Objectives 1 To know about the structural and

Objectives 1. To know about the structural and biochemical organizations of a mitochondrion 2. To understand the electrochemical reactions through which the chemical energy in food can be converted to chemical energy in ATP 3. To realize how the structural organizations of mitochondria have allowed the above electrochemical reactions to be carried out effectively 1
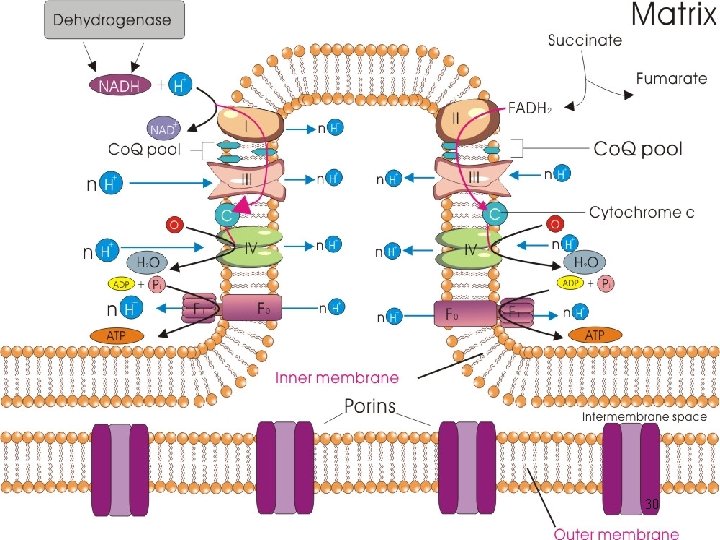
Energy Conversion (1): Mitochondria • Cellular respiration - Flow of electrons from reduced coenzymes to an electron acceptor; generation of ATP - NADH and FADH 2 from glycolysis, TCA cycle, oxidations, etc. - Ultimate electron acceptor is oxygen; reduced form as water (aerobic respiration); takes place with mitochondria in eukaryotic cells 2
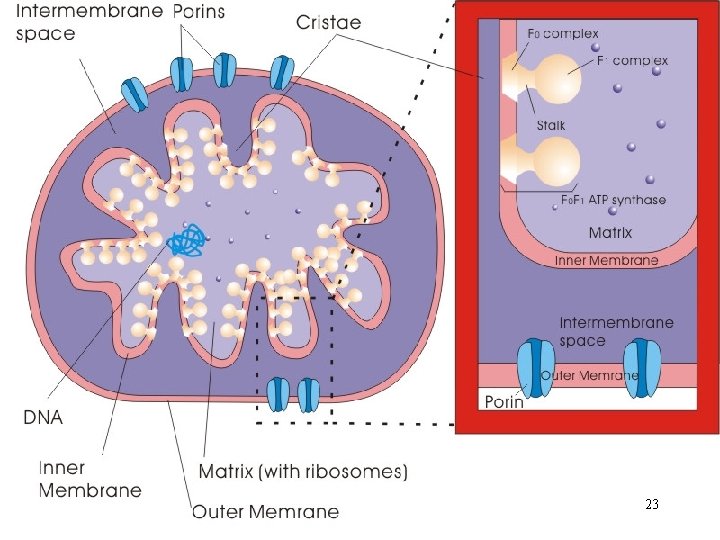
The Energy Powerhouse • Discrete sausage-shaped structures; the second largest organelle in most animal cells • A double-membrane organelle; outer membrane separated from inner membrane by intermembrane space A. Outer membrane • Not a significant permeability barrier for ions and small molecules; transmembrane proteins (porins) 3
B. Intermembrane space • Continuous with the cytosol C. Inner membrane • A permeability barrier to most solutes • Locale of the protein complexes of electron transport and ATP synthesis • Distinctive foldings (cristae); increase surface area to accommodate more the protein complexes 4

D. Matrix • Semi-fluid enclosed by inner membrane; - Enzymes for mitochondrial functions - A circular DNA molecule; coding for its own r. RNAs, t. RNAs, and a number of polypeptide subunits of innermembrane proteins (genetic competence) 5
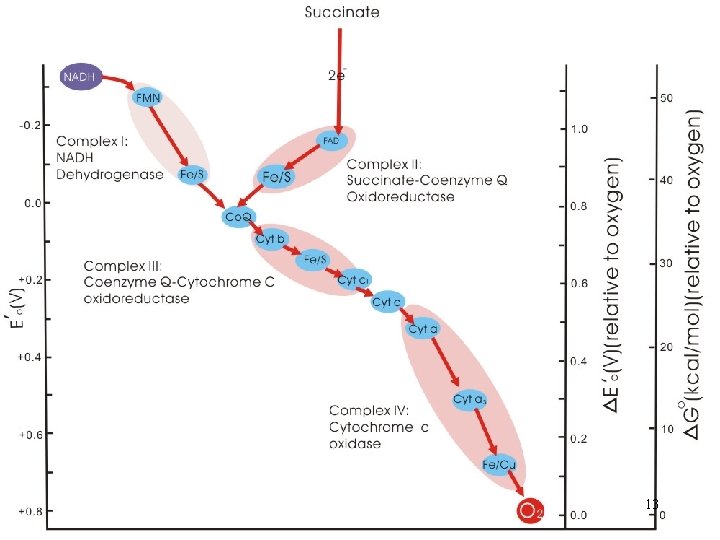
Electron Transport System (ETS) • Transfer of electrons from NADH and FADH 2 is highly exergonic • Multistep process; a series of reversibly oxidizable electron carriers; total free energy difference is released in increments to prevent excessive amount being released as heat (energy conservation for ATP) • 4 different kinds of carriers: : 6
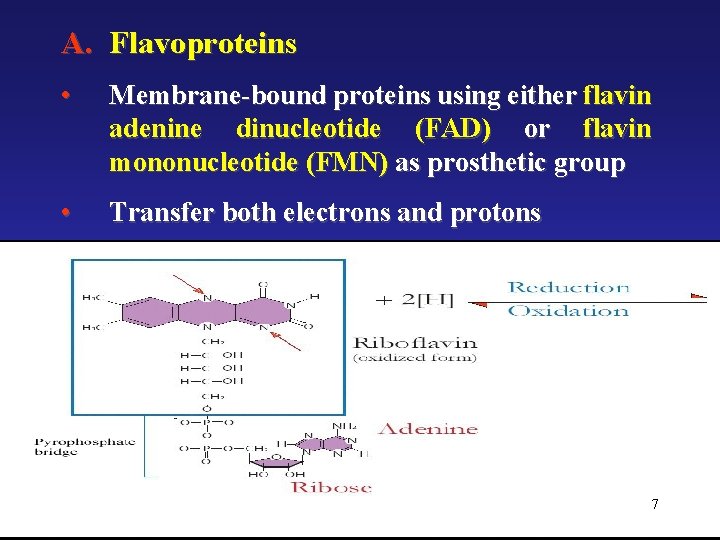
A. Flavoproteins • Membrane-bound proteins using either flavin adenine dinucleotide (FAD) or flavin mononucleotide (FMN) as prosthetic group • Transfer both electrons and protons 7
B. Iron-Sulfur Proteins • Proteins containing iron-sulfur (Fe/S) centers; iron and sulfur atoms complexed with cysteine groups of the protein • Alternates between Fe 2+(ferrous) • Do not pick up and release protons the Fe 3+(ferric) and C. Cytochromes (Cyt) • Contain iron; part of a porphyrin prosthetic group (heme) 8
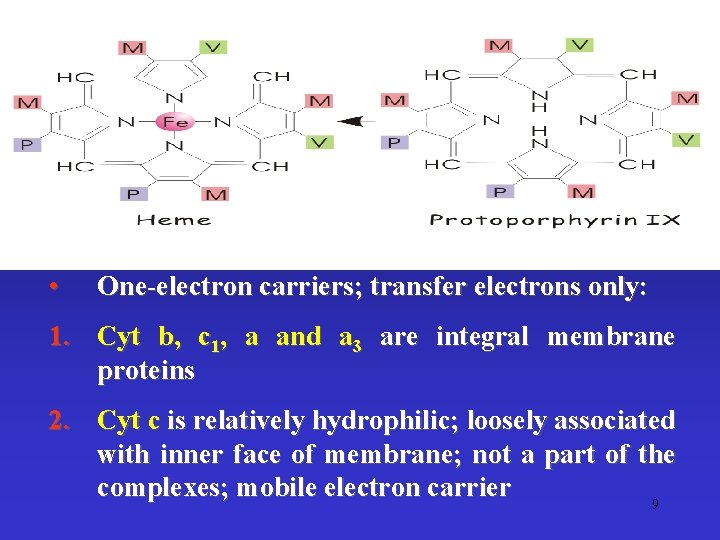
• One-electron carriers; transfer electrons only: 1. Cyt b, c 1, a and a 3 are integral membrane proteins 2. Cyt c is relatively hydrophilic; loosely associated with inner face of membrane; not a part of the complexes; mobile electron carrier 9

3. Cyt a and a 3 • Copper - containing - cytochromes (bimetallic iron-copper (Fe/Cu) center) • Components of cytochrome c oxidase • Keeping an O 2 molecule bound to the oxidase complex; completely picked up the four electrons and four protons 10
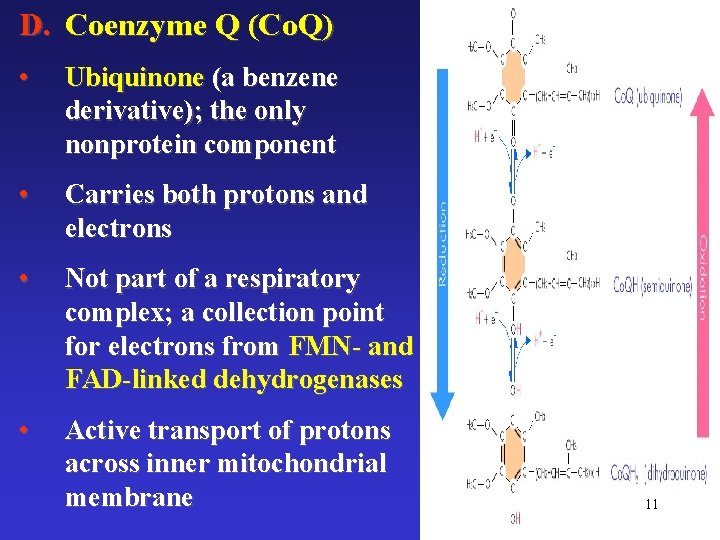
D. Coenzyme Q (Co. Q) • Ubiquinone (a benzene derivative); the only nonprotein component • Carries both protons and electrons • Not part of a respiratory complex; a collection point for electrons from FMN- and FAD-linked dehydrogenases • Active transport of protons across inner mitochondrial membrane 11
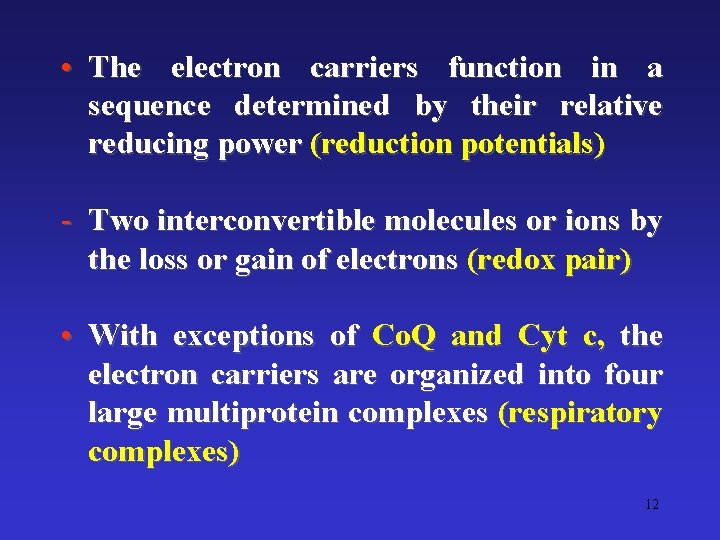
• The electron carriers function in a sequence determined by their relative reducing power (reduction potentials) - Two interconvertible molecules or ions by the loss or gain of electrons (redox pair) • With exceptions of Co. Q and Cyt c, the electron carriers are organized into four large multiprotein complexes (respiratory complexes) 12
13
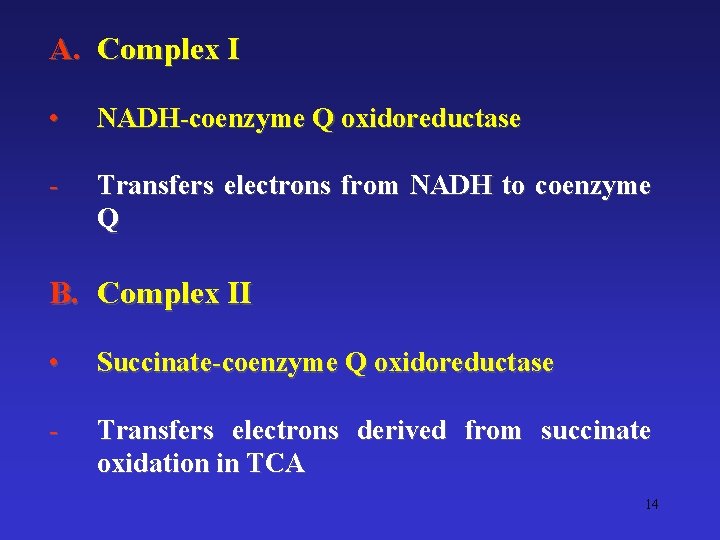
A. Complex I • NADH-coenzyme Q oxidoreductase - Transfers electrons from NADH to coenzyme Q B. Complex II • Succinate-coenzyme Q oxidoreductase - Transfers electrons derived from succinate oxidation in TCA 14
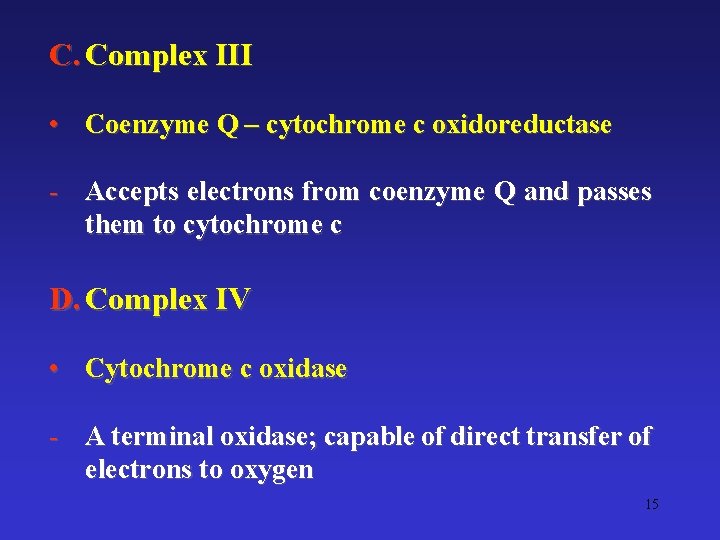
C. Complex III • Coenzyme Q – cytochrome c oxidoreductase - Accepts electrons from coenzyme Q and passes them to cytochrome c D. Complex IV • Cytochrome c oxidase - A terminal oxidase; capable of direct transfer of electrons to oxygen 15
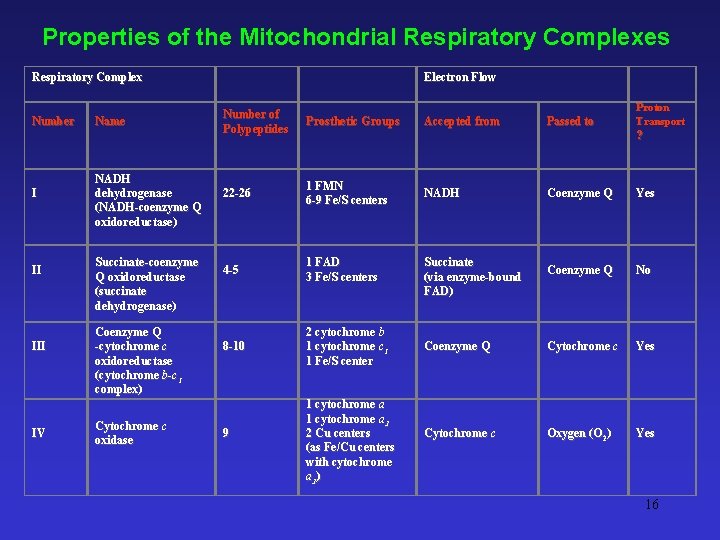
Properties of the Mitochondrial Respiratory Complexes Respiratory Complex Number I II IV Name NADH dehydrogenase (NADH-coenzyme Q oxidoreductase) Succinate-coenzyme Q oxidoreductase (succinate dehydrogenase) Coenzyme Q -cytochrome c oxidoreductase (cytochrome b-c 1 complex) Cytochrome c oxidase Electron Flow Number of Polypeptides Prosthetic Groups 22 -26 1 FMN 6 -9 Fe/S centers 4 -5 1 FAD 3 Fe/S centers 8 -10 2 cytochrome b 1 cytochrome c 1 1 Fe/S center 9 Accepted from Passed to Proton Transport ? 1 cytochrome a 3 2 Cu centers (as Fe/Cu centers with cytochrome a 3) NADH Coenzyme Q Yes Coenzyme Q No Coenzyme Q Cytochrome c Yes Cytochrome c Oxygen (O 2) Yes Succinate (via enzyme-bound FAD) 16
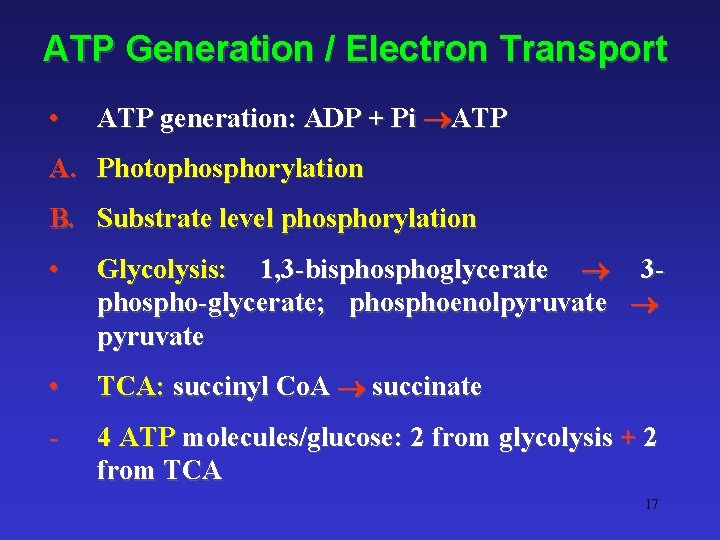
ATP Generation / Electron Transport • ATP generation: ADP + Pi ATP A. Photophosphorylation B. Substrate level phosphorylation • Glycolysis: 1, 3 -bisphoglycerate 3 phospho-glycerate; phosphoenolpyruvate • TCA: succinyl Co. A succinate - 4 ATP molecules/glucose: 2 from glycolysis + 2 from TCA 17
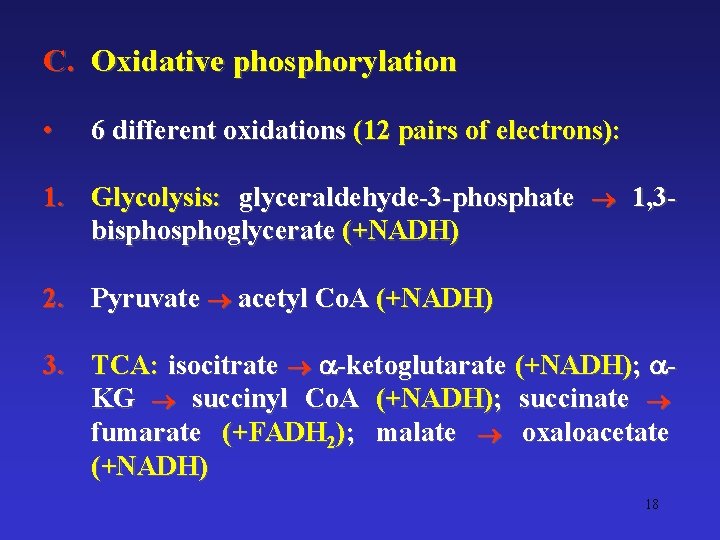
C. Oxidative phosphorylation • 6 different oxidations (12 pairs of electrons): 1. Glycolysis: glyceraldehyde-3 -phosphate 1, 3 bisphoglycerate (+NADH) 2. Pyruvate acetyl Co. A (+NADH) 3. TCA: isocitrate -ketoglutarate (+NADH); KG succinyl Co. A (+NADH); succinate fumarate (+FADH 2); malate oxaloacetate (+NADH) 18
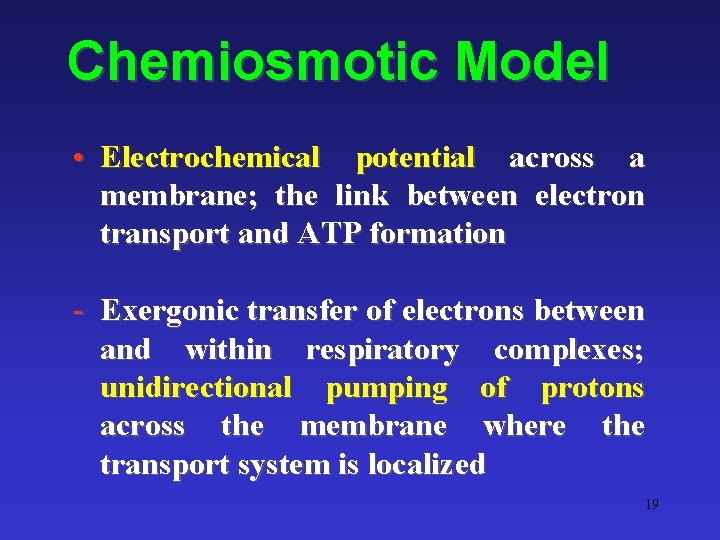
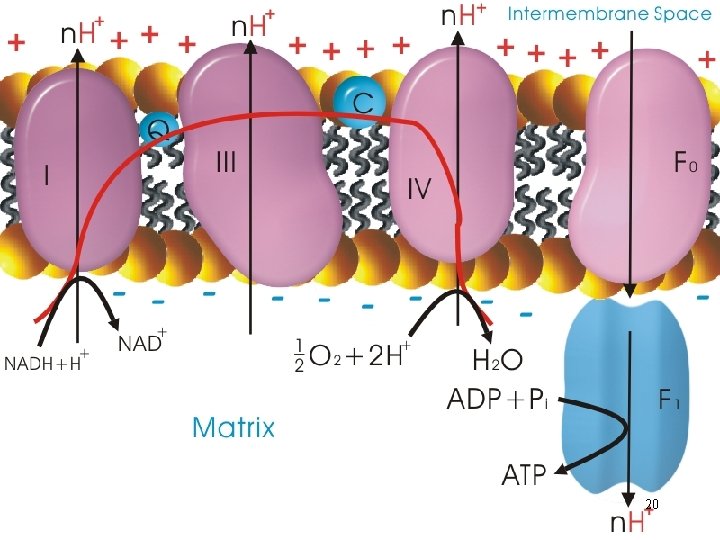
Chemiosmotic Model • Electrochemical potential across a membrane; the link between electron transport and ATP formation - Exergonic transfer of electrons between and within respiratory complexes; unidirectional pumping of protons across the membrane where the transport system is localized 19
20

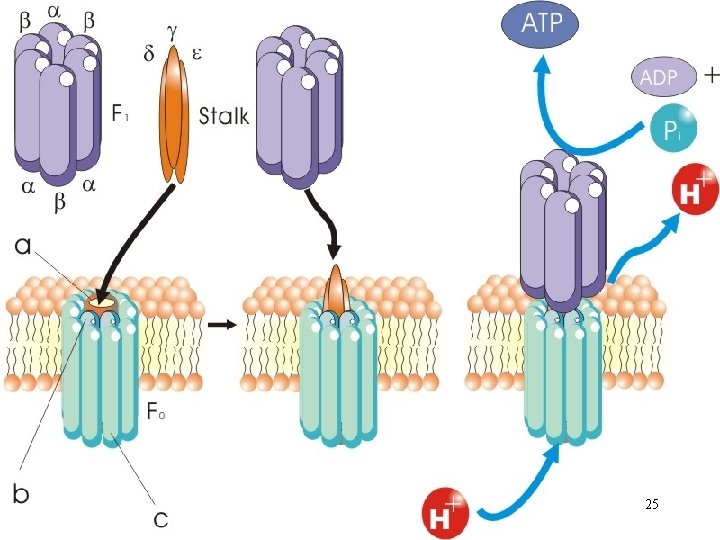
The F 0 F 1 Complex • A F-type ATPase; both ATPase and ATP synthase activities • Converts electrochemical energy (proton gradient) into potential chemical energy (ATP) A. F 1 complex • 3 and 3 polypeptides; 3 complexes (catalytic hexagon) 21
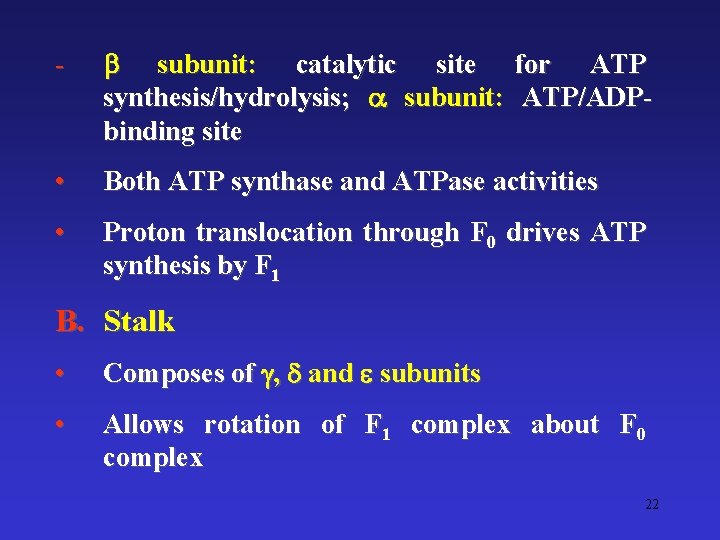
- subunit: catalytic site for ATP synthesis/hydrolysis; subunit: ATP/ADPbinding site • Both ATP synthase and ATPase activities • Proton translocation through F 0 drives ATP synthesis by F 1 B. Stalk • Composes of , and subunits • Allows rotation of F 1 complex about F 0 complex 22
23
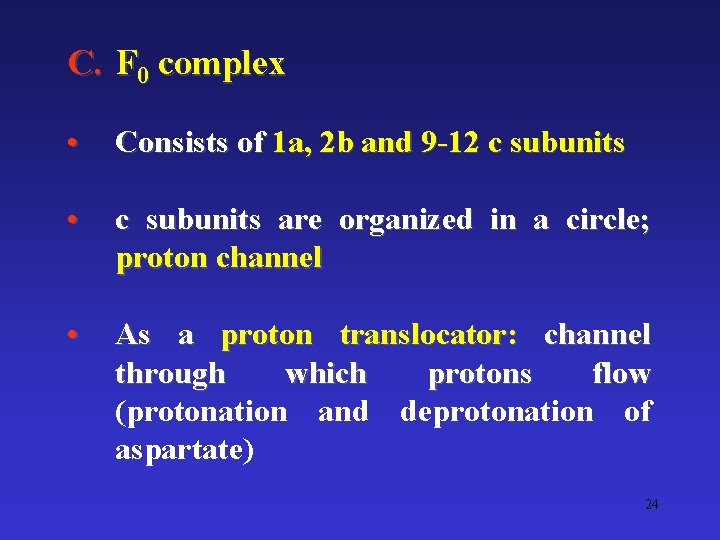
C. F 0 complex • Consists of 1 a, 2 b and 9 -12 c subunits • c subunits are organized in a circle; proton channel • As a proton translocator: channel through which protons flow (protonation and deprotonation of aspartate) 24
25
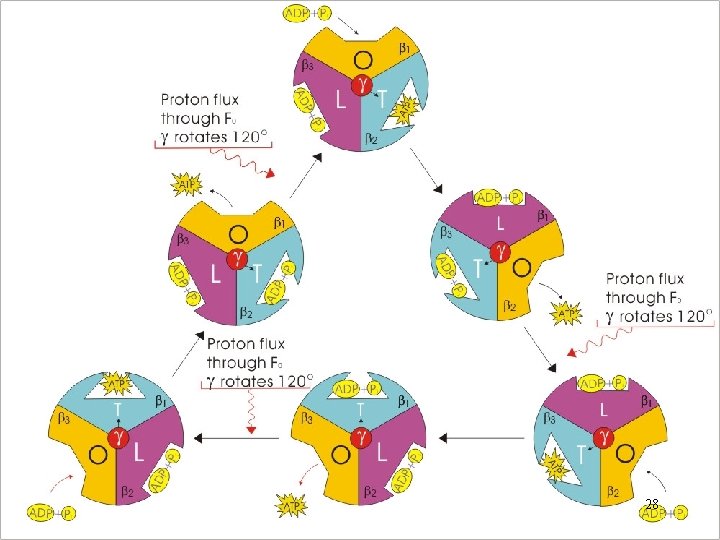
Binding Change Model • To explain how exergonic flow of protons through F 0 can drive endergonic phosphorylation of ADP to ATP • Electrochemical–to–mechanical–to–chemical transducer • Each of the three subunits exists in 3 different conformations at any point in time: 26

- (O)pen: little affinity; ADP and Pi are free to enter (ATP is free to leave) the catalytic site - (L)oose: higher affinity; lose binding of ADP and Pi - (T)ight: Packing the ADP and Pi together tightly; facilitating the condensation - O L T: 27
28
1. Flowing of protons flow through a channel in the a subunit of F 0 2. Rotation of the ring of c subunits; rotation of the attached subunit 3. Asymmetry of the subunit; different interactions with the three subunits at any point in time 4. Each subunit passes successively through the O, L, and T conformations as the subunit rotates 360º 29
30
- Slides: 30