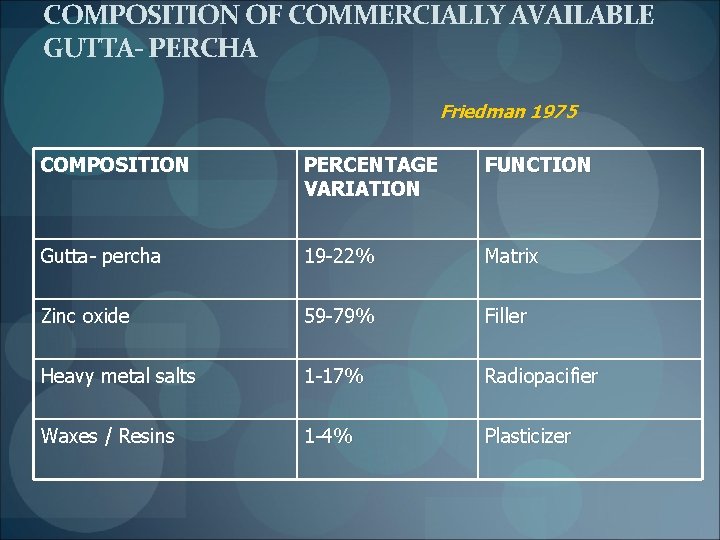
COMPOSITION OF COMMERCIALLY AVAILABLE GUTTA- PERCHA Friedman 1975 COMPOSITION PERCENTAGE VARIATION FUNCTION Gutta- percha 19 -22% Matrix Zinc oxide 59 -79% Filler Heavy metal salts 1 -17% Radiopacifier Waxes / Resins 1 -4% Plasticizer

PROPERTIES Softens at a temperature above 64 C Easily dissolved in chloroform and halothane Heat or solvent plasticized gutta percha, results in shrinkage of 1% -2% Dental gutta percha when heated from 37 o to 80 o. C and then cooled to 37 o. C there is a net loss of about 1. 4% in volume relative to precycle volume at 37 o. C Schilder H. 1985 1 mm thick gutta- percha has a radiopacity corresponding to 6. 44 mm aluminium

AGING (by Sorin and Oliet) Gutta percha oxidizes and becomes brittle when exposed to light and air Prevention Store in a cool dry place Rejuvenation Immersing cone in hot water (55 C) for 1 -2 sec and immediately immersing in cold tap water (22 o. C) for 5 -10 sec ( Solomon M Sorin. J Endod 1979)

STERILIZATION OF GUTTA PERCHA CONES 5. 25% or 5% Na. OCl for 1 min (vegetative microorganisms and spores) Disinfected by 1% Na. OCl – 1 min 0. 5% Na. OCl – 5 min Hydrogen peroxide – 3% Chlorhexidine – 2%(5 min) After disinfection, gutta percha cones must be rinsed in ethyl alcohol to remove crystallized Na. OCl before obturation

FORMS OF GUTTA PERCHA CONES / POINTS Core points (standard cones) Auxiliary points (non – standardized cones) Standardized gutta percha cone Nonstandardized gutta percha

Coloring agent – erythrosin Marciano, 1993
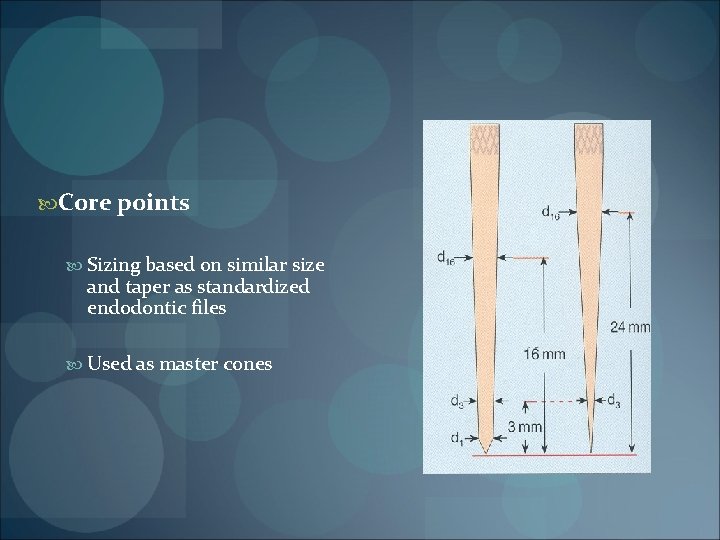
Core points Sizing based on similar size and taper as standardized endodontic files Used as master cones
Larger tolerance ( 0. 05 mm) than endodontic files (± 0. 02 mm) ENDO GAUGE or GUTTA GAUGE

Auxiliary points Have a larger taper pointed tip Tolerance is 0. 05 mm Length - 30 mm 2 mm Used as Accessory points during lateral compaction Master cones in warm vertical compaction and variable tapered preparations Are also standardized but in a very different system
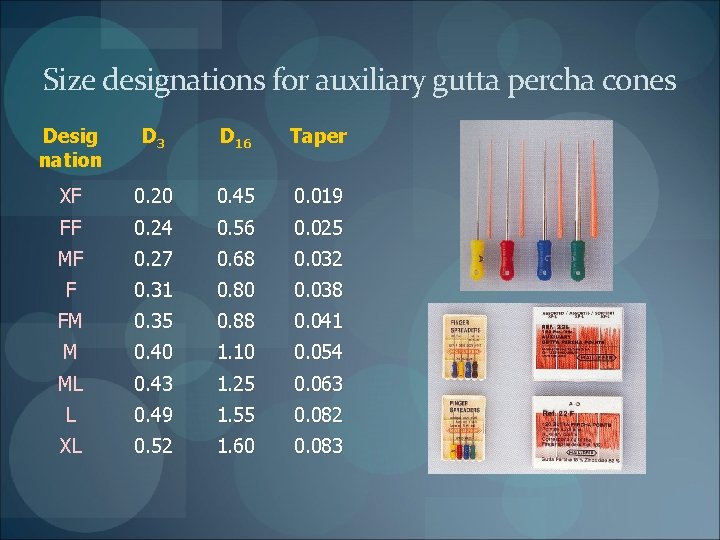
Size designations for auxiliary gutta percha cones Desig nation D 3 D 16 Taper XF 0. 20 0. 45 0. 019 FF 0. 24 0. 56 0. 025 MF 0. 27 0. 68 0. 032 F 0. 31 0. 80 0. 038 FM 0. 35 0. 88 0. 041 M 0. 40 1. 10 0. 054 ML 0. 43 1. 25 0. 063 L 0. 49 1. 55 0. 082 XL 0. 52 1. 60 0. 083

Greater taper gutta percha points

GUTTA PERCHA PELLETS / BARS For use in thermoplasticized gutta percha e. g. Obtura system SYRINGES As low viscosity gutta percha to be coated on carriers e. g. Alpha. Seal, Success. Fil

PRE COATED CORE CARRIER GUTTA PERCHA Stainless steel, titanium or plastic carrier precoated with alpha phase gutta percha e. g. Thermafil GUTTA PERCHA SEALERS Dissolving gutta percha in chloroform / eucalyptol e. g. chloropercha, eucapercha

ANTIBACTERIAL GUTTA PERCHA CONES IODOFORM CONTAINING GUTTA PERCHA MGP or MEDICATED GUTTA PERCHA (Lone Star Technologies, U. S. A) Developed by H. Martin, T. R. Martin – 1999 Contains 10% iodoform Iodoform is centrally located –takes 24 hrs to leach to the surface Remains inert until it comes in contact with tissue fluids that activate the free iodine Antimicrobial activity against § § § Streptococcus viridans, sanguis Staphylococcus aureus Bacteroides fragilis

CALCIUM HYDROXIDE CONTAINING GUTTA PERCHA - CALCIUM HYDROXID PLUS (Roeko, Germany) - HYGENIC CALCIUM HYDROXIDE POINTS Have a high percentage (40 -60%) of calcium hydroxide in a matrix of bio-inert gutta percha

ROEKO's Calcium Hydroxid PLUS Points § greater release of Ca(OH)2 § more effective over longer period Technique § Moisture in the canal activates the Calcium Hydroxide and the p. H in the canal rises to a level of 12 + within minutes § Average treatment time is 1 to 3 weeks § Once Ca(OH)2 has leached out, the point is no longer useful as a filling material and must be removed Available in § packages of 60 points each, ISO sizes 15 through 140 § 3 assortment boxes, 15 -40, 45 -80 and 90 -140, 10 points each size

Advantages § Clean: § No smearing around the access cavity during insertion § Removable without any residue § Time-saving: § § The points are ready to use No mixing Easy to apply Easy to remove § Safe: § The insertion of the points down to the apex is easy § Ensures that calcium hydroxide is released throughout the canal

CHLORHEXIDINE – IMPREGNATED GUTTA PERCHA ROEKO ACTIV POINTS (Roeko, Langenau, Germany) Gutta percha matrix embedded with 5% chlorhexidine diacetate For use as an intracanal medication § § temporary root canal filling prevention of reinfection ISO shaped points Radiopaque
Technique § An Activ point corresponding to the last used root canal instrument, or one size smaller, should be marked with the predetermined length and applied into the canal without condensation § A drop of moisture (e. g. sterile H 2 O) may be used together with the Activ point to accelerate the release of CHX § Further dissociation will be initiated by moisture flowing into the canal through the dentine tubules and apex

Advantage § Ease of introduction § It is firm for easy application yet flexible to follow the curves of the canal. § Ease of removal § It can easily be removed with tweezers or a probe even after 3 weeks § The stability of Activ point is not affected by the release of chlorhexidine in moisture § No residue is left in the canal

When chlorhexidine comes into contact with moisture it releases cations which combine with the anionic molecules on the surface of the cell walls of the bacteria causing osmosis to malfunction (Petereit & Kirch, W. , 1998). Did not possess an in vitro inhibitory activity against Enterococcus faecalis (Lui et al, 2004)

ADVANTAGES OF GUTTA PERCHA COMPACTIBILITY Adapts to the root canal walls BIOLOGICALLY INERT least reactive minimal toxicity minimal tissue irritability least allergenic well tolerated by periradicular tissues DIMENSIONAL STABILITY BECOMES PLASTIC WHEN WARMED HAS KNOWN SOLVENTS Chloroform Xylol DOES NOT DISCOLOUR THE TOOTH

DISADVANTAGES UNDERGOES SHRINKAGE WHEN PLASTICIZED DOES NOT POSSESS ADHESIVE QUALITIES LACK OF RIGIDITY UNDERGOES VERTICAL DISTORTION DURING COMPACTION Needs a definite apical constriction / stop

RESILON (Resilon Research LLC, Madison, CT, U. S. A) Thermoplastic synthetic polymer – based root canal filling material Consists of Resin core material polymers of polyester Difunctional methacrylate resin Bioactive glass Fillers and radiopacifiers Bismuth oxychloride Barium sulfate Overall filler content 65% by weight

It is used in conjunction with SELF – ETCHING PRIMER EPIPHANY PRIMER (Pentron Clinical Technologies) SEALER EPIPHANY ROOT CANAL SEALANT (Pentron Clinical Technologies) §Dual curable resin – based sealer

Self etch primer Sulphonic acid terminated functional monomer HEMA Water Polymerisation initiater Resin sealer Bis GMA ethoxylated Bis GMA UDMA hydrophilic difunctional metharcylate fillers

Performs like gutta percha and has the same handling characteristics Is biocompatible Also insoluble in water Easily retrievable for retreatment purposes Softened with heat Dissolved with solvents like chloroform

Available as Master cones in all ISO sizes 0. 04, 0. 06 taper Accessory cones – in different sizes Pellets – used for backfill in warm thermoplasticized techniques

Can be used for both warm and cold obturation techniques Can be thermoplasticized, but at a lower temperature With the Obtura gun Reduce the temperature by 20 degrees (i. e. approx. 150 170 o. C) System B - 150 o. C at a power of 10
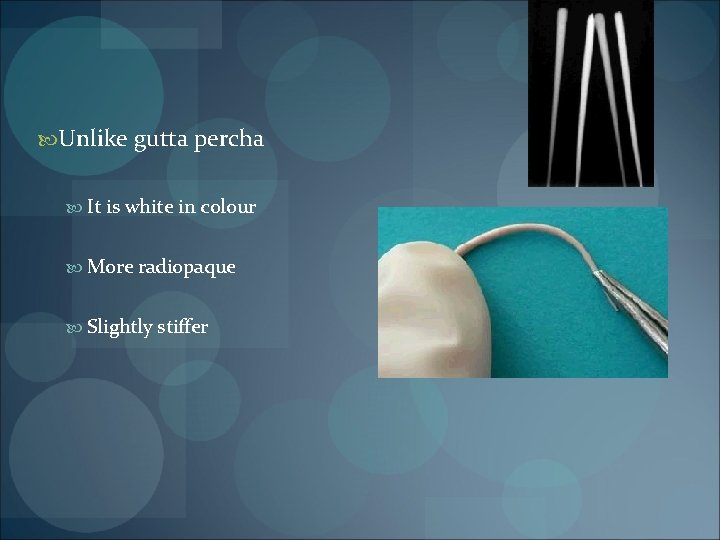
Unlike gutta percha It is white in colour More radiopaque Slightly stiffer
Advantages Adheres to the sealer Excellent sealing capability due to creation of a “monobloc” which adheres to the dentin walls Resists leakage six times more Strengthens the root by approximately 20% Provides an immediate coronal seal Shrinks only 0. 5% even heated

SOLID-CORE MATERIALS CEMENT/PASTE FILLS

HYDRON First described by Wichterle and Lim For use as a biocompatible implant material Introduced as a root canal filling in 1978 By Goldman and associates Is a polymer of hydroxy- ethyl- methacrylate (i. e. , poly – HEMA) Is a hydrophilic acrylic resin Undergoes polymerization in an aqueous environment Is self polymerizing Is rapid setting sets in 10 minutes Radiopaque addition of barium sulfate Injected into root canal using a special syringe and needle, that allows placement in thin and/or curved canals

When inserted into root canal Moisture from periapical tissues Polymerization Swells Increases sealing ability Plastic conforms to shape of root canal

Disadvantages Concerns of tissue toxicity by the unset material Lack of homogeneity Questionable ability to seal the root canal system Clinical use – proved unsatisfactory

BIOCALEX 6. 9 ( Biodent, Montreal, Quebec) Calcium oxide material The French Paste By Pierre D. Bernard, 1967 -Under the Ocalex CALCIUM OXIDE EXPANSION TECHNIQUE OCALEXIQUE ROOT CANAL THERAPY recent FDA approval – 2000

Used as the sole obturating material treating infected and purulent pulp More recently introduced because of concern of cross reactivity to gutta percha in individuals allergic to latex Powder and liquid heavy calcium oxide zinc oxide ethylene glycol water
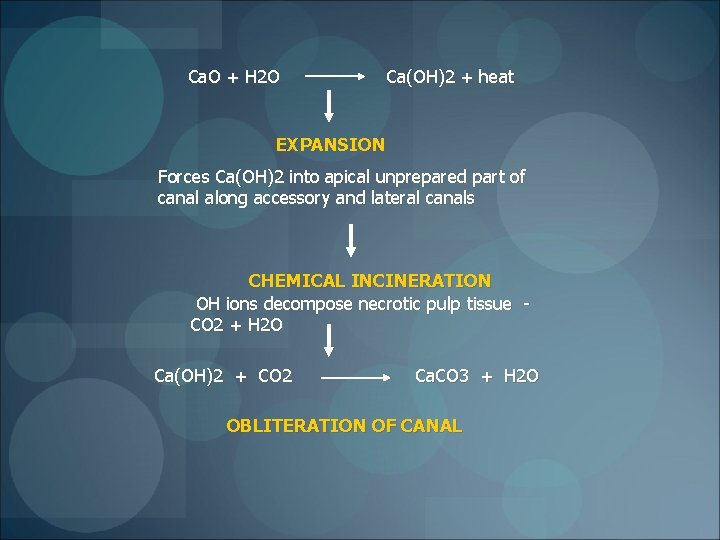
Ca. O + H 2 O Ca(OH)2 + heat EXPANSION Forces Ca(OH)2 into apical unprepared part of canal along accessory and lateral canals CHEMICAL INCINERATION OH ions decompose necrotic pulp tissue CO 2 + H 2 O Ca(OH)2 + CO 2 Ca. CO 3 + H 2 O OBLITERATION OF CANAL

§Calcium carbonate will form and remain insoluble only as long as the milieu is basic §So in an infected root canal, ►environment is made acidic by bacterial metabolic products ►Ca. O will generate Ca(OH)2 ►buffer and disinfect §When the infection comes under control ►the fluids return to physiological p. H ►the reaction of Ca. O turns to generation of calcium carbonate ► material hardens in place § If the periapical infection reasserts itself and again produces acid, ►the previously hardened calcium carbonate will redissolve ►yielding more Ca(OH)2 to fight the infection ►Self–regulating system, a material that is both an active disinfectant and an obturator, as required by the surrounding conditions

Evolution BIOCALEX 4 Introduced in 1967 Powder- calcium oxide Mixed to a slurry with § Ethylene glycol § Ethyl alcohol § Distilled water Expansion of 200 to 280%
Endocal - 10 (Biodent, Montreal) substituting yttrium oxide for the zinc oxide Includes § 10 vials of 1 g powder § 10 ml bottle Ocalexic solution

Indications When whole pulp is necrotic with or without periapical lesion Narrow canals Canal blocked by organic tissue Pronounced apical curvature Contra indications Vital pulp tissue Acute phase of periapical inflammation

Advantages High p. H of calcium hydroxide Bactericidal action Stimulates osteoblastic action Biocompatible Enhanced sealing Promotion of significant intratubular calcium diffusion
Disadvantage Can cause potential root fracture (Goldberg et al 2004)
RESORCINOL – FORMALDEHYDE (RF) RESIN THERAPY ( RUSSIAN RED CEMENT) JOE 2003; 7: 435 – 441 Available as FOREDENT (Dental A S, Czech Republic) Methods for using this therapy were described in 1957 and have been widely used since 1960 Consists of Formaldehyde / alcohol - liquid Resorcinol - powder Sodium hydroxide – catalyst Zinc oxide / barium sulfate – radiopacity (optional)

Assumed that pulp tissue will be fixed and bacteria destroyed apical to the level of the resin placement Hence canals are frequently not instrumented or obturated to their full length When 10% sodium hydroxide is added to the mixture, polymerization occurs Forms a “brick – hard red” material that has no known solvent
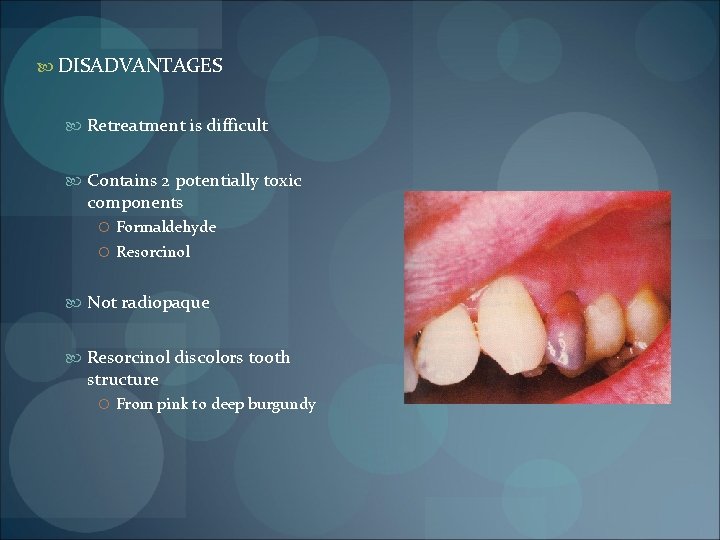
DISADVANTAGES Retreatment is difficult Contains 2 potentially toxic components Formaldehyde Resorcinol Not radiopaque Resorcinol discolors tooth structure From pink to deep burgundy

MINERAL TRIOXIDE AGGREGATE By Mahmoud Torabinejad in 1993 Available as Pro. Root MTA (Dentsply) Gray MTA Off- white MTA Mainly used for obturation of apical third Open apex cases Powder consists of fine, hydrophilic particles in the presence of water creates a colloidal gel solidifying within 4 hours – 7 hours water: powder ratio of 1: 3 increased water: powder mixing ratios could account for increased solubility and porosity of the material Fridland et al 2003

Properties Good sealing ability Extremely biocompatible Histologically Induction of osteoid like material Low cyotoxicity Has a much longer working time In moist environment sets in about 7 hours

GRAY COLORED FORMULA OFF – WHITE COLORED FORMULA Tricalcium silicate Bismuth oxide (mineral oxides) Dicalcium silicate Tricalcium aluminate Tetracalcium aluminoferrite Calcium sulfate dehydrate Lacks the tetracalcium aluminoferrite

Original MTA gray in color occasional staining White MTA Off – white, for esthetically sensitive areas

CALCIUM – PHOSPHATE CEMENT By W. E Brown and L. C Chow Developed and patented at the American Dental Association (ADA) Paffenbarger Research Centre 2 calcium phosphate powders Acidic – dicalcium phosphate dihydrate / anhydrous dicalcium phosphate Basic – Tetracalcium phosphate When mixed with water sets into a hardened mass hydroxyapatite Sets within 5 minutes By adding glycerin to the mixture Setting time can be extended Can be extruded from a 19 gauge needle

Final set cement As radiopaque as bone Nearly insoluble in water, saliva and blood Readily soluble in strong acids Tissue response-mild irritation New bone formation

DISADVANTAGES OF PASTE FILLS Toxicity – from components of some paste that either leach out of the paste or are in contact with the periradicular tissues Porosities in paste fills Most pastes resorb in time resulting in leakage, percolation and strong possibility of ultimate endodontic failure Systemic recovery of certain components in blood samples and various vital organs Antigenic chemical components – causing immunologic response Apical control of pastes fills is all but impossible especially when no apical stop is present or a root perforation exists

REFERENCES E Nicholls 3 rd edition Stephen Cohen 8 th and 9 th edition Grossman 11 th edition John I Ingle 5 th edition Franklin S Weine 6 th edition
- Slides: 78