Objective To perform metric conversions Chapter 1 Section

Objective • To perform metric conversions.

Chapter 1 Section 1: The Metric System (The International System Of Units or SI System)

What is SI? • SI stands for Syteme internationale d’Unites • Used by scientists since 1960 to set a consistent worldwide standard.

SI Base Units Quantity SI Base Unit Symbol Length (l) meter m Mass (m) kilogram kg Temperature (T) Kelvin K Time (t) second s Amount of Substance (n) mole mol

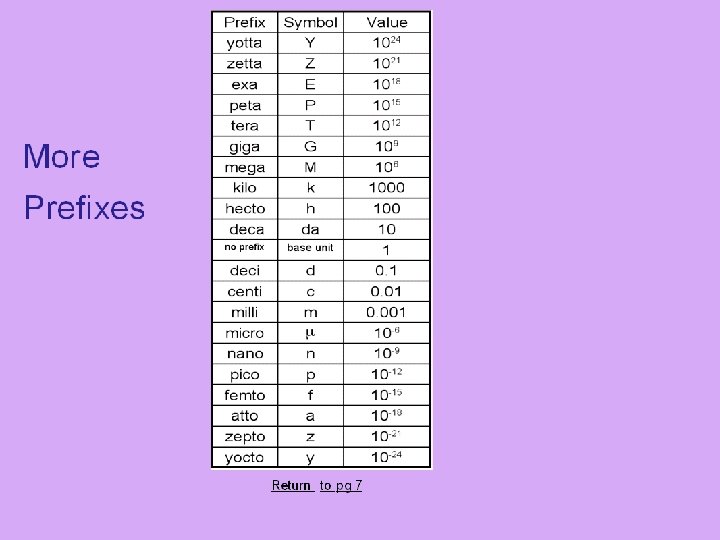
SI Prefixes Prefix kilo (k) hecto (h) Symbol Value k 1000 h 100 deka (da) da 10 deci (d) d 0. 1 centi (c) c 0. 01 milli (m) m 0. 001

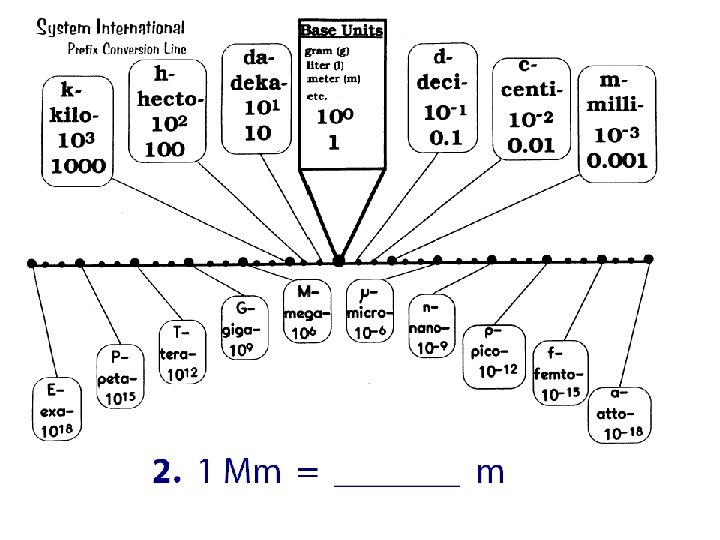
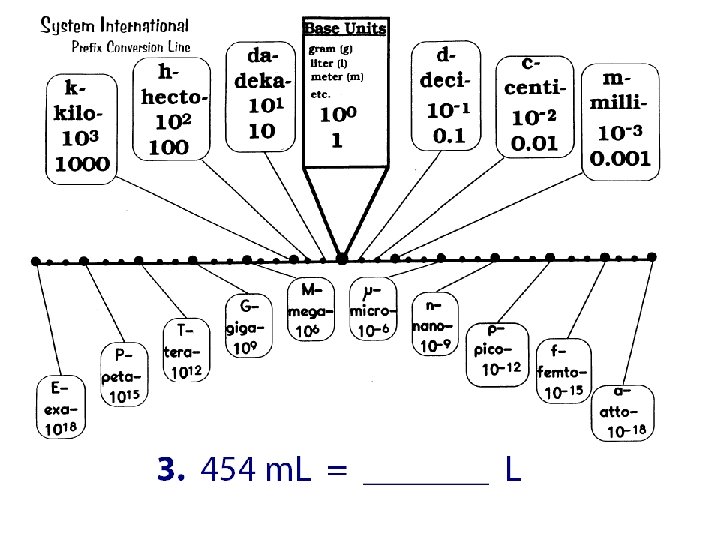

Practice Convert: 1. 253 m. L to liters 2. 1258 cm to m 3. 15 g to kg 4. 17. 3 m to cm 5. 2. 56 m to km 6. 5. 13 m to mm 7. 273 Gg to mg 8. 7. 2 L to n. L 9. 56 cm to m 10. 870 m. L to GL 11. 0. 539 mm to km 12. 200 mm to cm

Convert: 1. 253 m. L to liters 2. 1258 cm to m 3. 15 g to kg 4. 17. 3 m to cm 5. 2. 56 m to km 6. 5. 13 m to mm 7. 273 Gg to mg 8. 7. 2 L to n. L 9. 56 cm to m 10. 870 m. L to GL 11. 0. 539 mm to km 12. 200 mm to cm 1. 2. 3. 4. 5. 6. 7. 273, 000, 000 mg 8. 7, 200, 000 n. L 9. 0. 56 m 10. 0. 00000870 GL 11. 0. 00000539 km 12. 20 cm 0. 253 L 12. 58 m 0. 015 kg 1730 cm 0. 00256 km 5130 mm

Figure out which of the following measurements is largest: a. ) 0. 500 L b. ) 5000 m. L c. ) 0. 050 k. L d. ) 50. 0 c. L Hint: Convert each answer to the same unit!
- Slides: 12