Observation What is evidence Any observation of Natural

Observation - What is evidence? • Any observation of Natural Phenomena – Qualitative • Use your senses • Ex. Texture, color, smell, taste, sound – Quantitative • Measured values • Ex. Weight, volume, length, temperature • Measurements, Verifiable observations, etc. • Utilizing the Scientific Method, this is how we know what we know!

Organization of Matter • Continuous – Aristotle • Discontinuous – Democritus • (470 -400 B. C. ) – Dalton • (1808) – Joseph Gay-Lussac • (1805)

States of Matter Solid: high degree of interparticle forces Liquid: significant degree of interparticle forces Gases: very low degree of interparticle forces Water can be found in the solid, liquid, and vapor (gaseous) forms simultaneously.

Changes of Matter Physical: a change which does NOT alter the composition of a substance. i. e. : cutting, breaking, tearing, phase changes (melting, boiling), etc. Chemical: a change which alters the composition of a substance. i. e. : “rusting”, burning, “chemically reacting”, etc. The green color of the Statue of Liberty results from the reaction of copper with the components of air.

"Good" versus "Bad" Properties for a Chemical Substance
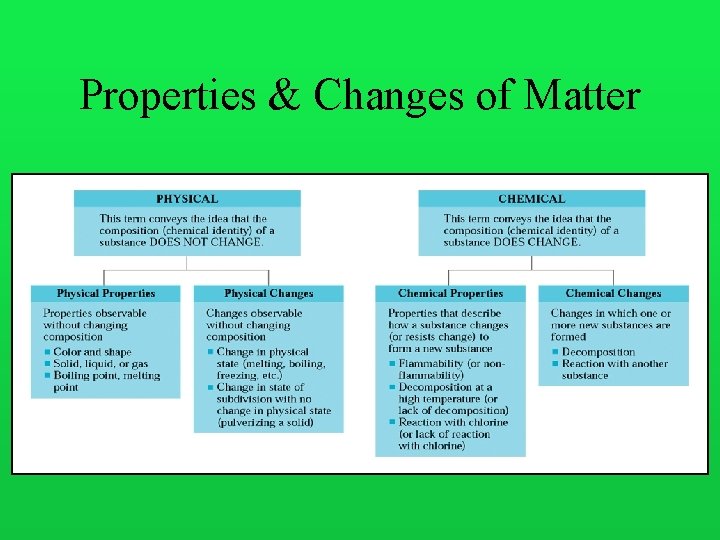
Properties & Changes of Matter
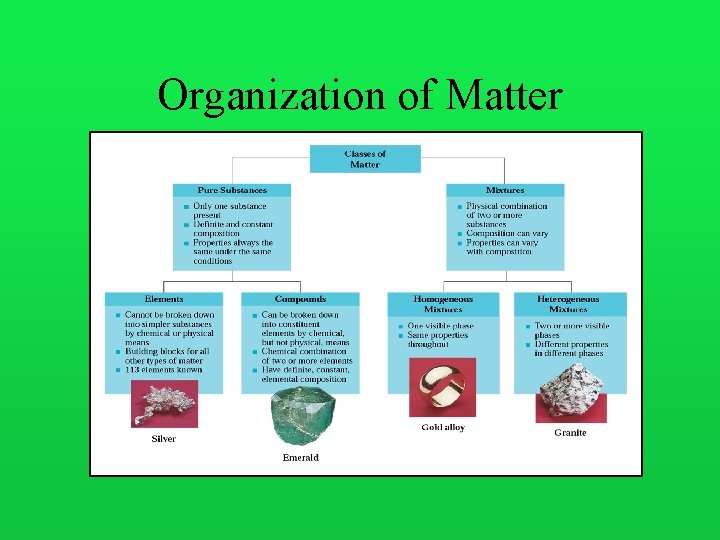
Organization of Matter

How could the appearance of each type of chemical change? Outward physical appearance of naturally occurring elements Hg As S Bi Mg I 2
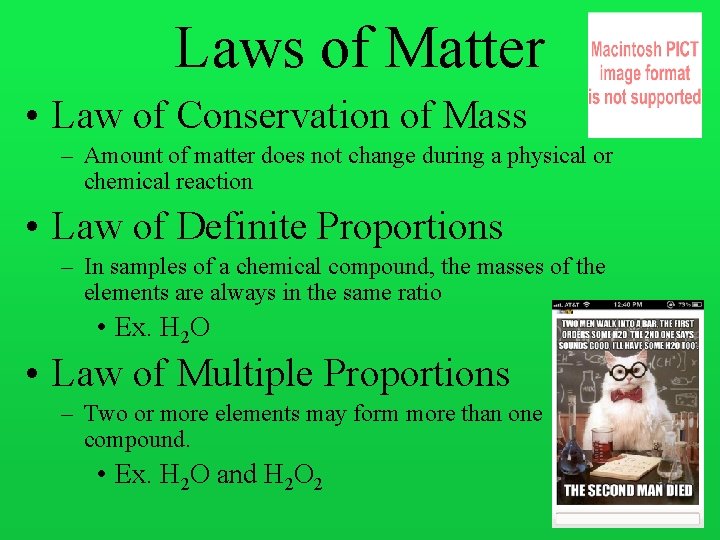
Laws of Matter • Law of Conservation of Mass – Amount of matter does not change during a physical or chemical reaction • Law of Definite Proportions – In samples of a chemical compound, the masses of the elements are always in the same ratio • Ex. H 2 O • Law of Multiple Proportions – Two or more elements may form more than one compound. • Ex. H 2 O and H 2 O 2
Studying Matter - Chemistry • Requires a scientific approach – Scientific method – Nature of science • Requires use of a measurement system and tools for measuring • Requires displaying and explaining experimental results – Variables (dependent vs. independent) & controls
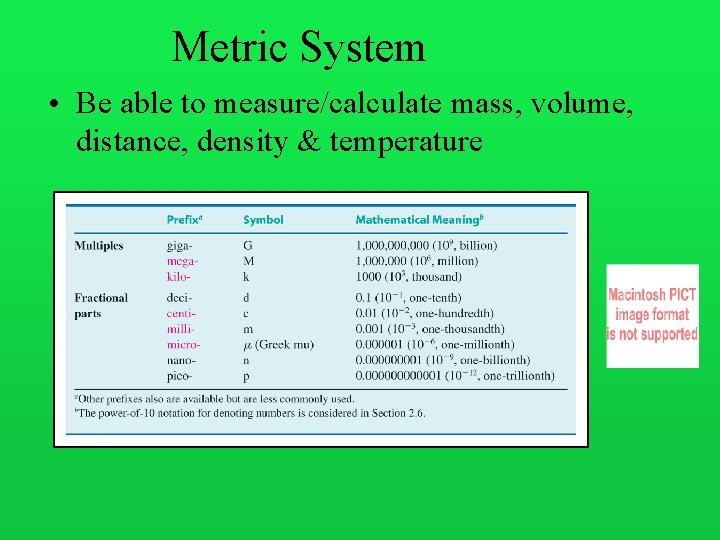
Metric System • Be able to measure/calculate mass, volume, distance, density & temperature
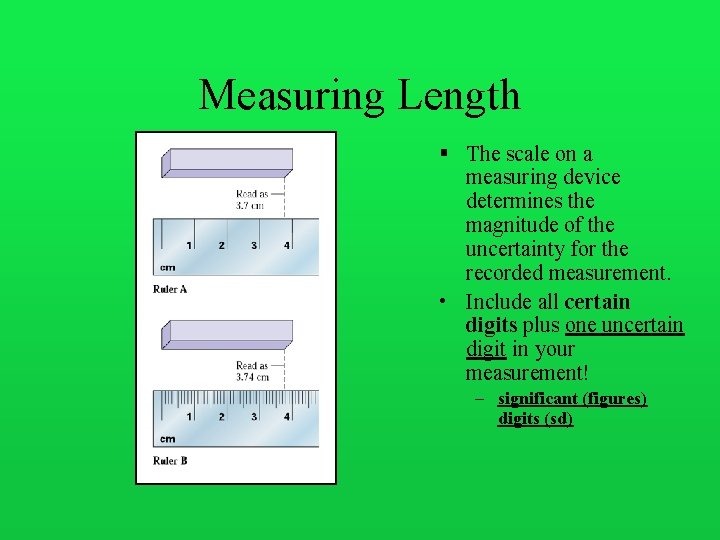
Measuring Length § The scale on a measuring device determines the magnitude of the uncertainty for the recorded measurement. • Include all certain digits plus one uncertain digit in your measurement! – significant (figures) digits (sd)

Volume/Space • Liter • Milliliter • Microliter (L) (m. L) ( L) 1 L = 1 dm 3 1 m. L = 1 cm 3 = 1 cc 1 L = 1 mm 3

Measuring Volume • Note the type of liquid • Indicate which kind of meniscus it is (concave or convex) – Concave: read the bottom of the meniscus (WATER) – Convex: read the top of the meniscus (MERCURY) • Include all certain digits plus one uncertain digit in your measurement! – sd

Mass/Weight • A gram is defined as the mass of 1 ml of water at 4�˚C. Therefore, water has a density of 1 g/m. L at 4˚ C.

Measuring Mass • Check that the scale is TARED prior to placing anything on it. • Include all certain digits plus one uncertain digit in your measurement! – sd
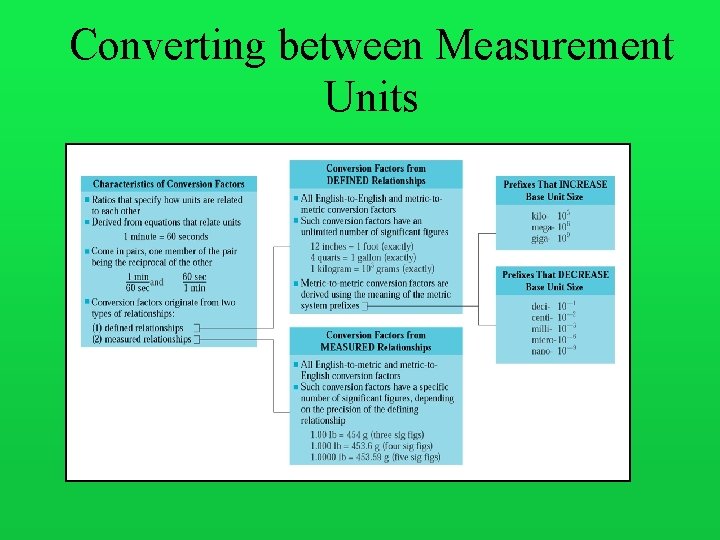
Converting between Measurement Units
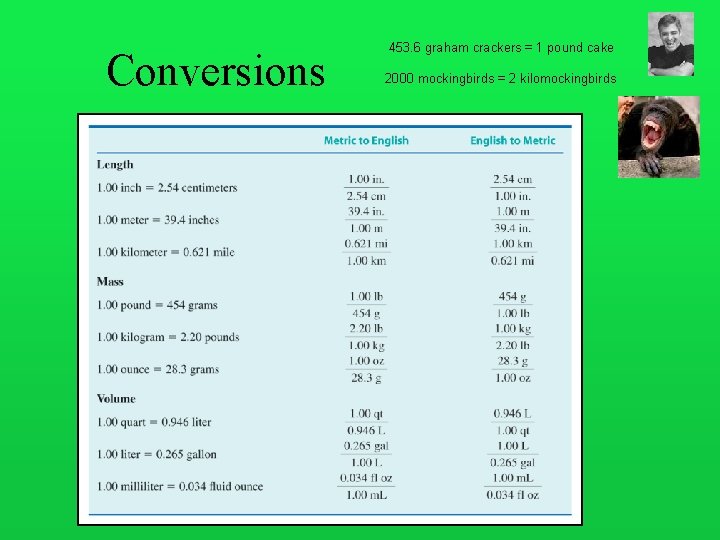
Conversions 453. 6 graham crackers = 1 pound cake 2000 mockingbirds = 2 kilomockingbirds
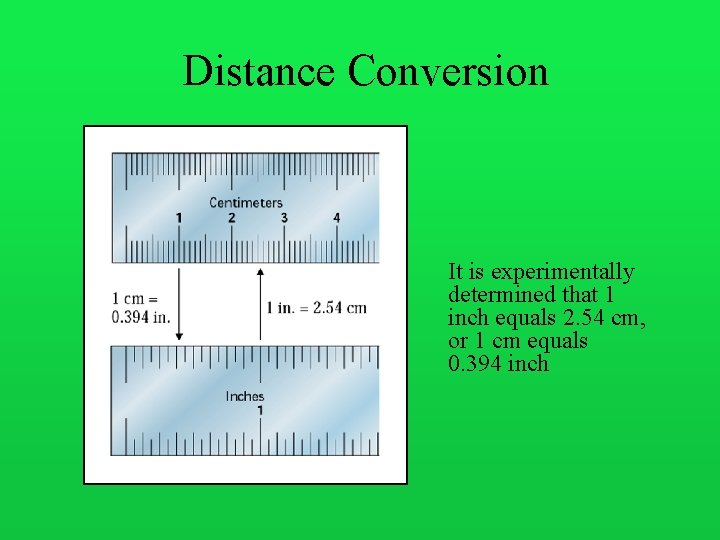
Distance Conversion It is experimentally determined that 1 inch equals 2. 54 cm, or 1 cm equals 0. 394 inch
- Slides: 19