Objectives 1 I can classify matter into substances

Objectives: 1. I can classify matter into substances and mixtures. 2. I can identify and distinguish between physical and chemical properties. 3. I can identify and distinguish between the two types of forces that affect the nature of molecules. 4. I can understand distinguish between physical and chemical changes. 5. I can identify techniques used to separate mixtures.

Chemistry: 1. 23. 18 Due: • Scientific Skills Packet • Classify Matter-Define Terms Objectives: • Review Scientific Skills Packet • I can classify matter. • I can distinguish between physical and chemical properties

What do Chemists study? • Study matter: the properties of matter and changes matter can undergo.

How do Chemists Define Matter? One or more atoms that take up space and has mass.
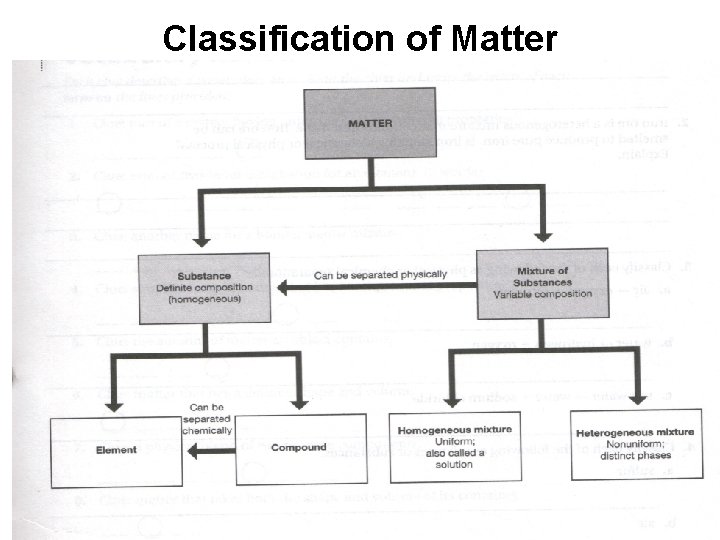
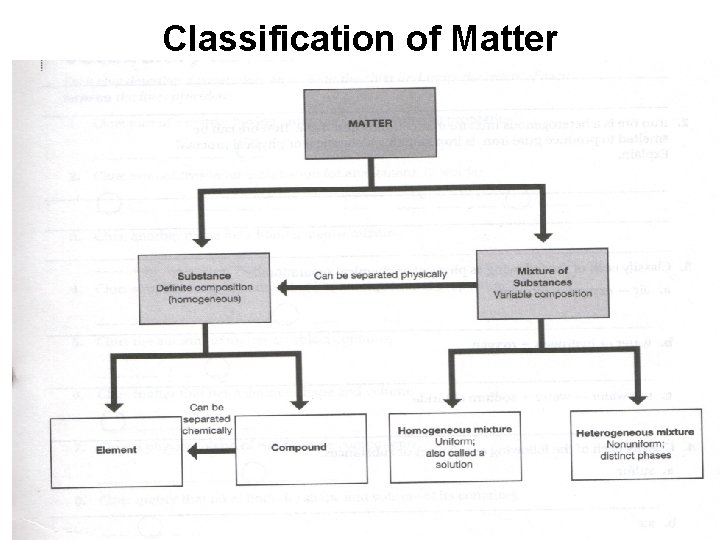
Classification of Matter

Classifying Matter: Substances vs. Mixtures Substance Mixture Composed of one type of substance • Composed of several types of substances. • Has a fixed (the same) chemical make-up. • Uniform throughout the sample. • Either an element or a compound. • Ex. Copper wire (Cu) Sugar (C 12 H 22 O 11) • Substances are physically combined • Chemical make-up can vary in type of substances and amount. • Either homogenous or heterogenous mixtures • Ex. Pizza Salt Water (Na. Cl + H 2 O)
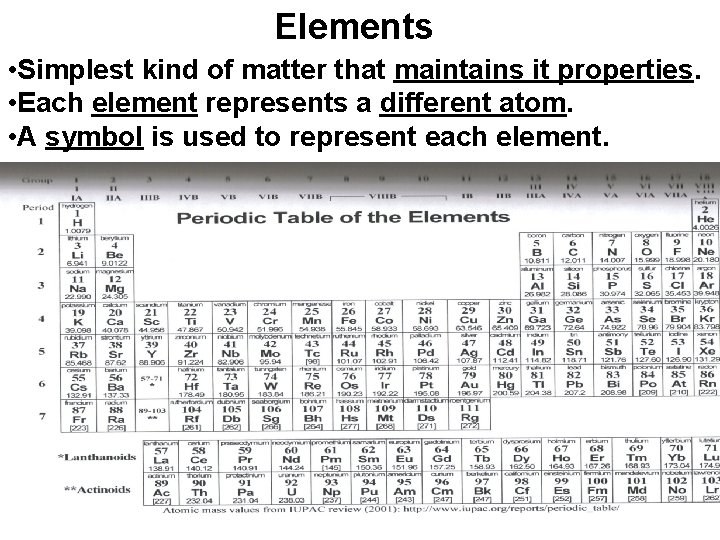
Elements • Simplest kind of matter that maintains it properties. • Each element represents a different atom. • A symbol is used to represent each element.

Chemistry: 1. 24. 18 Due: • Classify Matter Packet Objectives: • I can classify matter-Classification Lab • I can distinguish between physical and chemical properties/changes.
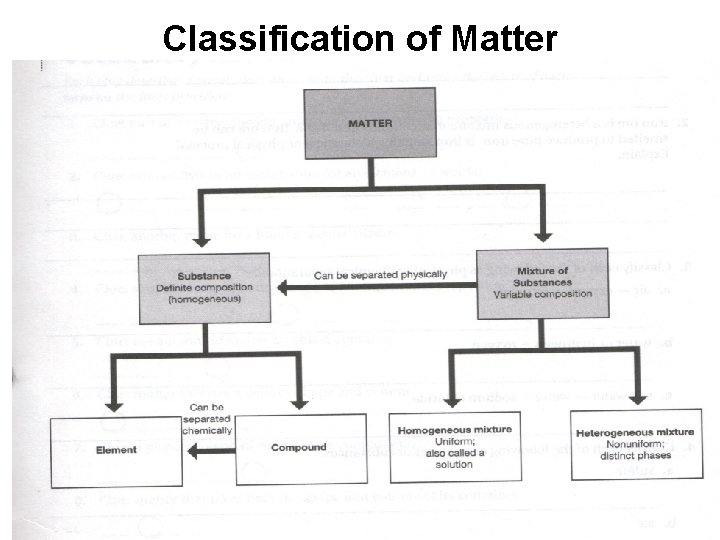
Classification of Matter
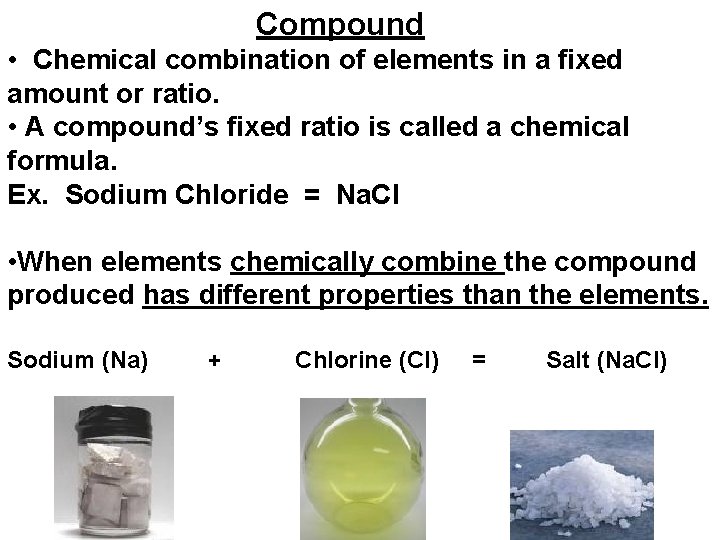
Compound • Chemical combination of elements in a fixed amount or ratio. • A compound’s fixed ratio is called a chemical formula. Ex. Sodium Chloride = Na. Cl • When elements chemically combine the compound produced has different properties than the elements. Sodium (Na) + Chlorine (Cl) = Salt (Na. Cl)

Mixture • Substances (elements or compounds) are physically mixed together to produce a mixture. • The composition and amount of each substance in a mixture varies from one sample to another. • The properties of each substance in a mixture is preserved.

Homogenous Mixtures *Several substances are physically mixed, so properties of each type of substance is preserved. *The substances mix well, so observe one phase (same throughout) in a homo mixture. *Homo mixtures are also called solutions.

Heterogenous Mixtures * Several substances are physically mixed, so properties of each type of substance is preserved. *The substances do no mix well, so observe different phases (parts) in a hetero mixture.

Matter’s Chemical Make-Up The make-up of matter is represented with a symbol or formula. • It can help classify matter. • It can determine if substances are physically or chemically combined in a sample of matter. Element Sodium: (Na) Compound Salt: Na. Cl Mixture Salt Water: Na. Cl + H 2 O

Classifying Matter Lab • I can classify matter.

Chemistry: 1. 25. 18 Due: • Classify Matter Packet-review with Venn. Diagram Objectives: • I can classify matter. • I can distinguish between physical and chemical properties/changes. • I can identify the forces affected during a physical and chemical change.

Classifying Matter Lab Review • How did you know the difference between an element and a compound? • How did you know the difference between a compound a mixture? • How did you know the difference between a hetero and homo mix?
Classification of Matter
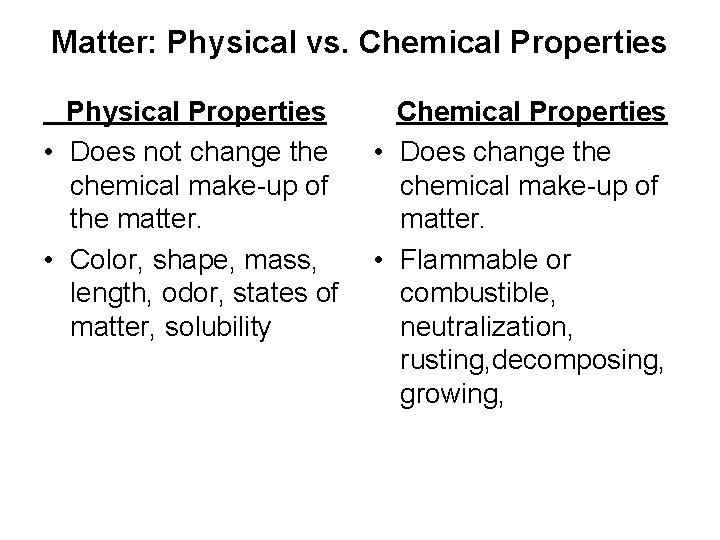
Matter: Physical vs. Chemical Properties Physical Properties • Does not change the chemical make-up of the matter. • Color, shape, mass, length, odor, states of matter, solubility Chemical Properties • Does change the chemical make-up of matter. • Flammable or combustible, neutralization, rusting, decomposing, growing,

Solubility-physical property • The degree one substance dissolves in another. Soluble Salt added to water Insoluble
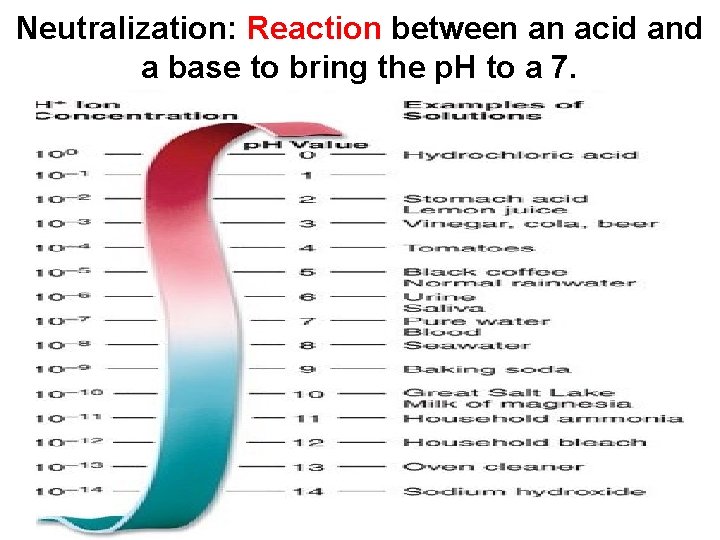
Neutralization: Reaction between an acid and a base to bring the p. H to a 7.
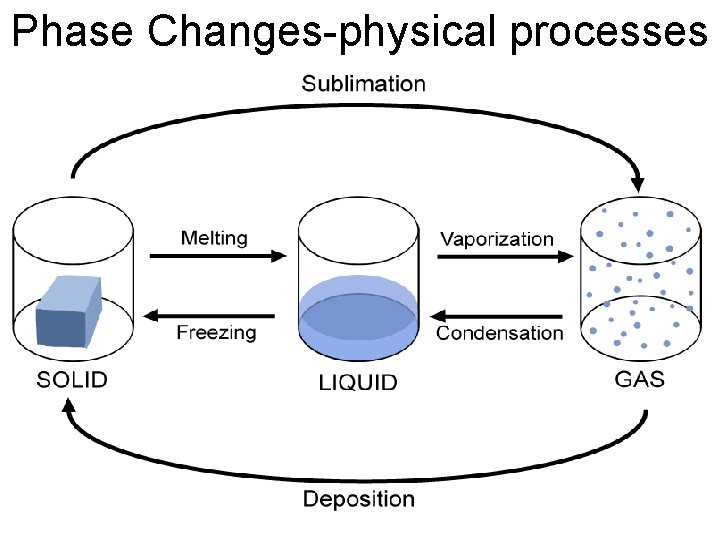
Phase Changes-physical processes
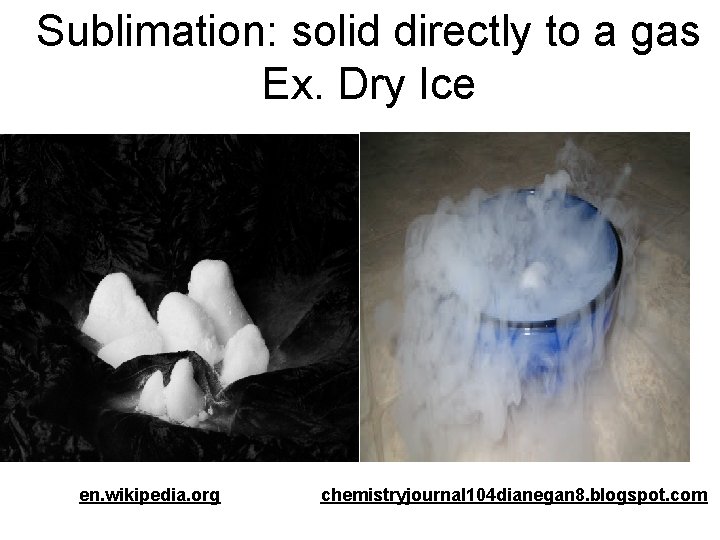
Sublimation: solid directly to a gas Ex. Dry Ice en. wikipedia. org chemistryjournal 104 dianegan 8. blogspot. com

Deposition-gas directly to a solid Ex. Metallic Coating Formation of hail www 2. volstate. edu

Chemical vs. Physical Change Chemical Change: • Matter’s chemical make-up changes. • New matter is produced with new properties. • Ex. Iron Rust (Fe. O) Physical Change: • Physical properties change, but chemical properties are preserved. • Matter’s chemical make-up stays the same. • Ex. cutting paper

Types of Physical Changes • • • Cutting Tearing Dissolving Phase Changes Absorbing
Types of Chemical Changes • • Combustion Decaying Digesting Baking Neutralization (acidic and basic chemicals) Growing Spoiled Reacting

Chemistry: 1. 26. 18 Due: • Physical vs. Chemical Properties/Changes Objectives: • I can classify matter. -quiz • I can distinguish between physical and chemical properties/changes. Worked on pre-lab.
Chemistry: 1. 29. 18 Infinite Campus: • Classification of Matter Quiz-15 pts. • Classification of Matter Lab-17 pts. Due: • Matter Review and Phase Change graph Objectives: • I can distinguish between physical/ chemical properties/changes- Lab

Physical vs. Chemical Change Lab • • Must wear goggles Follow procedures Using 0. 5 M HCl instead of 6 M HCl Used matches in tub NOT waste basket Do not waste matches Follow waste procedures. Clean equipment prior to leaving lab
Chemistry: 1. 30. 18 Infinite Campus: • Measurement Lab -12 pts. Due: • Separation Technique processes • Matter Review and Graphing Sheet Objectives: • I can distinguish between physical/ chemical properties/changes- Lab • I can identify techniques used to separate substances from a mixture

Physical vs. Chemical Change Lab • • Must wear goggles Follow procedures Using 0. 5 M HCl instead of 6 M HCl Used matches in tub NOT waste basket Do not waste matches Follow waste procedures. Clean equipment prior to leaving lab

Chemical vs. Physical Propertis/ Changes Lab Conclusion: 1. Identify one physical change you observed in the lab and explain why. 2. Identify one chemical change you observed in the lab and explain why. 3. Did you observe a temperature change with any of the experiments?

Chem I : 1. 31. 18 Infinite Campus: • Physical vs. Chemical Change Lab Homework: Separation Techniques of Mixtures Objectives: I can identify forces affected during physical and chemical changes. I can identify and predict which separation technique is best for each mixture.
Mixtures and Separation Methods • Separation Methods: *Filtration *Extraction *Distillation *Chromatography
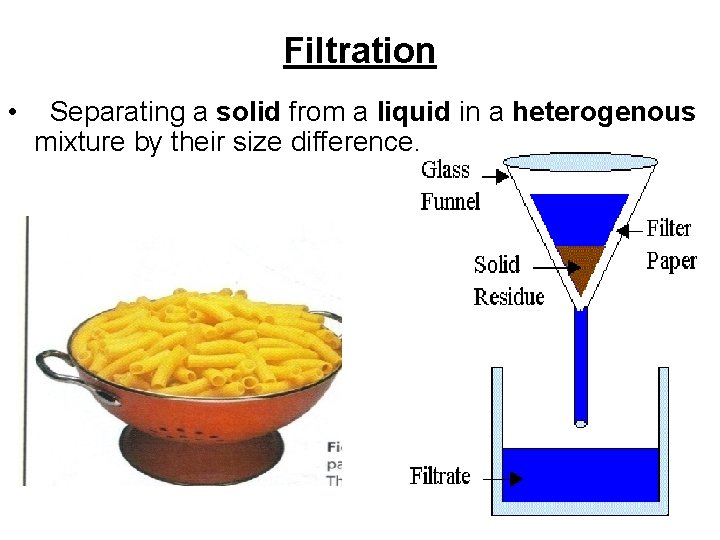
Filtration • Separating a solid from a liquid in a heterogenous mixture by their size difference.

Extraction 1. Extraction- separating substances in a heterogenous mixture from one another based on their physical properties. Physically separate oil from water because they are not soluble with one another. How could we separate iron fillings from sand?
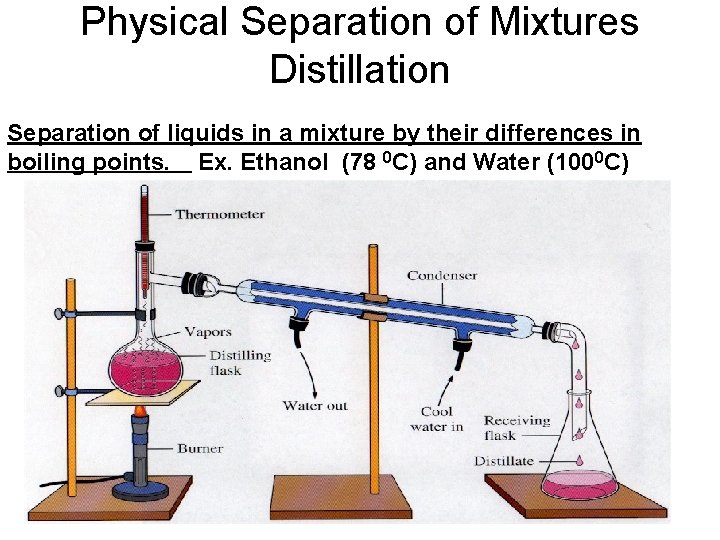
Physical Separation of Mixtures Distillation Separation of liquids in a mixture by their differences in boiling points. Ex. Ethanol (78 0 C) and Water (1000 C)
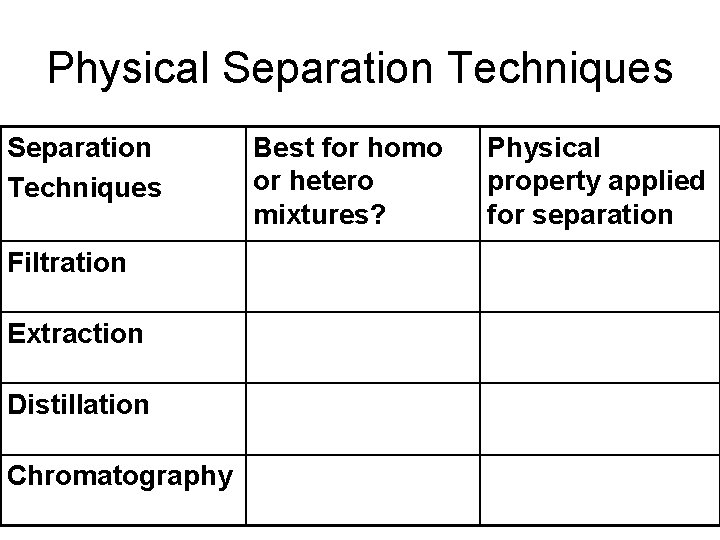
Physical Separation Techniques Filtration Extraction Distillation Chromatography Best for homo or hetero mixtures? Physical property applied for separation
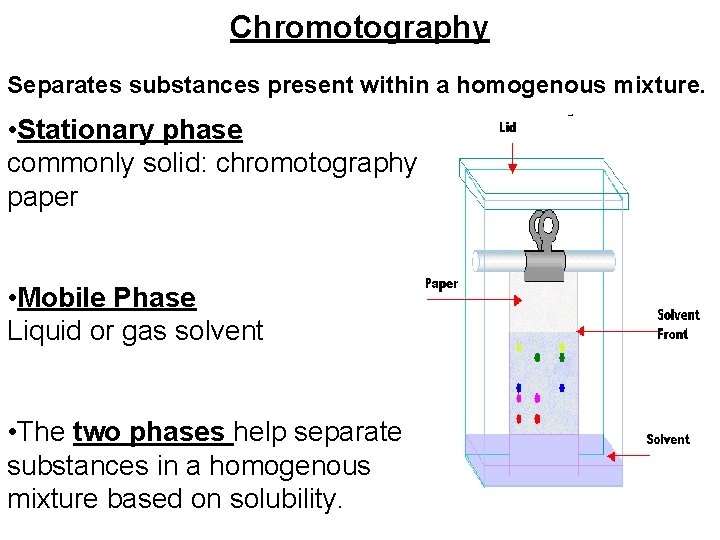
Chromotography Separates substances present within a homogenous mixture. • Stationary phase commonly solid: chromotography paper • Mobile Phase Liquid or gas solvent • The two phases help separate substances in a homogenous mixture based on solubility.

Physical vs. Chemical Changes Lab
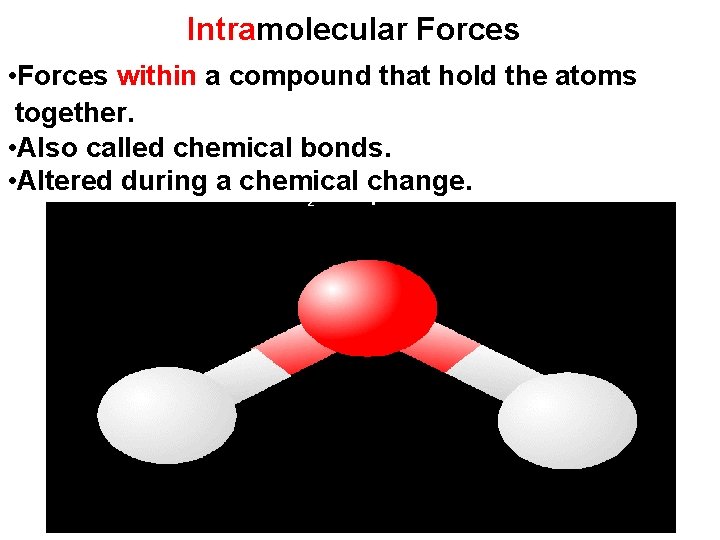
Intramolecular Forces • Forces within a compound that hold the atoms together. • Also called chemical bonds. • Altered during a chemical change. H O compound 2
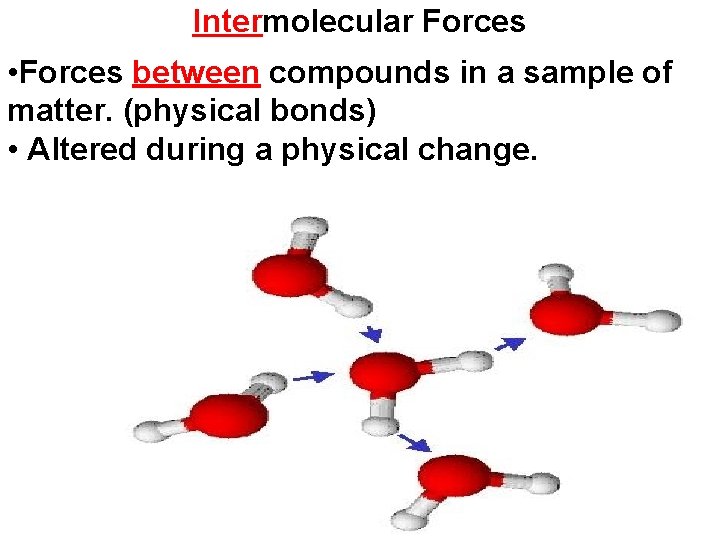
Intermolecular Forces • Forces between compounds in a sample of matter. (physical bonds) • Altered during a physical change.
Marshmallow Lab: Modeling Physical vs Chemical Changes Intramolecular Forces. Intermolecular Forces
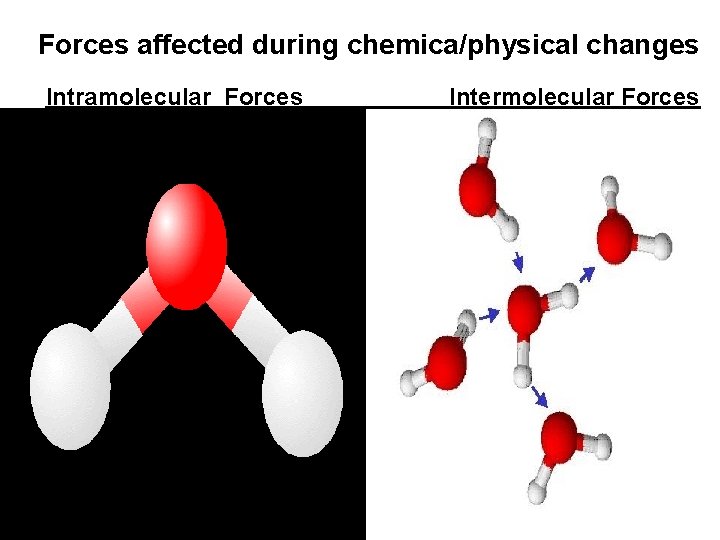
Forces affected during chemica/physical changes Intramolecular Forces. Intermolecular Forces

Conservation of Matter and Energy Matter: Matter undergoes changes, but the atoms are conserved. Energy: Energy is also conserved during changes that matter undergoes. If energy increases for a system, then its surroundings must decrease in energy by the same amount.
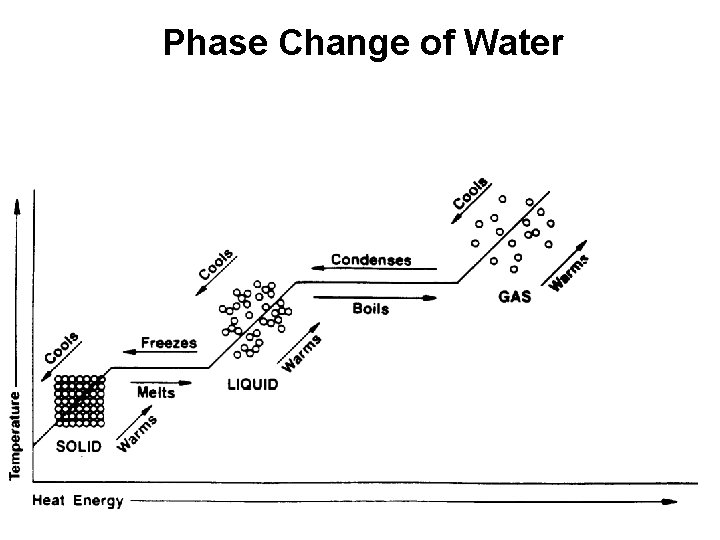
Phase Change of Water
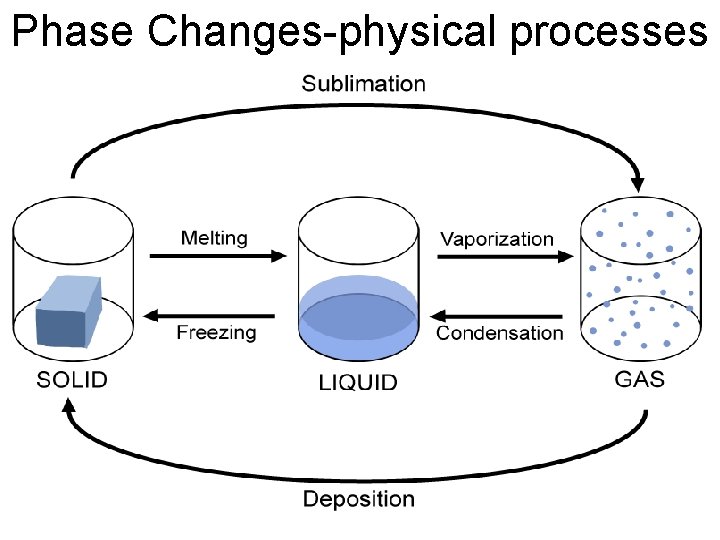
Phase Changes-physical processes
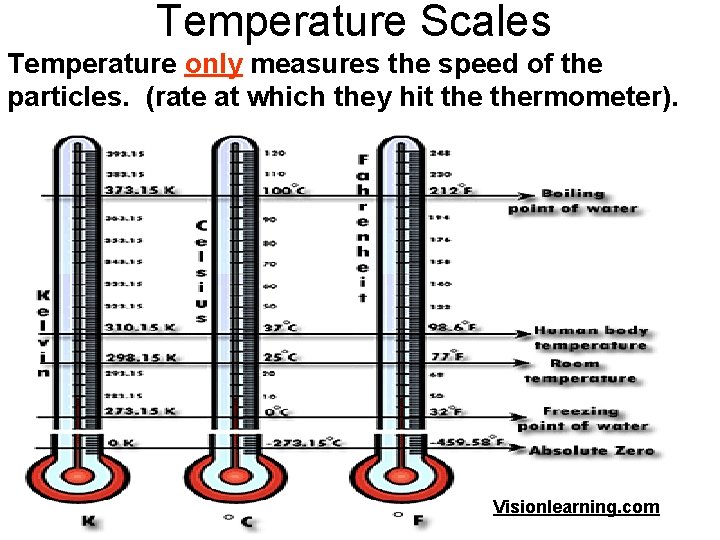
Temperature Scales Temperature only measures the speed of the particles. (rate at which they hit thermometer). Visionlearning. com
Heat Energy Flow Energy flows between: • The System: The matter you are studying/measuring. • The Surrounding : The matter (environment) around the system. Energy Processes: • Endothermic Process: When more heat is absorbed by the system. • Exothermic Process: When more heat is released by the system.
- Slides: 50