Nuevas opciones teraputicas en CCR diseminado 2005 Sorafenib

Nuevas opciones terapéuticas en CCR diseminado
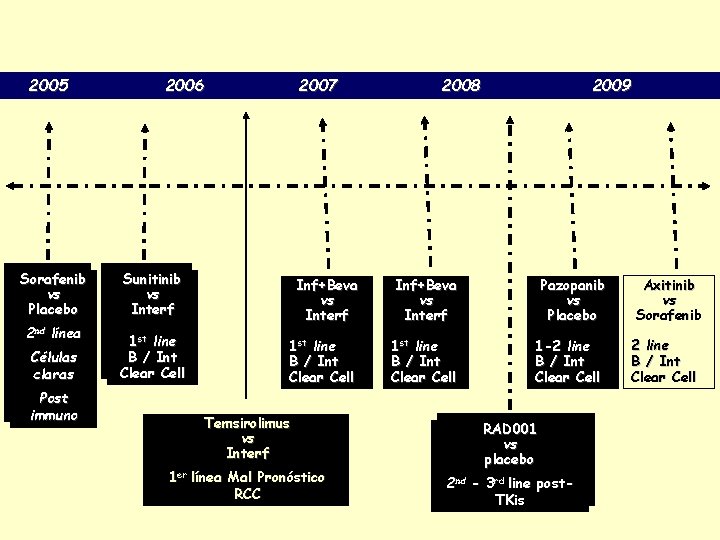
2005 Sorafenib vs Placebo 2 nd línea Células claras Post immuno 2006 2007 2008 Sunitinib vs Interf Inf+Beva vs Interf 1 st line B / Int Clear Cell Temsirolimus vs Interf 1 er línea Mal Pronóstico RCC 2009 Pazopanib vs Placebo 1 -2 line B / Int Clear Cell RAD 001 vs placebo 2 nd - 3 rd line post. TKis Axitinib vs Sorafenib 2 line B / Int Clear Cell
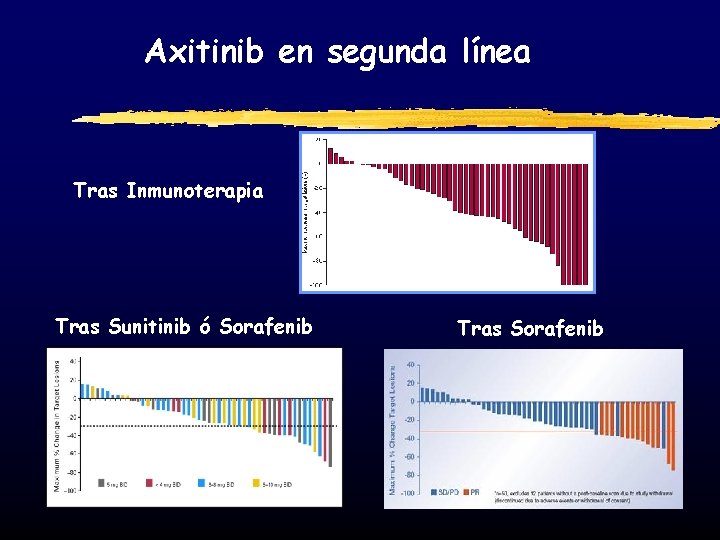
Axitinib en segunda línea Tras Inmunoterapia Tras Sunitinib ó Sorafenib Tras Sorafenib
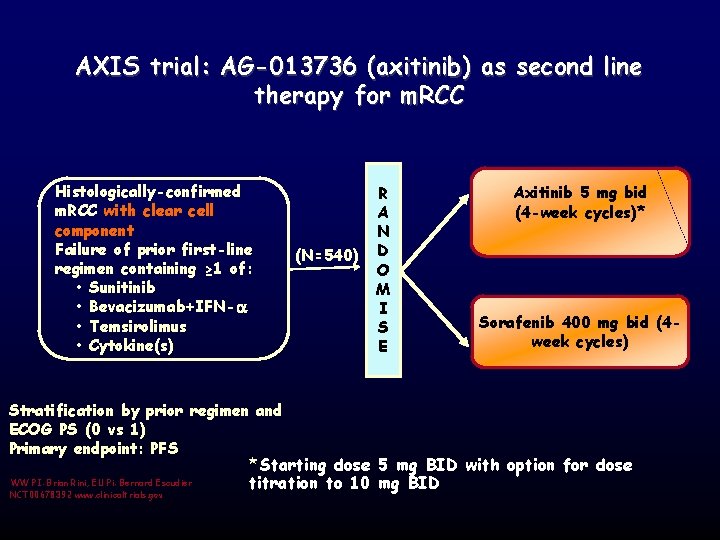
AXIS trial: AG-013736 (axitinib) as second line therapy for m. RCC Histologically-confirmed m. RCC with clear cell component Failure of prior first-line regimen containing ≥ 1 of: • Sunitinib • Bevacizumab+IFN- • Temsirolimus • Cytokine(s) (N=540) R A N D O M I S E Stratification by prior regimen and ECOG PS (0 vs 1) Primary endpoint: PFS Axitinib 5 mg bid (4 -week cycles)* Sorafenib 400 mg bid (4 week cycles) *Starting dose 5 mg BID with option for dose WW PI: Brian Rini; EU Pi: Bernard Escudier NCT 00678392 www. clinicaltrials. gov titration to 10 mg BID
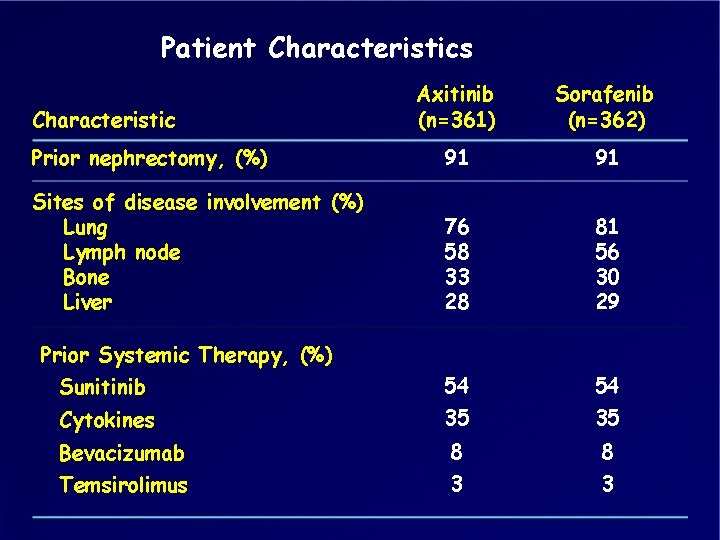
Patient Characteristics Axitinib (n=361) Sorafenib (n=362) Prior nephrectomy, (%) 91 91 Sites of disease involvement (%) Lung Lymph node Bone Liver 76 58 33 28 81 56 30 29 Sunitinib 54 54 Cytokines 35 35 Bevacizumab 8 8 Temsirolimus 3 3 Characteristic Prior Systemic Therapy, (%)
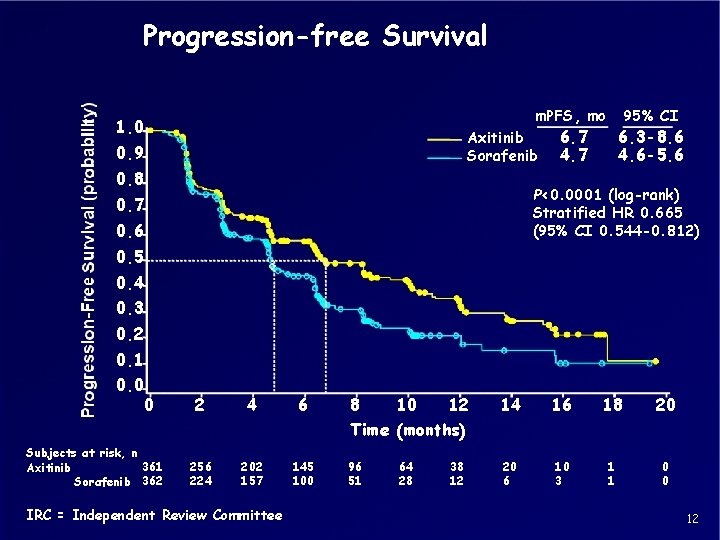
Progression-free Survival m. PFS, mo 1. 0 Axitinib Sorafenib 0. 9 0. 8 95% CI 6. 7 4. 7 6. 3 -8. 6 4. 6 -5. 6 P<0. 0001 (log-rank) Stratified HR 0. 665 (95% CI 0. 544 -0. 812) 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0. 0 0 2 4 6 8 10 12 14 16 18 20 20 6 10 3 1 1 0 0 Time (months) Subjects at risk, n 361 Axitinib Sorafenib 362 256 224 202 157 IRC = Independent Review Committee 145 100 96 51 64 28 38 12 12
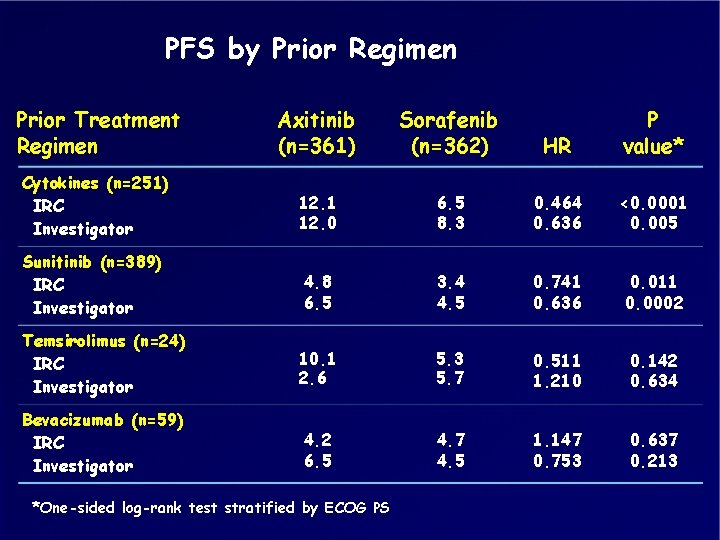
PFS by Prior Regimen Prior Treatment Regimen Axitinib (n=361) Sorafenib (n=362) HR P value* Cytokines (n=251) IRC Investigator 12. 1 12. 0 6. 5 8. 3 0. 464 0. 636 <0. 0001 0. 005 Sunitinib (n=389) IRC Investigator 4. 8 6. 5 3. 4 4. 5 0. 741 0. 636 0. 011 0. 0002 Temsirolimus (n=24) IRC Investigator 10. 1 2. 6 5. 3 5. 7 0. 511 1. 210 0. 142 0. 634 Bevacizumab (n=59) IRC Investigator 4. 2 6. 5 4. 7 4. 5 1. 147 0. 753 0. 637 0. 213 *One-sided log-rank test stratified by ECOG PS
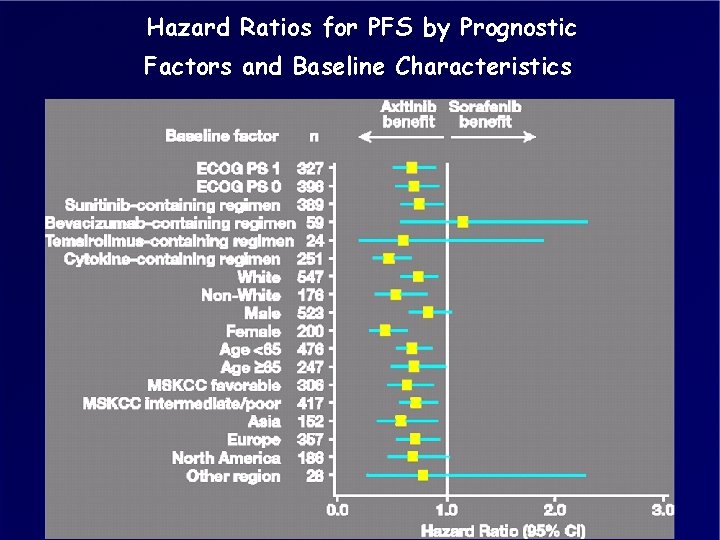
Hazard Ratios for PFS by Prognostic Factors and Baseline Characteristics
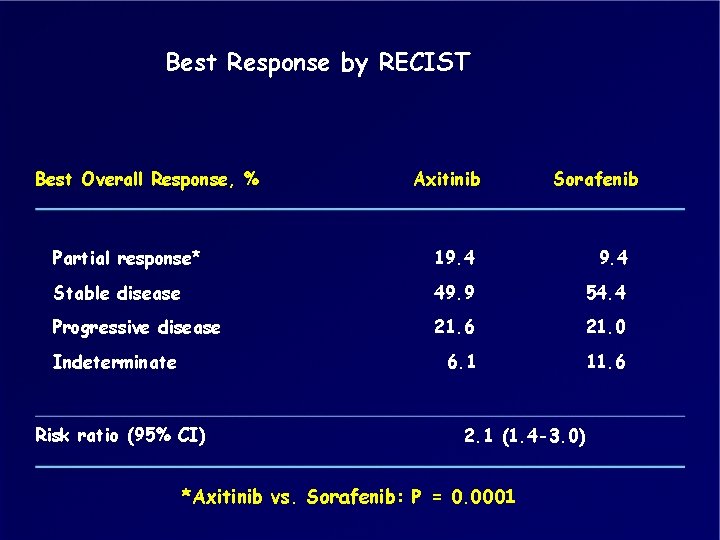
Best Response by RECIST Best Overall Response, % Axitinib Sorafenib Partial response* 19. 4 Stable disease 49. 9 54. 4 Progressive disease 21. 6 21. 0 6. 1 11. 6 Indeterminate Risk ratio (95% CI) 2. 1 (1. 4 -3. 0) *Axitinib vs. Sorafenib: P = 0. 0001
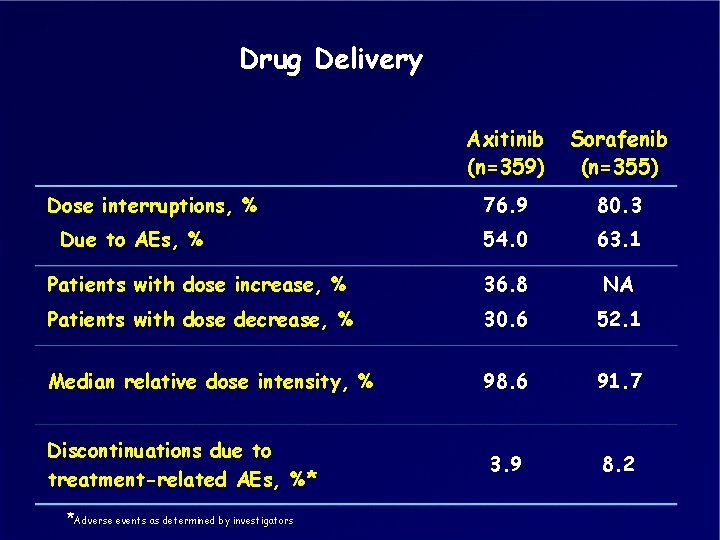
Drug Delivery Axitinib (n=359) Sorafenib (n=355) 76. 9 80. 3 54. 0 63. 1 Patients with dose increase, % 36. 8 NA Patients with dose decrease, % 30. 6 52. 1 Median relative dose intensity, % 98. 6 91. 7 Discontinuations due to treatment-related AEs, %* 3. 9 8. 2 Dose interruptions, % Due to AEs, % *Adverse events as determined by investigators
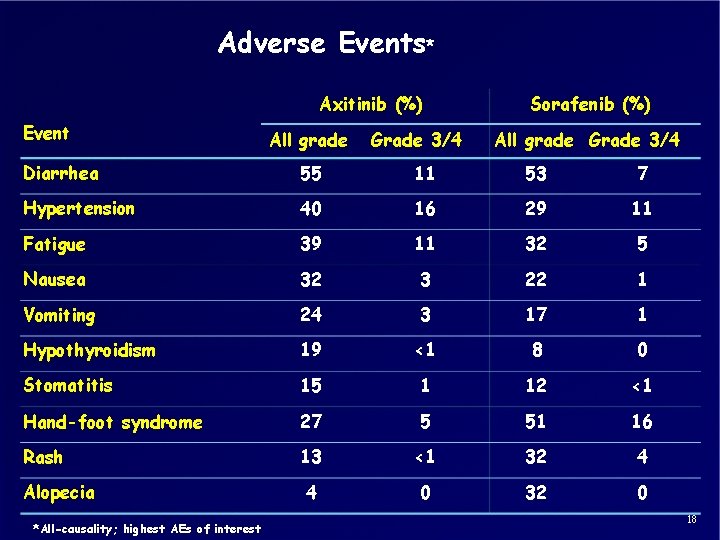
Adverse Events* Axitinib (%) Event All grade Grade 3/4 Sorafenib (%) All grade Grade 3/4 Diarrhea 55 11 53 7 Hypertension 40 16 29 11 Fatigue 39 11 32 5 Nausea 32 3 22 1 Vomiting 24 3 17 1 Hypothyroidism 19 <1 8 0 Stomatitis 15 1 12 <1 Hand-foot syndrome 27 5 51 16 Rash 13 <1 32 4 4 0 32 0 Alopecia *All-causality; highest AEs of interest 18
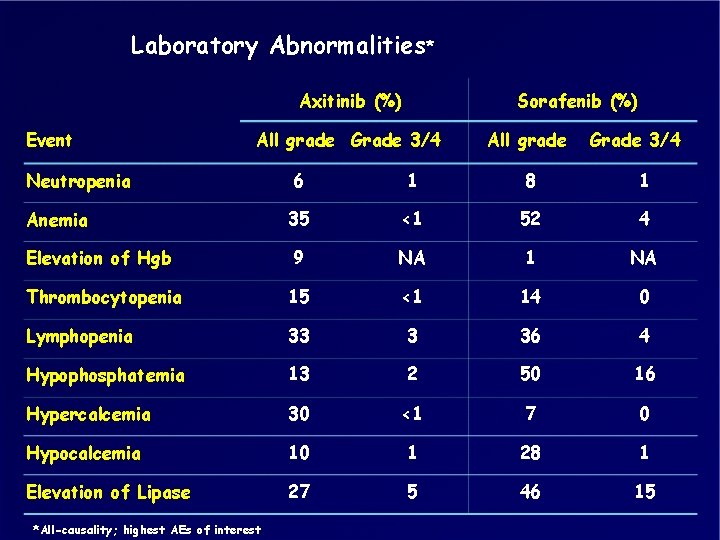
Laboratory Abnormalities* Axitinib (%) Event Sorafenib (%) All grade Grade 3/4 Neutropenia 6 1 8 1 Anemia 35 <1 52 4 Elevation of Hgb 9 NA 1 NA Thrombocytopenia 15 <1 14 0 Lymphopenia 33 3 36 4 Hypophosphatemia 13 2 50 16 Hypercalcemia 30 <1 7 0 Hypocalcemia 10 1 28 1 Elevation of Lipase 27 5 46 15 *All-causality; highest AEs of interest
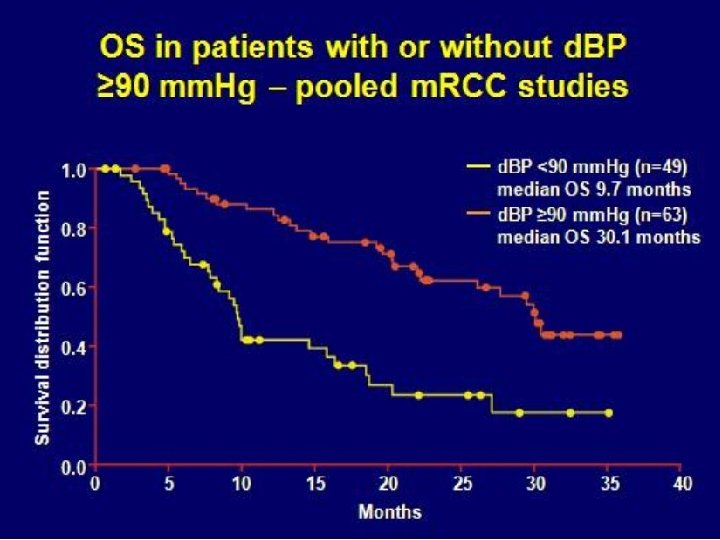

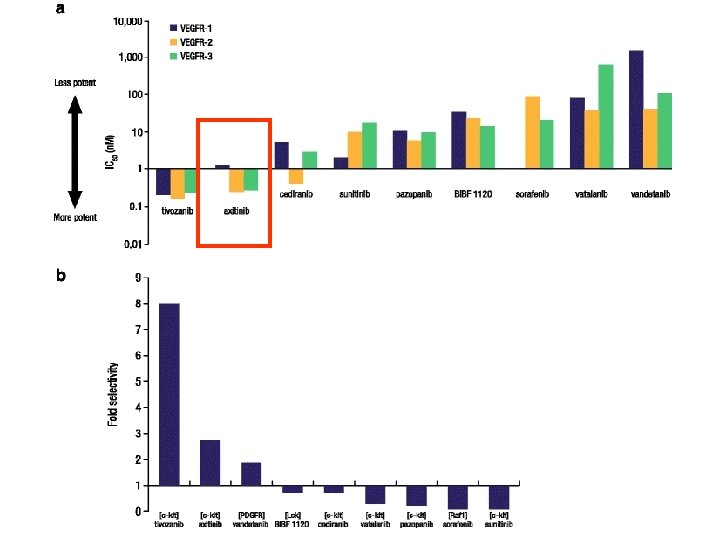
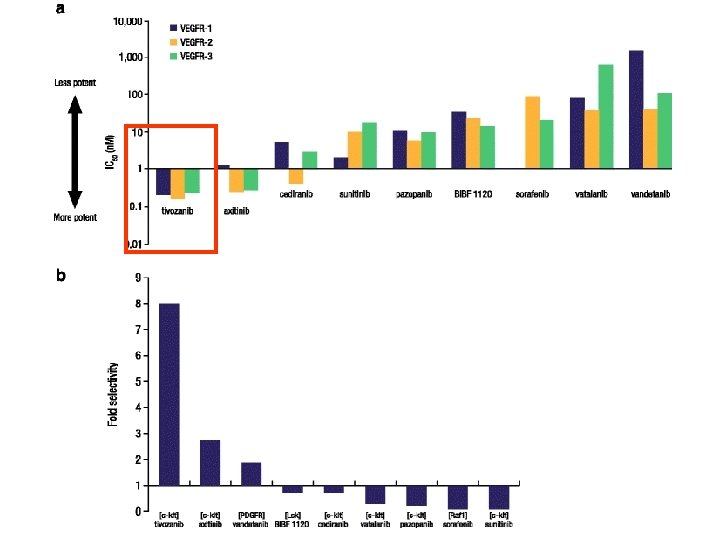
Conclusions These data support the hypothesis that more potent biochemical targeting of the VEGF receptor is associated with superior clinical activity in RCC
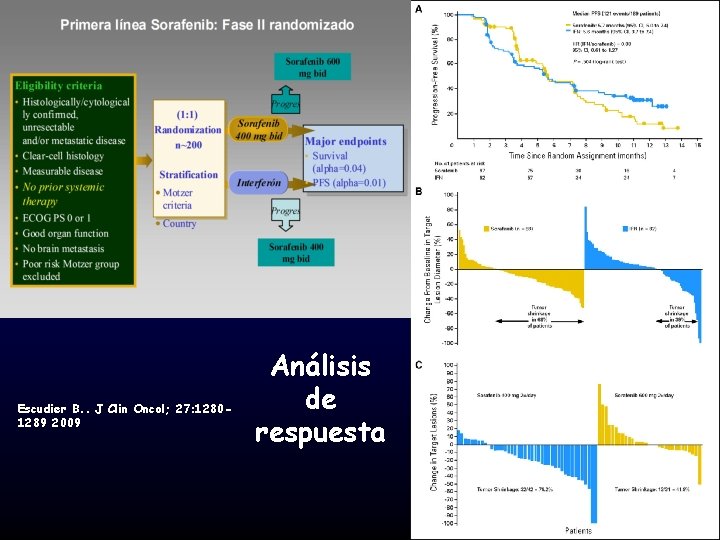
Escudier B. . J Clin Oncol; 27: 12801289 2009 Análisis de respuesta
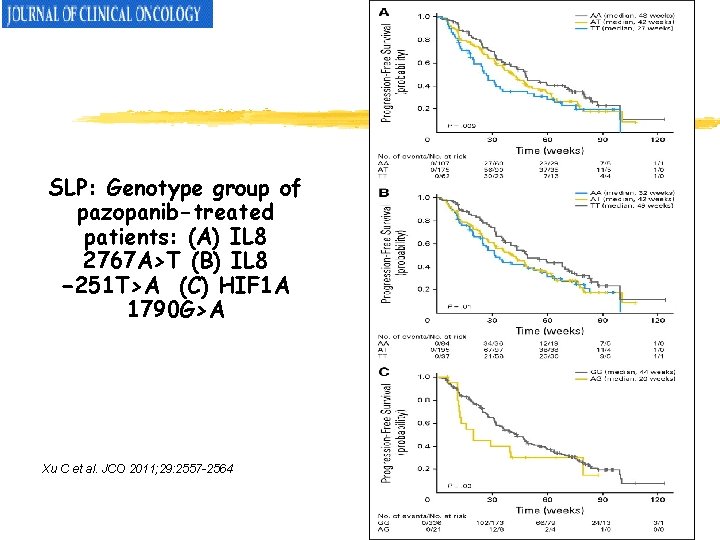
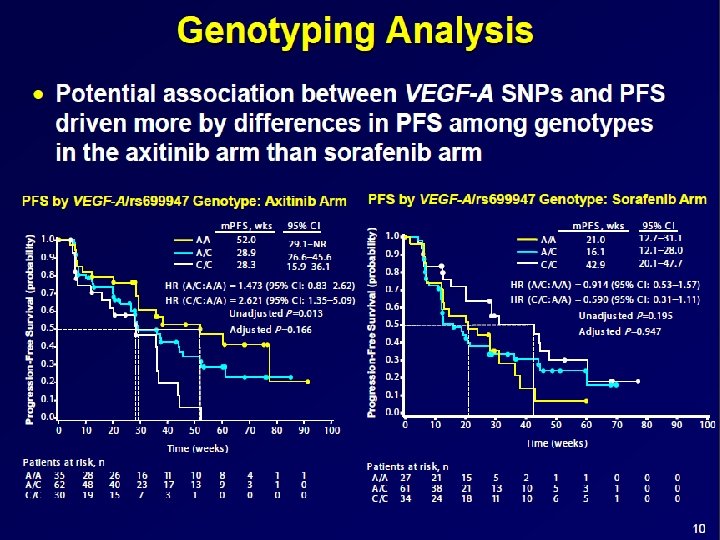
SLP: Genotype group of pazopanib-treated patients: (A) IL 8 2767 A>T (B) IL 8 − 251 T>A (C) HIF 1 A 1790 G>A Xu C et al. JCO 2011; 29: 2557 -2564
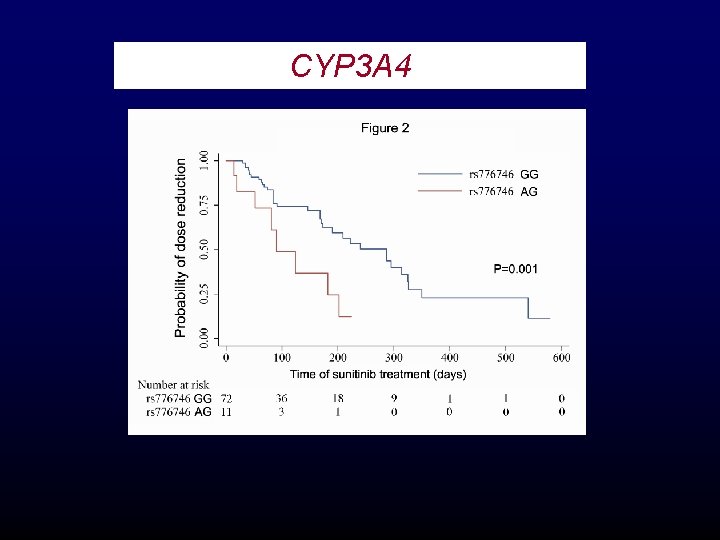
CYP 3 A 4
El plateau de las nuevas terapias? % Spv m. TOR Citoquinas + Cx Anti. ANGI Cronología
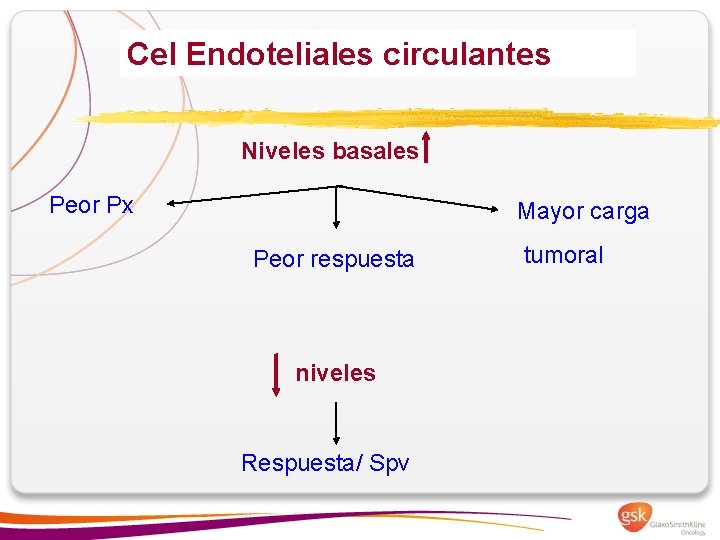
Cel Endoteliales circulantes Niveles basales Peor Px Mayor carga Peor respuesta niveles Respuesta/ Spv tumoral

• Estudio prospectivo ( 1º linea) • 75 pacientes en RP/EE • CEC como predictoras precoces progresión
Tivozanib: Ensayo discontinuación
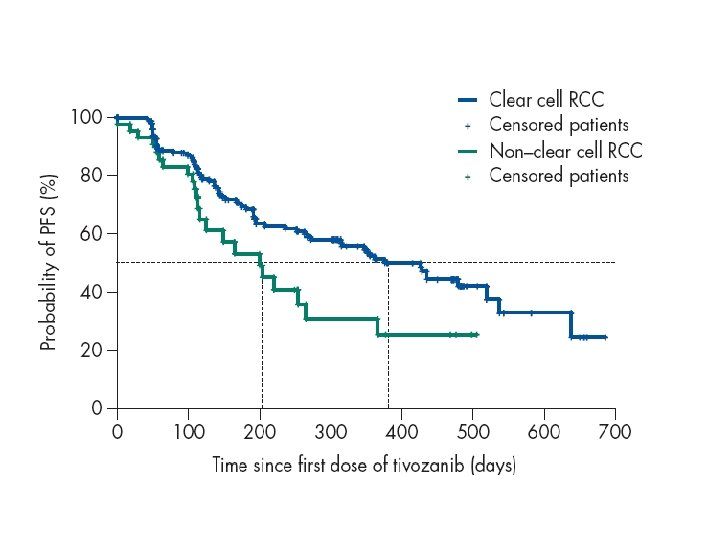
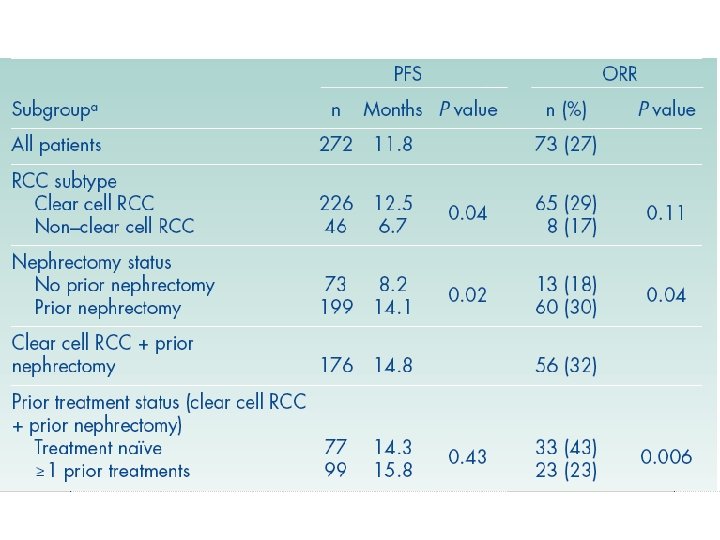
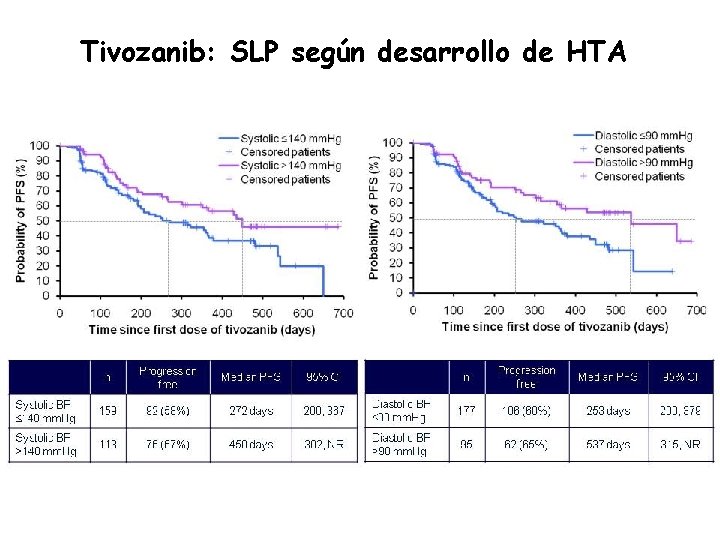
Tivozanib: SLP según desarrollo de HTA

Tivozanib: Conclusiones fase II • HTA como marcador de respuesta • Tolerabilidad superior a otros Itks (mucositis 4%!!!!) • Combinabilidad: fase II con everolimus y temsirolimus (único Itk) • Más potente
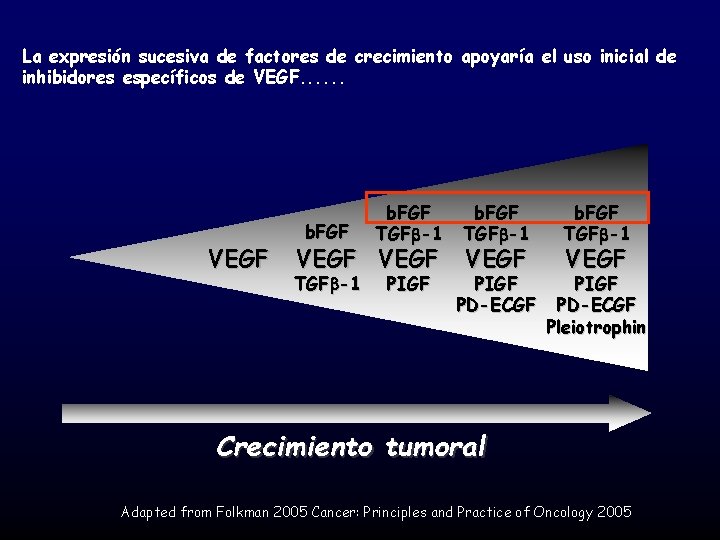
La expresión sucesiva de factores de crecimiento apoyaría el uso inicial de inhibidores específicos de VEGF. . . VEGF b. FGF TGF -1 PIGF PD-ECGF Pleiotrophin VEGF Crecimiento tumoral Adapted from Folkman 2005 Cancer: Principles and Practice of Oncology 2005
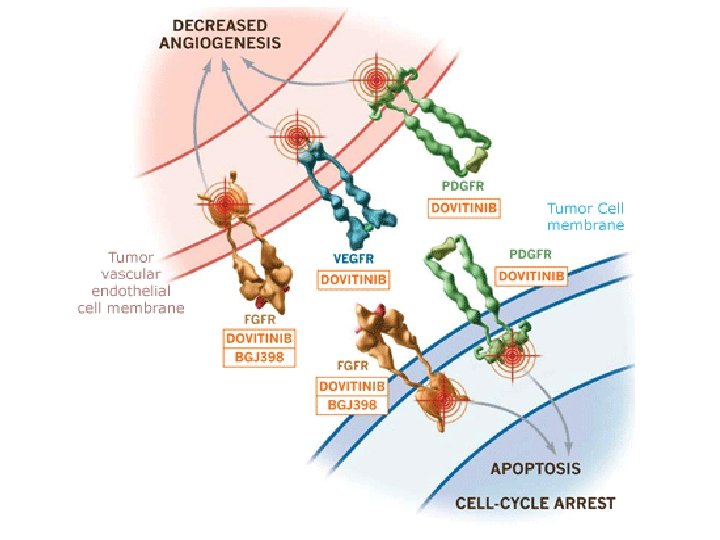
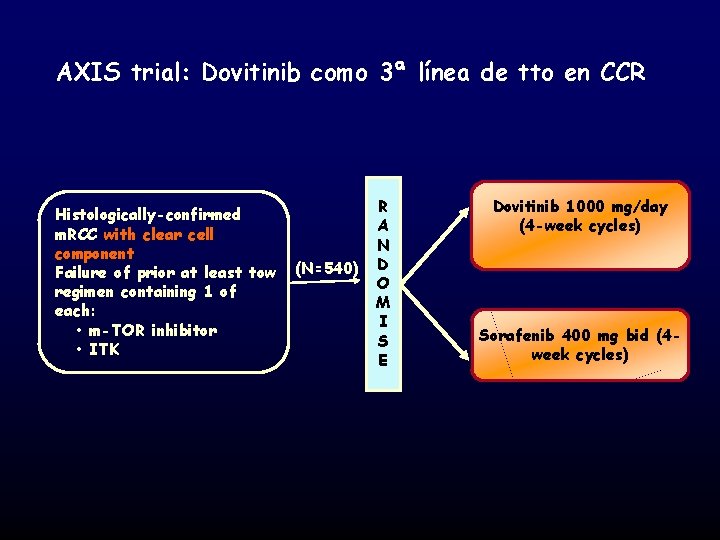
AXIS trial: Dovitinib como 3ª línea de tto en CCR Histologically-confirmed m. RCC with clear cell component Failure of prior at least tow regimen containing 1 of each: • m-TOR inhibitor • ITK (N=540) R A N D O M I S E Dovitinib 1000 mg/day (4 -week cycles) Sorafenib 400 mg bid (4 week cycles)
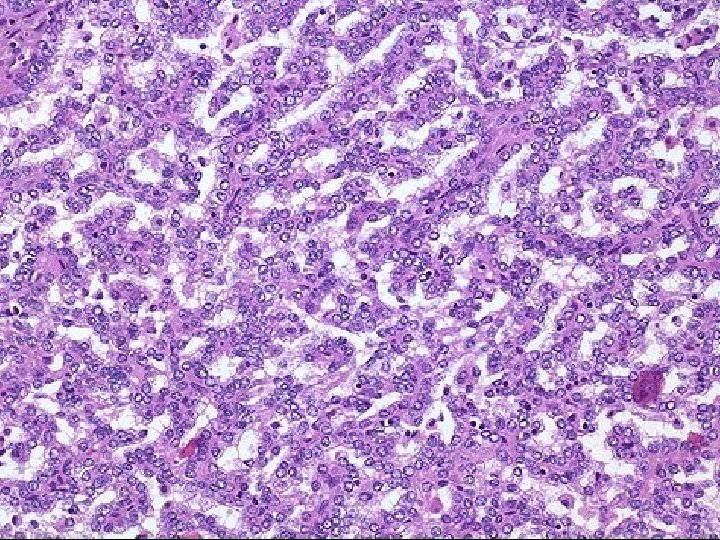
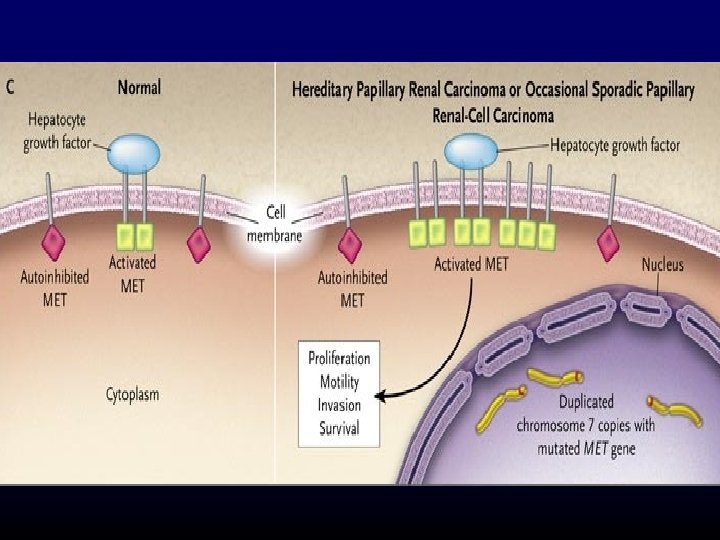

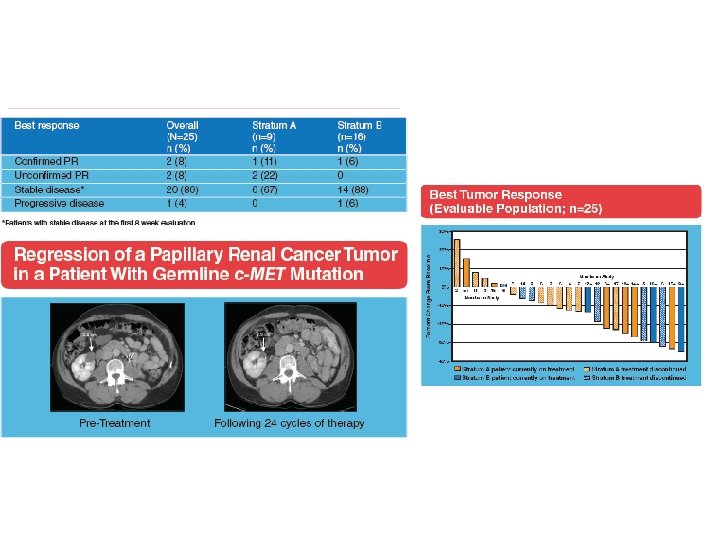
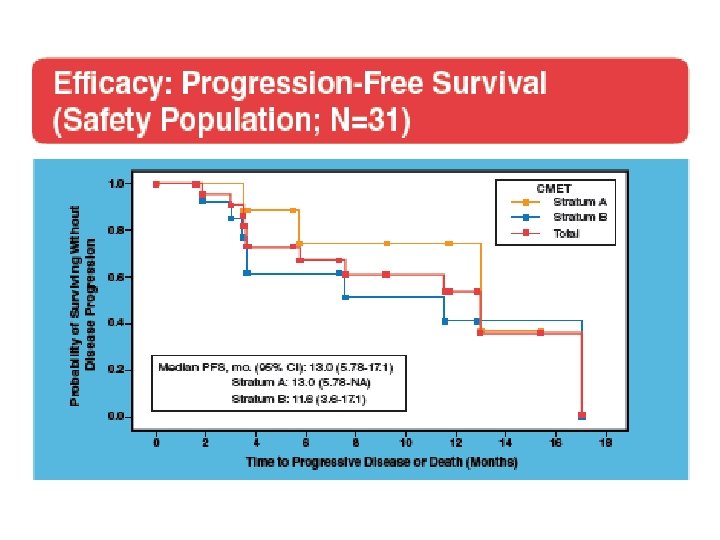
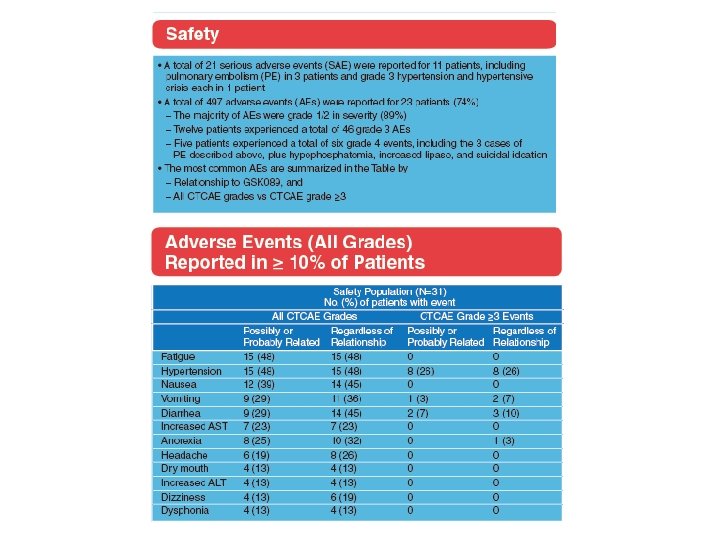
A phase II study of the dual MET-VEGFR 2 inhibitor GSK 1363089 (formerly XL 880) in patients with papillary renal carcinoma Objectives and study design Efficacy (ORR) and safety of GSK 1363089 in PRCC Key results n=31 patients 2 Stratum: (A) patients with evidence of c. MET activation (n=9) and (B) no evidence of c. MET activation (n=16) ORR: 8 %, Strat A: 11 %, Strat B: 6 % PFS: 13 months, Strat A: 13 months, Strat B 11. 6 months 74 % of patients experienced AEs Conclusions In this interim analysis of an ongoing trial, GSK 1363089 demonstrated antitumour efficacy in patients with PRC GSK 1363089 was generally well tolerated, with manageable toxicities Citation Srinivasan R, et al. A phase II study of the dual MET-VEGFR 2 inhibitor GSK 1363089 (formerly XL 880) in patients with papillary renal carcinoma (PRC). J Clin Oncol 26: 2008 (May 20 suppl; abstr 5103)

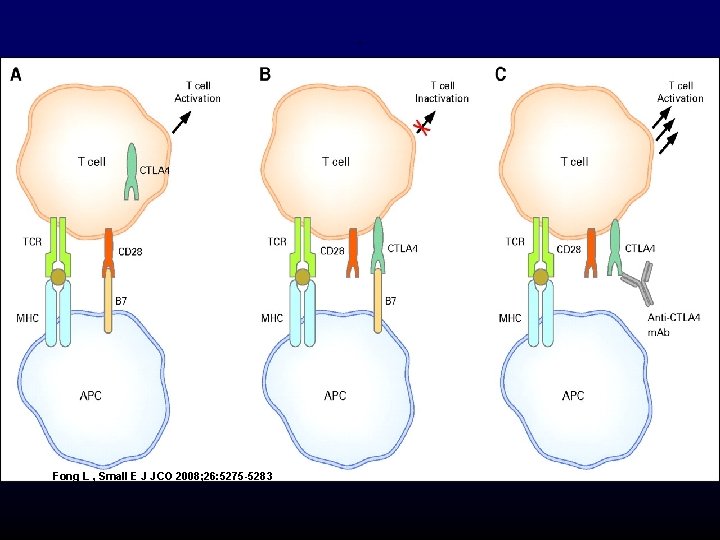
. Fong L , Small E J JCO 2008; 26: 5275 -5283
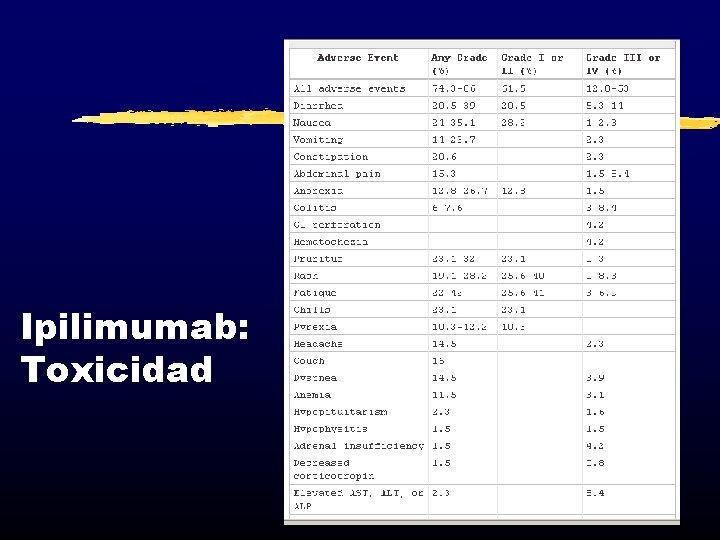
Ipilimumab: Toxicidad
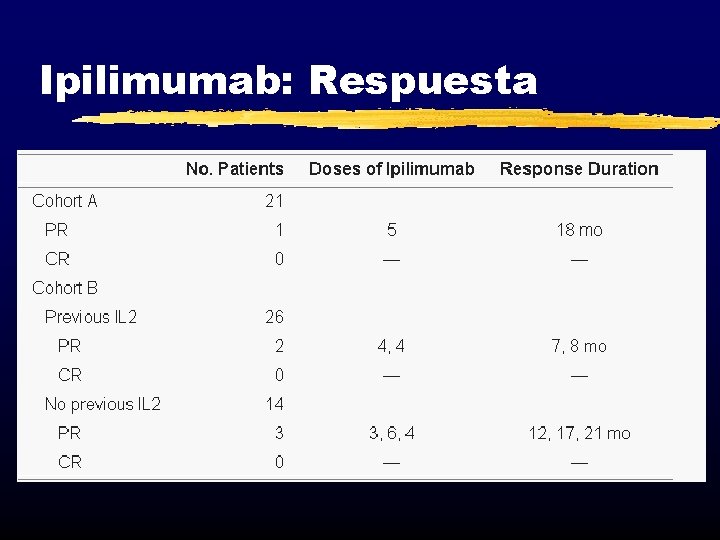
Ipilimumab: Respuesta

Conclusiones En los próximos meses: resultados de más ensayos randomizados en 1ª-2ª línea y estudios de combinación El panorama CCR cambiará, no radicalmente, quizá, pero cambiará, con estos resultados Necesidad de centrarnos en individualizar el tratamiento

Gracias
- Slides: 45