Objectives Drug Development To identify the drug development
Objectives: Drug Development To identify the drug development process and the role of Food and Drug Administration (FDA). Identify different factors affecting dosage form design. 1

New Molecule Animal/ Plant Chemical Genetic engineering Preclinical testing Preformulation: Physicochemical properties Safety and bioactivity (in vivo and in vitro) Gila monster Investigation of New Drug Application (IND) Clinical trial: Phases I, III Research and Development : Long term animal toxicity Scale up manufacturing Package and label design New Drug Application (NDA) 2 FDA Approval and Post-marketing Surveillance
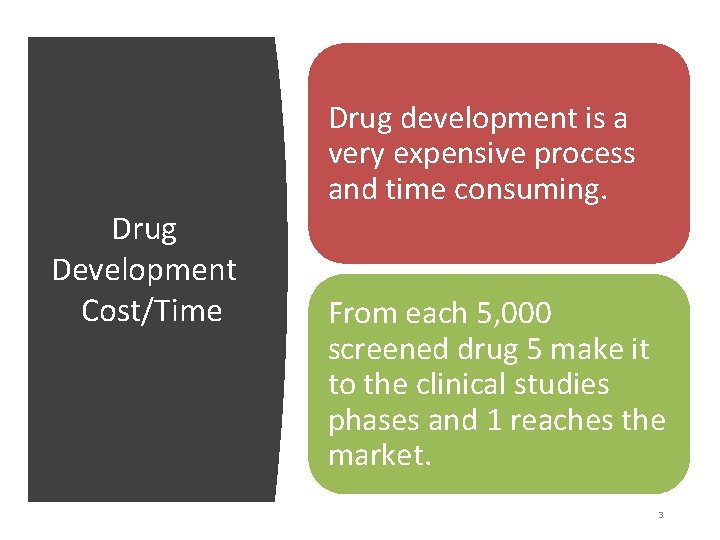
Drug Development Cost/Time Drug development is a very expensive process and time consuming. From each 5, 000 screened drug 5 make it to the clinical studies phases and 1 reaches the market. 3

4 Preclinical research • After the identification of a promising molecule, Pharmacologists run animal test to identify the ADME of the drug, its toxicity and to get an idea about the rate of metabolism (ADME/Tox).
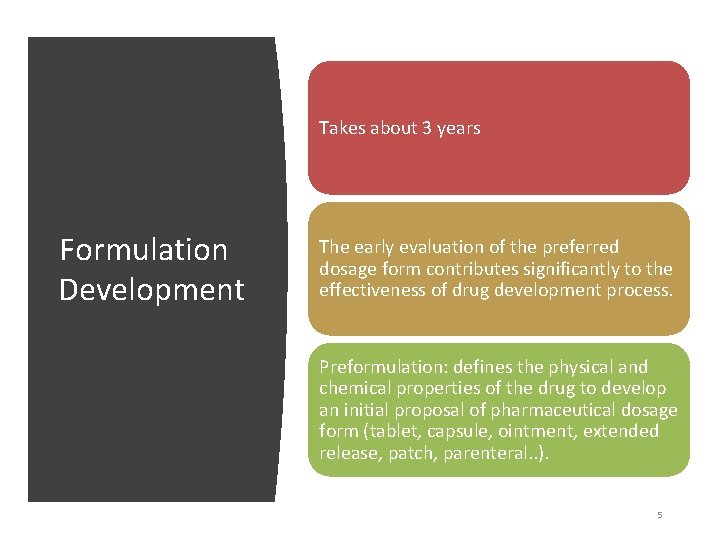
Takes about 3 years Formulation Development The early evaluation of the preferred dosage form contributes significantly to the effectiveness of drug development process. Preformulation: defines the physical and chemical properties of the drug to develop an initial proposal of pharmaceutical dosage form (tablet, capsule, ointment, extended release, patch, parenteral. . ). 5
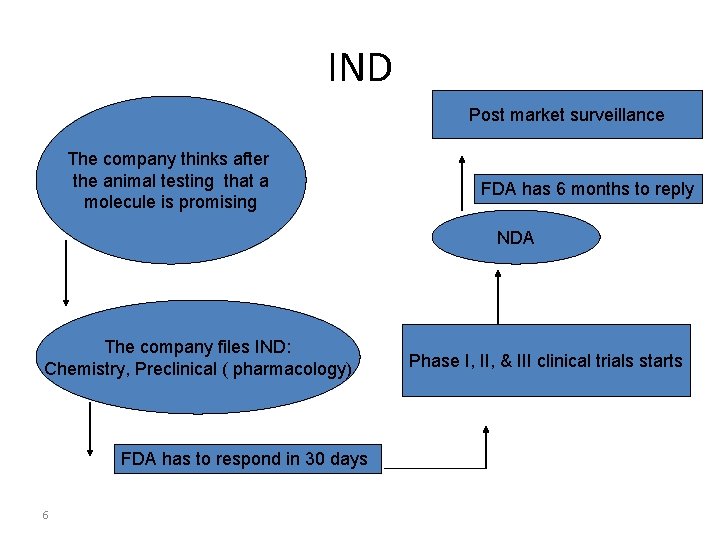
IND Post market surveillance The company thinks after the animal testing that a molecule is promising FDA has 6 months to reply NDA The company files IND: Chemistry, Preclinical ( pharmacology) FDA has to respond in 30 days 6 Phase I, II, & III clinical trials starts

First exposure of the IND to humans In healthy volunteers Closely monitored Phase I Takes a year 20 -80 volunteers Goal: To establish the tolerance of the drug. 7
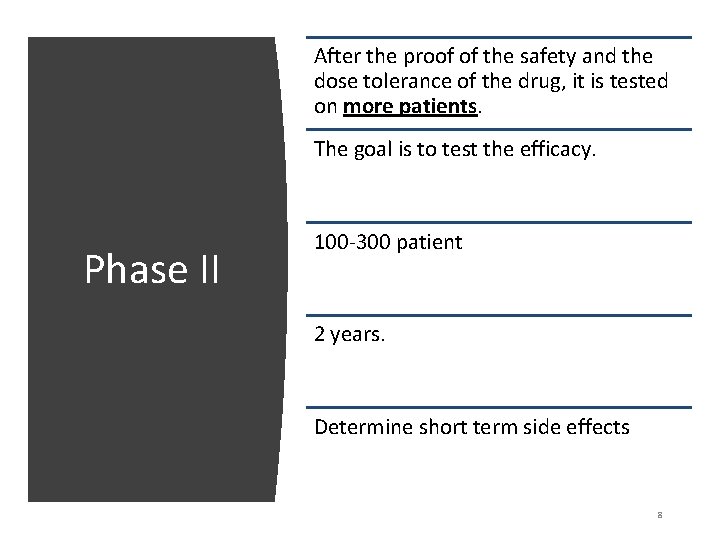
After the proof of the safety and the dose tolerance of the drug, it is tested on more patients. The goal is to test the efficacy. Phase II 100 -300 patient 2 years. Determine short term side effects 8
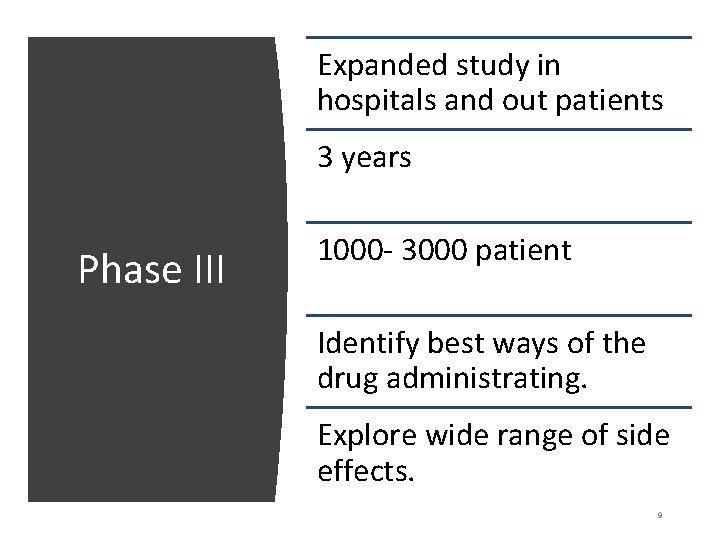
Expanded study in hospitals and out patients 3 years Phase III 1000 - 3000 patient Identify best ways of the drug administrating. Explore wide range of side effects. 9

ANDA • Abbreviated New Drug Application. • It is submitted of an application to license a generic drug of an approved marketed drug (not biological). WHY? • Biological products: Any small change in the biological manufacturing may change the whole product, as small as the media temperature, solvents and so on. 10

homework • Visit the FDA website http: //FDA/ • Visit the USP website 11

Formulation Consideration Drug substance is rarely administered alone. • Inactive (previously called inert) ingredients called excipients are usually needed. 12
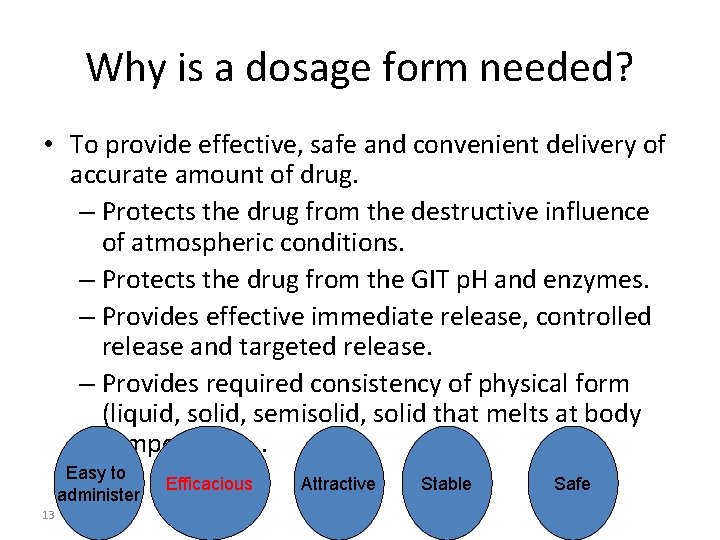
Why is a dosage form needed? • To provide effective, safe and convenient delivery of accurate amount of drug. – Protects the drug from the destructive influence of atmospheric conditions. – Protects the drug from the GIT p. H and enzymes. – Provides effective immediate release, controlled release and targeted release. – Provides required consistency of physical form (liquid, solid, semisolid, solid that melts at body temperature. . . Easy to administer 13 Efficacious Attractive Stable Safe

Formulation Consideration • Excipients: solubilize, suspend, preserve, dilute, emulsify, improve patient acceptance, protect by coating, target the drug to desired site, local drug delivery, and to provide controlled release and targeted dosage forms. 14
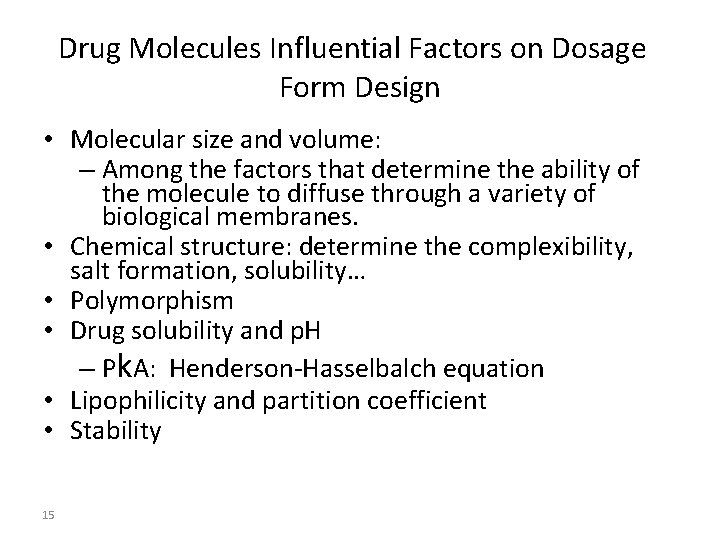
Drug Molecules Influential Factors on Dosage Form Design • Molecular size and volume: – Among the factors that determine the ability of the molecule to diffuse through a variety of biological membranes. • Chemical structure: determine the complexibility, salt formation, solubility… • Polymorphism • Drug solubility and p. H – Pk. A: Henderson-Hasselbalch equation • Lipophilicity and partition coefficient • Stability 15
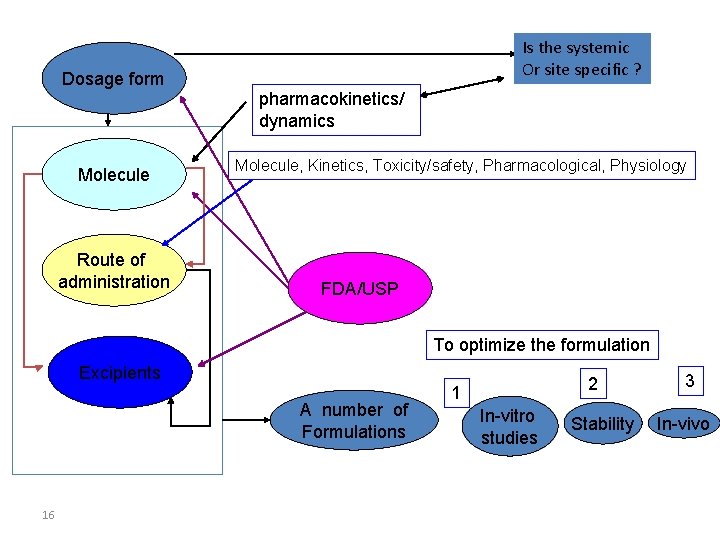
Is the systemic Or site specific ? Dosage form pharmacokinetics/ dynamics Molecule Route of administration Molecule, Kinetics, Toxicity/safety, Pharmacological, Physiology FDA/USP To optimize the formulation Excipients A number of Formulations 16 2 1 In-vitro studies Stability 3 In-vivo
- Slides: 16