Objective TRA7 A Represent the reaction quotient Qc
Objective TRA-7. A Represent the reaction quotient Qc or Qp , for a reversible reaction, and the corresponding equilibrium expressions Kc = Qc or Kp = Qp. TRA-8. B Explain the relationships between Q, K, and the direction in which a reversible reaction will proceed to reach equilibrium.

Reaction quotient, Q For a system to be at equilibrium, all substances (even the solids and liquids that are excluded from the K expression) must be present.

Reaction quotient, Q When only “reactants” are provided, the system is clearly not at equilibrium. The forward reaction will occur at a faster rate than the reverse reaction until the system achieves equilibrium.

Reaction quotient, Q When only “products” are provided, the system is clearly not at equilibrium. The reverse reaction will occur at a faster rate than the forward reaction until the system achieves equilibrium.

Reaction quotient, Q But, what if all of the components are present initially? How will I determine a) if the system is at equilibrium, and b) if it isn’t, which reaction will happen more to reach equilibrium? To do this, you will solve for the reaction quotient, Q.

Reaction quotient, Q Q has the exact same formula as K. • Q is the ratio of products to reactants at any point during the reaction. • K is the ratio of products to reactants specifically at equilibrium. (SEE DIAGRAM ON BOARD)

Reaction quotient, Q If Q = K, the system is at equilibrium. The forward and reverse reaction are already occurring at the same rate.

Reaction quotient, Q If Q is less than K, the system needs to shift right to reach equilibrium. • The ratio of products to reactants is too small. • The forward reaction needs to happen faster than the reverse reaction. • Some phrases you will see: • • • “System must shift right” “Forward reaction is favored” “Products are favored”

Reaction quotient, Q If Q is greater than K, the system needs to shift left to reach equilibrium. • The ratio of products to reactants is too big. • The reverse reaction needs to happen faster than the forward reaction. • Some phrases you will see: • • • “System must shift left” “Reverse reaction is favored” “Reactants are favored”
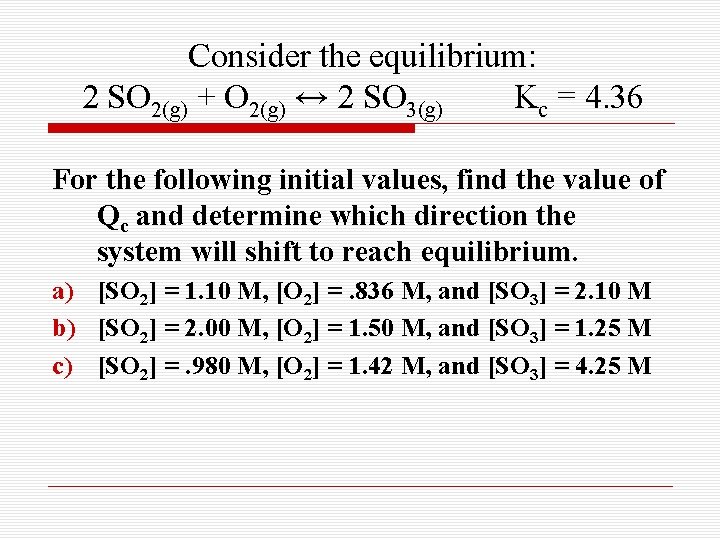
Consider the equilibrium: 2 SO 2(g) + O 2(g) ↔ 2 SO 3(g) Kc = 4. 36 For the following initial values, find the value of Qc and determine which direction the system will shift to reach equilibrium. a) [SO 2] = 1. 10 M, [O 2] =. 836 M, and [SO 3] = 2. 10 M b) [SO 2] = 2. 00 M, [O 2] = 1. 50 M, and [SO 3] = 1. 25 M c) [SO 2] =. 980 M, [O 2] = 1. 42 M, and [SO 3] = 4. 25 M
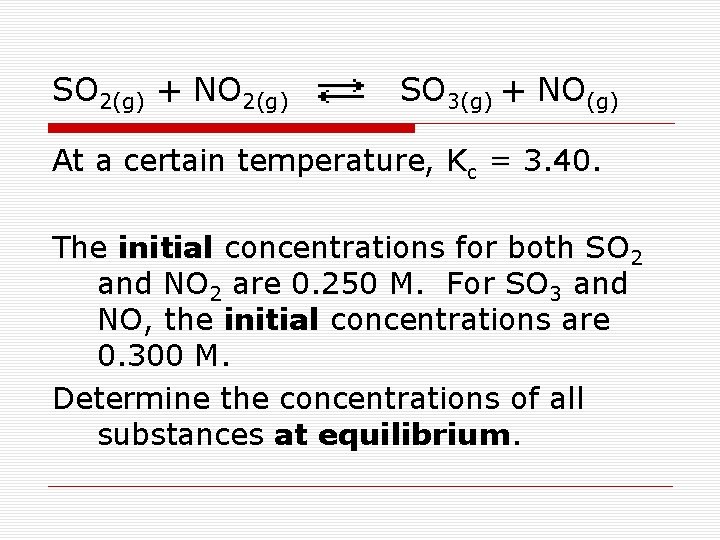
SO 2(g) + NO 2(g) SO 3(g) + NO(g) At a certain temperature, Kc = 3. 40. The initial concentrations for both SO 2 and NO 2 are 0. 250 M. For SO 3 and NO, the initial concentrations are 0. 300 M. Determine the concentrations of all substances at equilibrium.
- Slides: 12