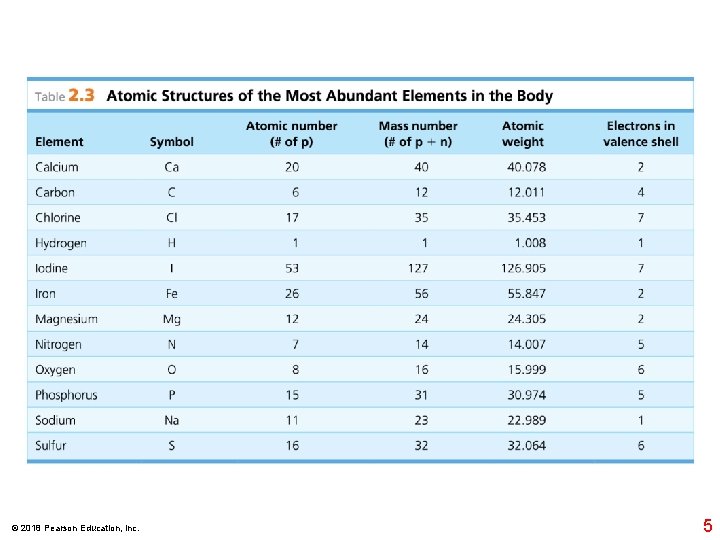
Nucleus Helium atom 2 protons p 2 neutrons






- Slides: 39

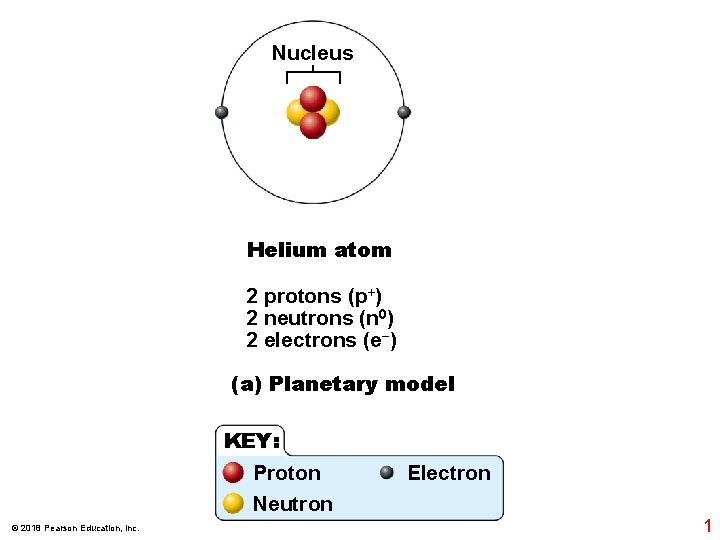
Nucleus Helium atom 2 protons (p+) 2 neutrons (n 0) 2 electrons (e−) (a) Planetary model KEY: Proton Neutron © 2018 Pearson Education, Inc. Electron 1

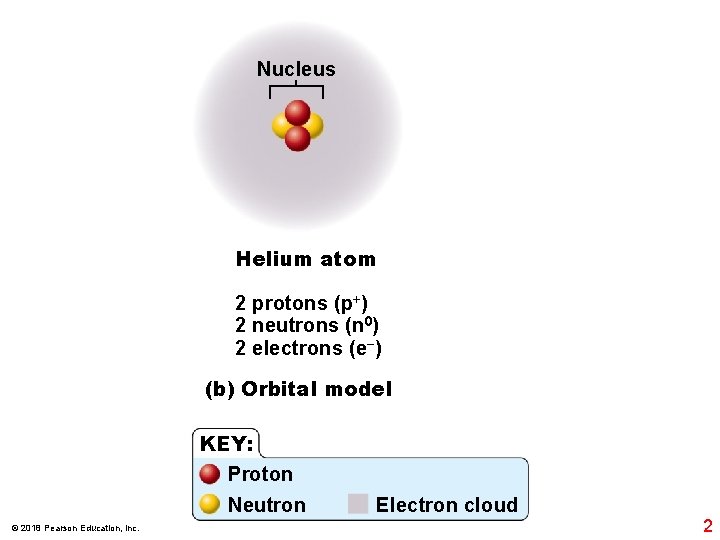
Nucleus Helium atom 2 protons (p+) 2 neutrons (n 0) 2 electrons (e−) (b) Orbital model KEY: Proton Neutron © 2018 Pearson Education, Inc. Electron cloud 2

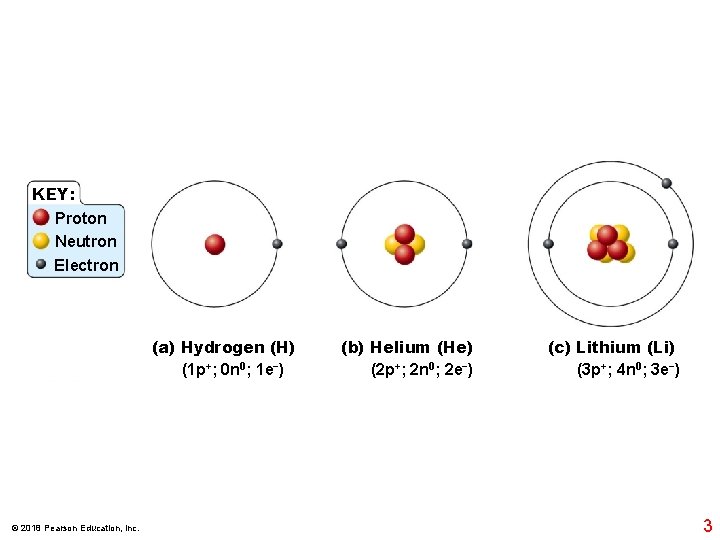
KEY: Proton Neutron Electron (a) Hydrogen (H) (1 p+; 0 n 0; 1 e−) © 2018 Pearson Education, Inc. (b) Helium (He) (2 p+; 2 n 0; 2 e−) (c) Lithium (Li) (3 p+; 4 n 0; 3 e−) 3

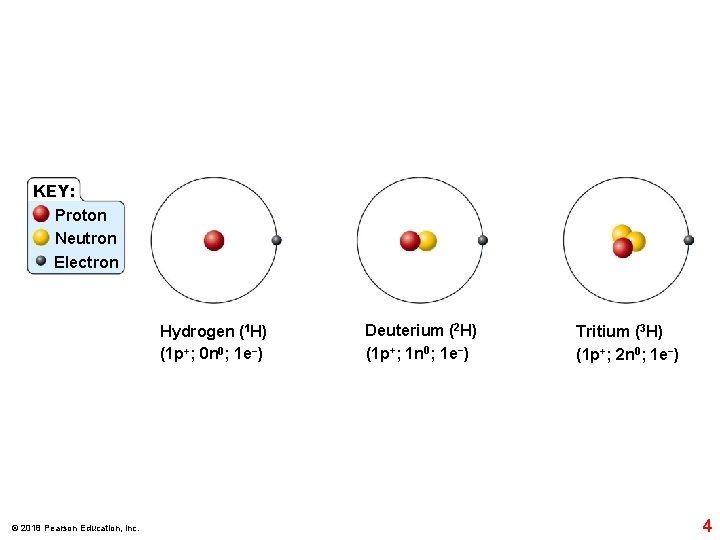
KEY: Proton Neutron Electron Hydrogen (1 H) (1 p+; 0 n 0; 1 e−) © 2018 Pearson Education, Inc. Deuterium (2 H) (1 p+; 1 n 0; 1 e−) Tritium (3 H) (1 p+; 2 n 0; 1 e−) 4

© 2018 Pearson Education, Inc. 5

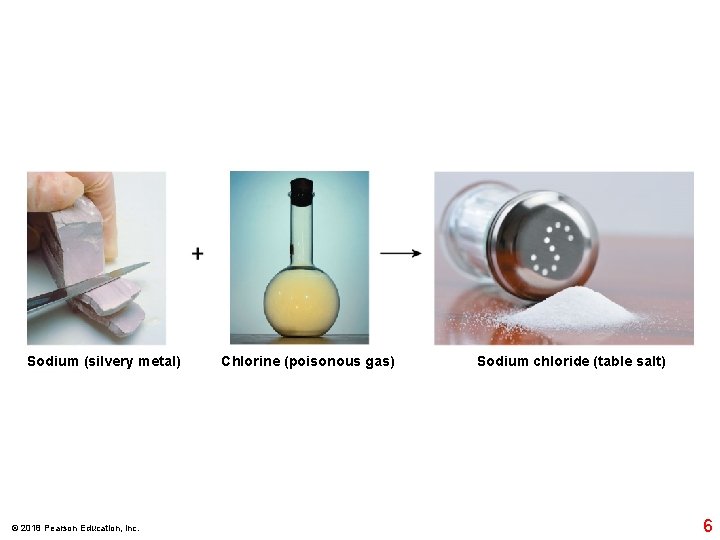
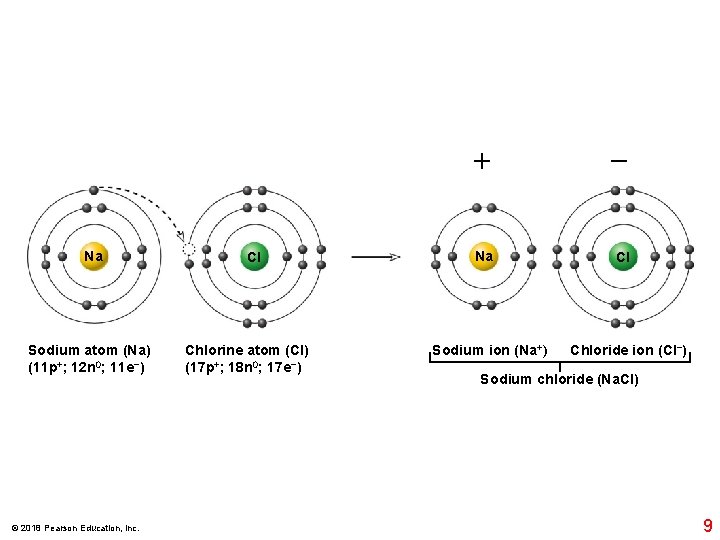
Sodium (silvery metal) © 2018 Pearson Education, Inc. Chlorine (poisonous gas) Sodium chloride (table salt) 6

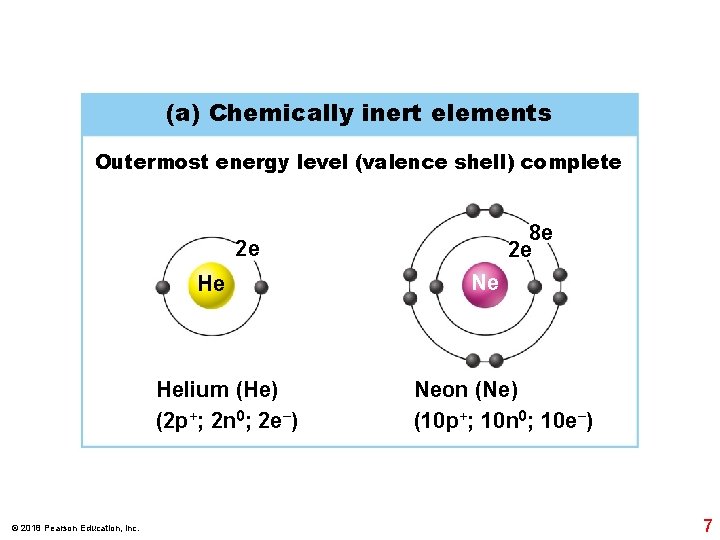
(a) Chemically inert elements Outermost energy level (valence shell) complete 8 e 2 e 2 e He Helium (He) (2 p+; 2 n 0; 2 e−) © 2018 Pearson Education, Inc. Ne Neon (Ne) (10 p+; 10 n 0; 10 e−) 7

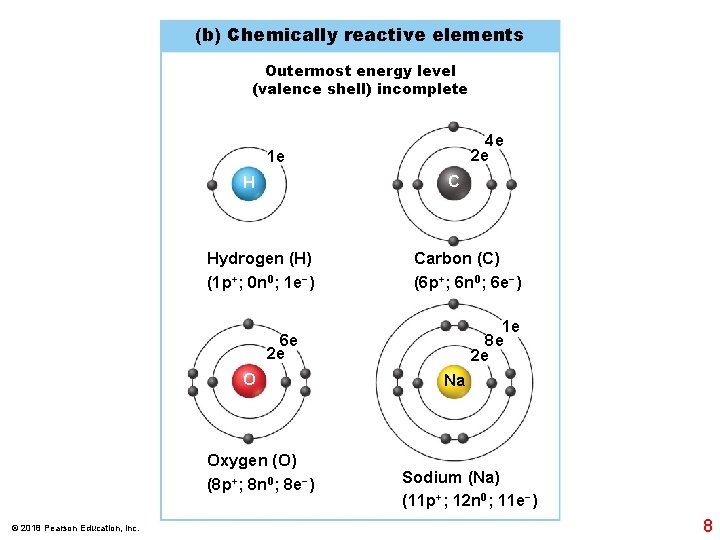
(b) Chemically reactive elements Outermost energy level (valence shell) incomplete 4 e 2 e 1 e C H Hydrogen (H) (1 p+; 0 n 0; 1 e−) Carbon (C) (6 p+; 6 n 0; 6 e−) 1 e 8 e 2 e 6 e 2 e O Oxygen (O) (8 p+; 8 n 0; 8 e−) © 2018 Pearson Education, Inc. Na Sodium (Na) (11 p+; 12 n 0; 11 e−) 8

Na Sodium atom (Na) (11 p+; 12 n 0; 11 e−) © 2018 Pearson Education, Inc. Cl Chlorine atom (Cl) (17 p+; 18 n 0; 17 e−) + − Na Cl Sodium ion (Na+) Chloride ion (Cl−) Sodium chloride (Na. Cl) 9

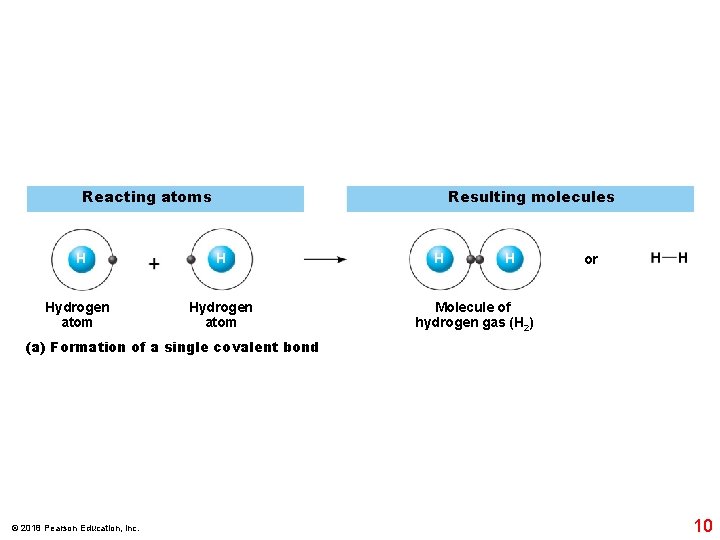
Reacting atoms Resulting molecules H H Hydrogen atom H H or Molecule of hydrogen gas (H 2) (a) Formation of a single covalent bond © 2018 Pearson Education, Inc. 10

Reacting atoms Resulting molecules O O Oxygen atom O O or Molecule of oxygen gas (O 2) (b) Formation of a double covalent bond © 2018 Pearson Education, Inc. 11

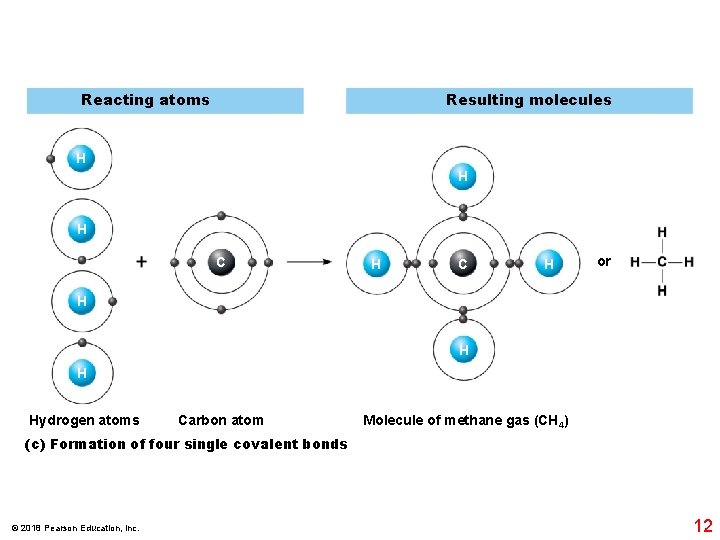
Reacting atoms Resulting molecules H H H C H or H Hydrogen atoms Carbon atom Molecule of methane gas (CH 4) (c) Formation of four single covalent bonds © 2018 Pearson Education, Inc. 12

(a) Carbon dioxide (CO 2) © 2018 Pearson Education, Inc. 13

δ− δ+ δ+ (b) Water (H 2 O) © 2018 Pearson Education, Inc. 14

H δ+ H O δ− Hydrogen bonds δ+ δ+ δ− δ− H O H δ− O δ+ δ+ H H O δ− H δ+ H (a) © 2018 Pearson Education, Inc. (b) 15

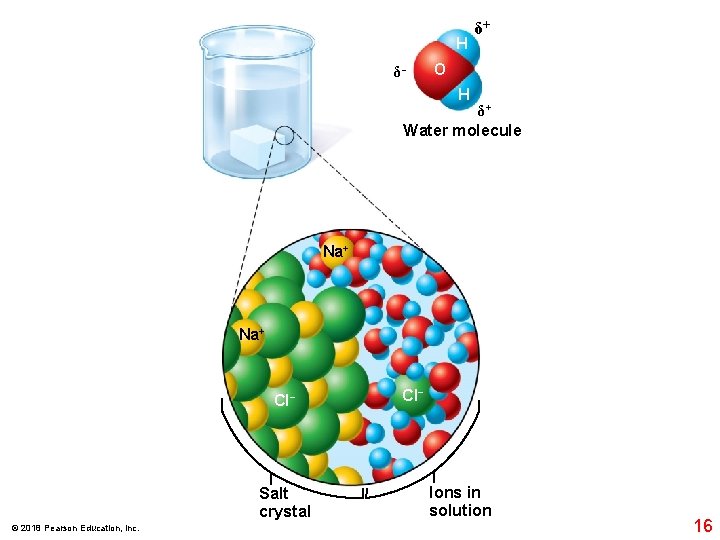
H δ− δ+ O H δ+ Water molecule Na+ Cl− Salt crystal © 2018 Pearson Education, Inc. Cl− Ions in solution 16

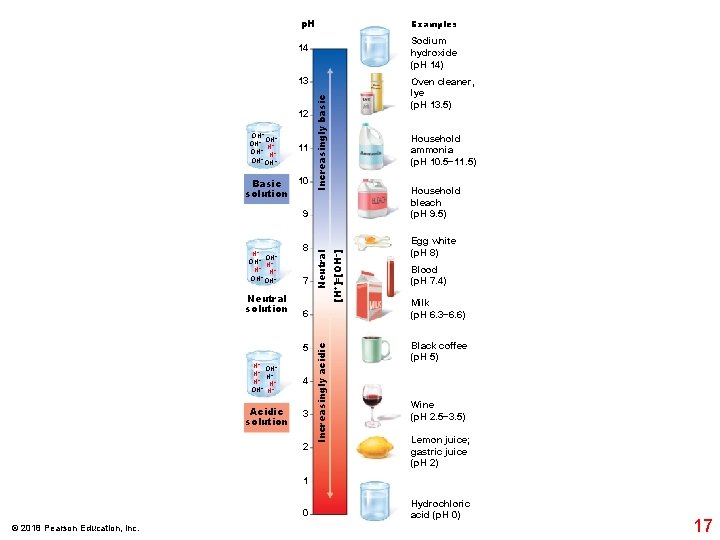
p. H Examples Sodium hydroxide (p. H 14) 14 13 OH− OH− H+ OH− Basic solution 11 10 Increasingly basic 12 Oven cleaner, lye (p. H 13. 5) Household ammonia (p. H 10. 5− 11. 5) Household bleach (p. H 9. 5) Neutral solution 7 6 5 H+ OH− H+ Acidic solution Neutral 8 4 3 2 Increasingly acidic H+ OH− H+ + H H+ OH− [H+]=[OH–] 9 Egg white (p. H 8) Blood (p. H 7. 4) Milk (p. H 6. 3− 6. 6) Black coffee (p. H 5) Wine (p. H 2. 5− 3. 5) Lemon juice; gastric juice (p. H 2) 1 0 © 2018 Pearson Education, Inc. Hydrochloric acid (p. H 0) 17

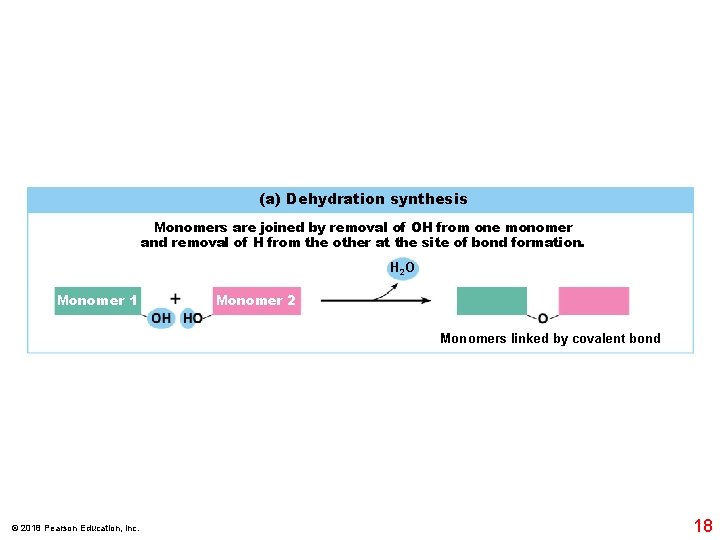
(a) Dehydration synthesis Monomers are joined by removal of OH from one monomer and removal of H from the other at the site of bond formation. H 2 O Monomer 1 Monomer 2 Monomers linked by covalent bond © 2018 Pearson Education, Inc. 18

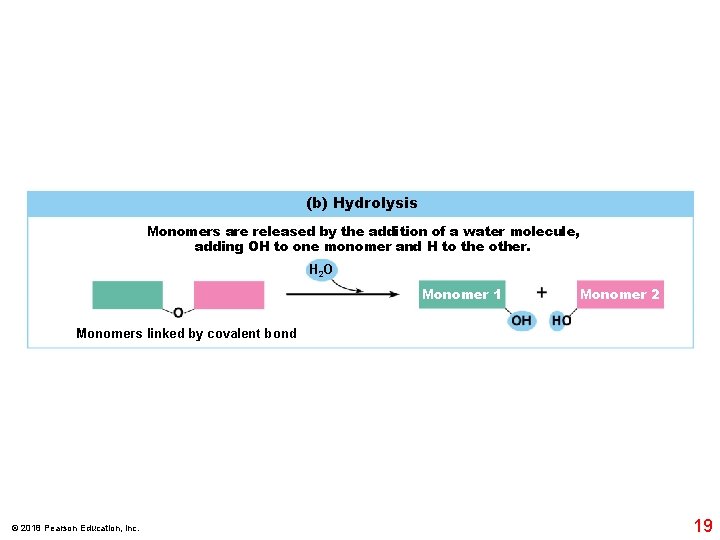
(b) Hydrolysis Monomers are released by the addition of a water molecule, adding OH to one monomer and H to the other. H 2 O Monomer 1 Monomer 2 Monomers linked by covalent bond © 2018 Pearson Education, Inc. 19

(a) Simple sugar (monosaccharide) © 2018 Pearson Education, Inc. 20

(b) Double sugar (disaccharide) © 2018 Pearson Education, Inc. 21

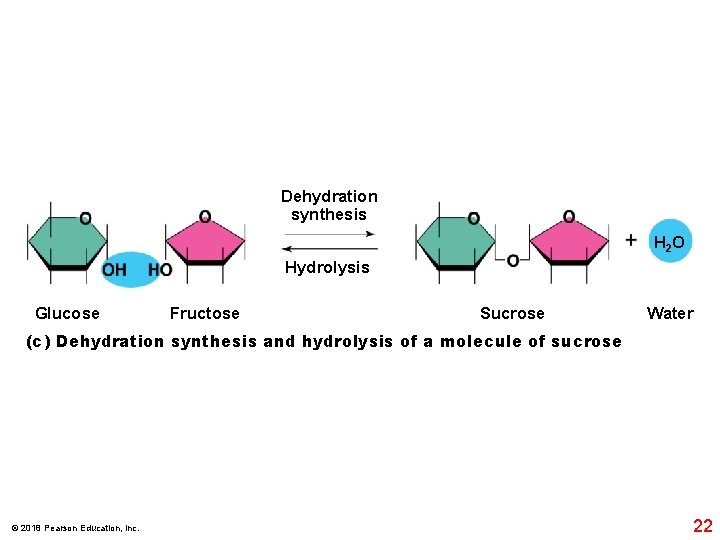
Dehydration synthesis H 2 O Hydrolysis Glucose Fructose Sucrose Water (c) Dehydration synthesis and hydrolysis of a molecule of sucrose © 2018 Pearson Education, Inc. 22

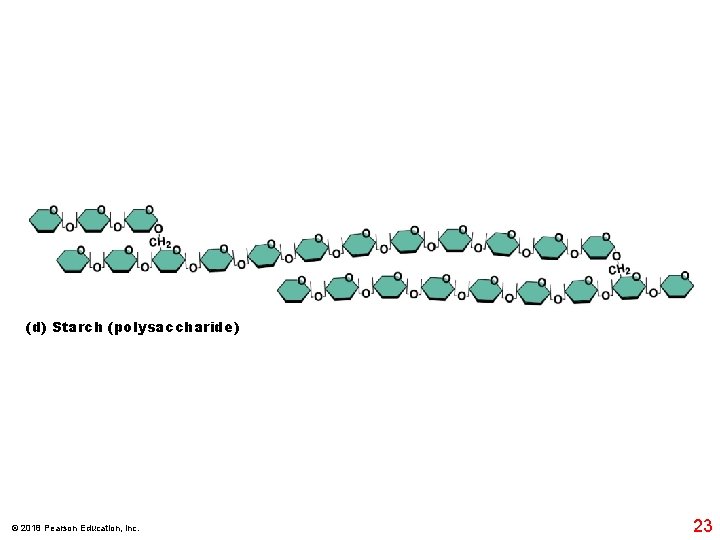
(d) Starch (polysaccharide) © 2018 Pearson Education, Inc. 23

Structural formula of a saturated fat molecule (a) Saturated fat. At room temperature, the molecules of a saturated fat such as this butter are packed closely together, forming a solid. © 2018 Pearson Education, Inc. 24

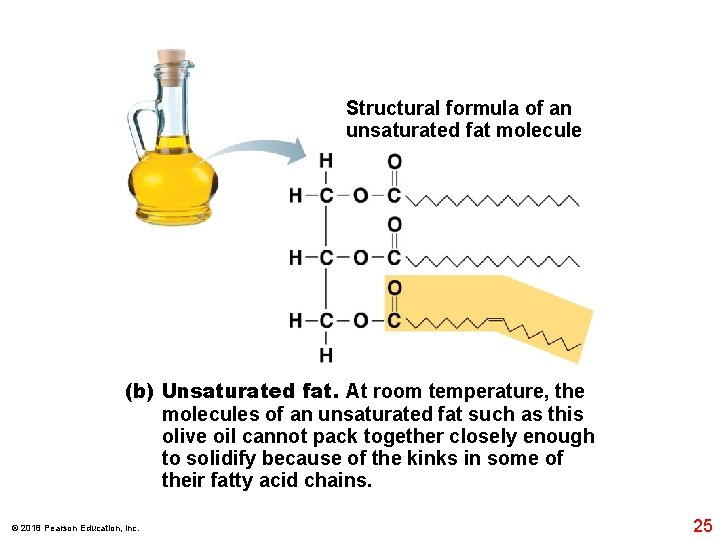
Structural formula of an unsaturated fat molecule (b) Unsaturated fat. At room temperature, the molecules of an unsaturated fat such as this olive oil cannot pack together closely enough to solidify because of the kinks in some of their fatty acid chains. © 2018 Pearson Education, Inc. 25

Polar “head” Nonpolar “tail” (schematic phospholipid) Phosphorus-containing group (polar head) Glycerol backbone 2 fatty acid chains (nonpolar tail) (b) Typical structure of a phospholipid molecule (phosphatidylcholine). Two fatty acid chains and a phosphorous-containing group are attached to a glycerol backbone. © 2018 Pearson Education, Inc. 26

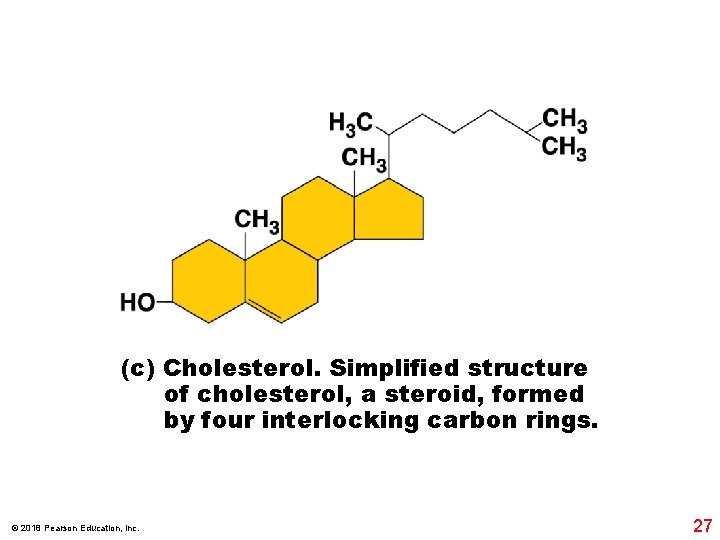
(c) Cholesterol. Simplified structure of cholesterol, a steroid, formed by four interlocking carbon rings. © 2018 Pearson Education, Inc. 27

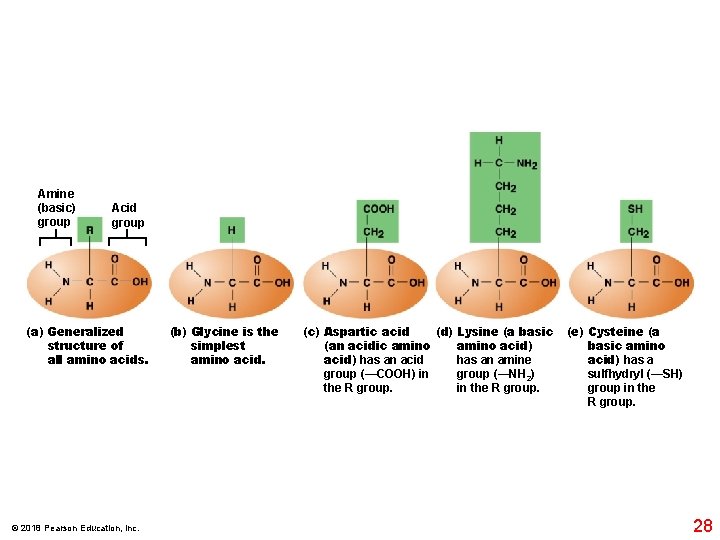
Amine (basic) group Acid group (a) Generalized structure of all amino acids. © 2018 Pearson Education, Inc. (b) Glycine is the simplest amino acid. (d) Lysine (a basic (c) Aspartic acid amino acid) (an acidic amino acid) has an acid has an amine group (—NH 2) group (—COOH) in in the R group. (e) Cysteine (a basic amino acid) has a sulfhydryl (—SH) group in the R group. 28

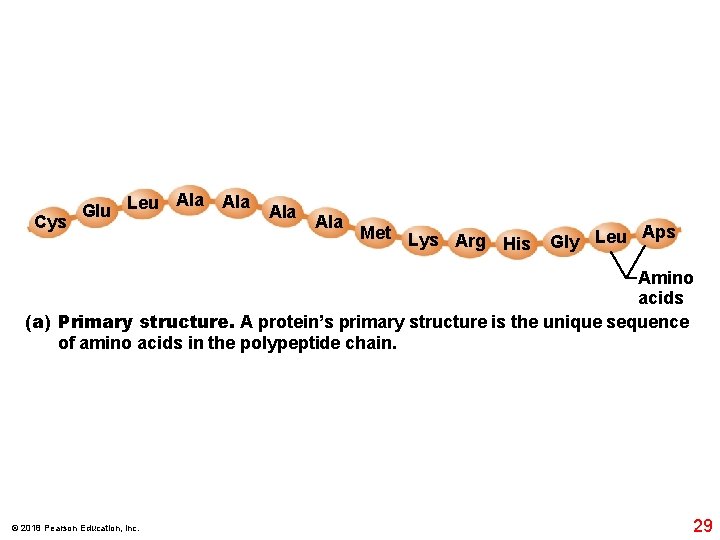
Cys Ala Glu Leu Ala Ala Met Lys Arg His Aps Gly Leu Amino acids (a) Primary structure. A protein’s primary structure is the unique sequence of amino acids in the polypeptide chain. © 2018 Pearson Education, Inc. 29

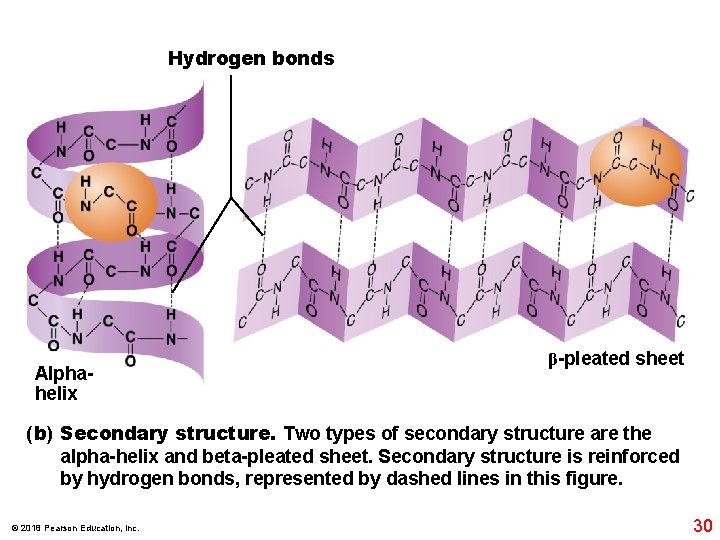
Hydrogen bonds Alphahelix β-pleated sheet (b) Secondary structure. Two types of secondary structure are the alpha-helix and beta-pleated sheet. Secondary structure is reinforced by hydrogen bonds, represented by dashed lines in this figure. © 2018 Pearson Education, Inc. 30

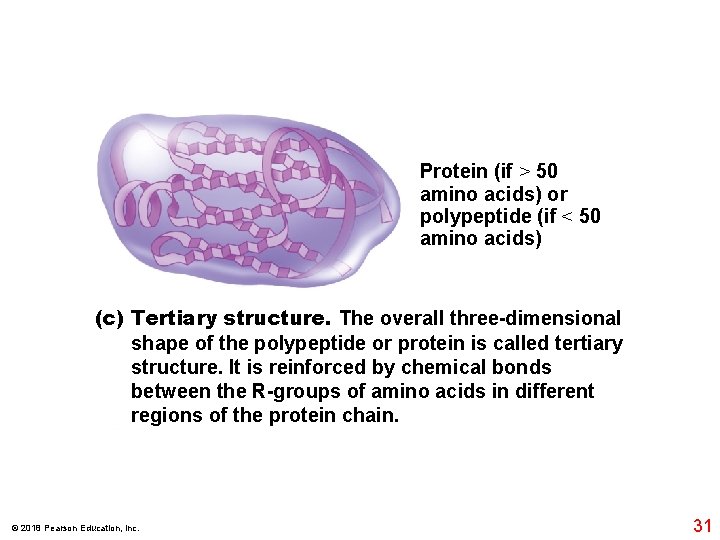
Protein (if > 50 amino acids) or polypeptide (if < 50 amino acids) (c) Tertiary structure. The overall three-dimensional shape of the polypeptide or protein is called tertiary structure. It is reinforced by chemical bonds between the R-groups of amino acids in different regions of the protein chain. © 2018 Pearson Education, Inc. 31

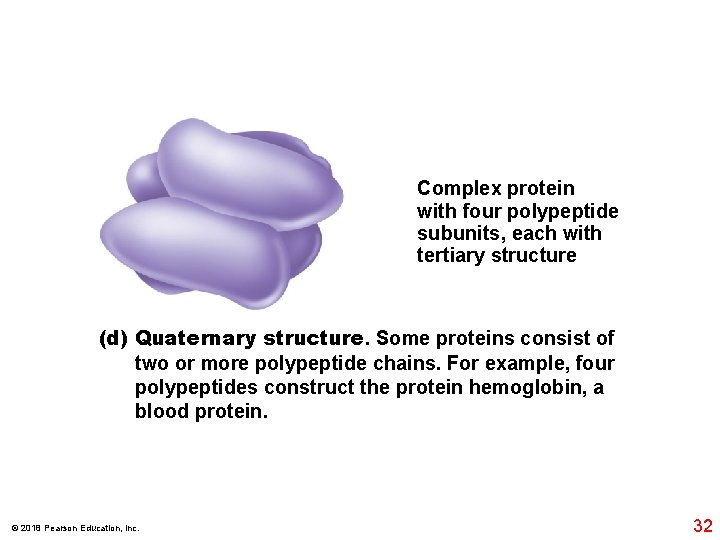
Complex protein with four polypeptide subunits, each with tertiary structure (d) Quaternary structure. Some proteins consist of two or more polypeptide chains. For example, four polypeptides construct the protein hemoglobin, a blood protein. © 2018 Pearson Education, Inc. 32

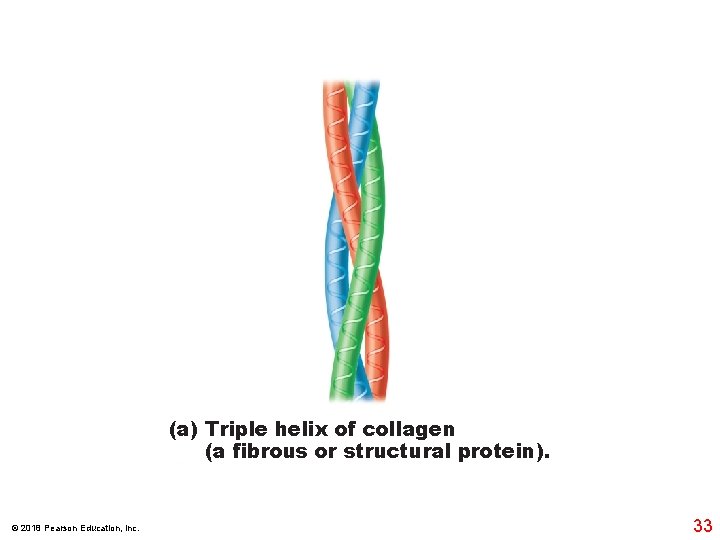
(a) Triple helix of collagen (a fibrous or structural protein). © 2018 Pearson Education, Inc. 33

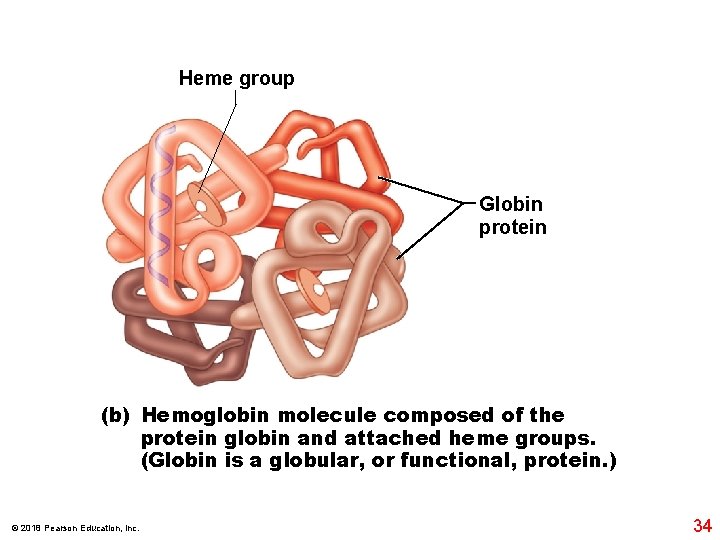
Heme group Globin protein (b) Hemoglobin molecule composed of the protein globin and attached heme groups. (Globin is a globular, or functional, protein. ) © 2018 Pearson Education, Inc. 34

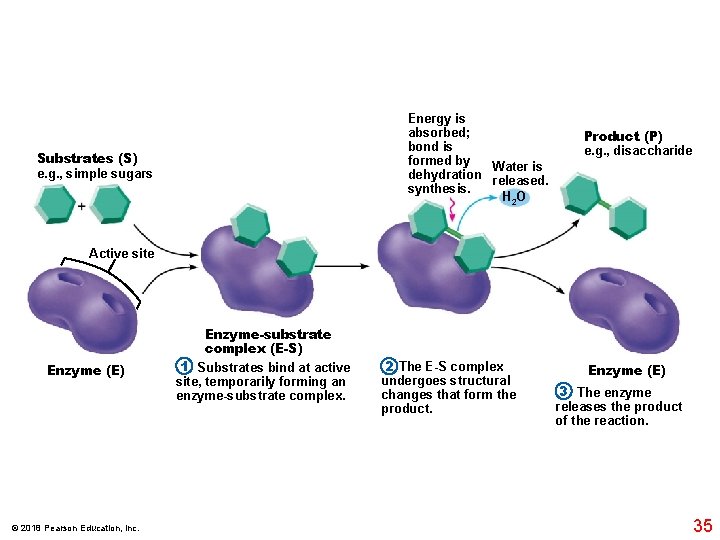
Energy is absorbed; bond is formed by Water is dehydration released. synthesis. H 2 O Substrates (S) e. g. , simple sugars Product (P) e. g. , disaccharide Active site Enzyme (E) © 2018 Pearson Education, Inc. Enzyme-substrate complex (E-S) 1 Substrates bind at active site, temporarily forming an enzyme-substrate complex. 2 The E-S complex undergoes structural changes that form the product. Enzyme (E) 3 The enzyme releases the product of the reaction. 35

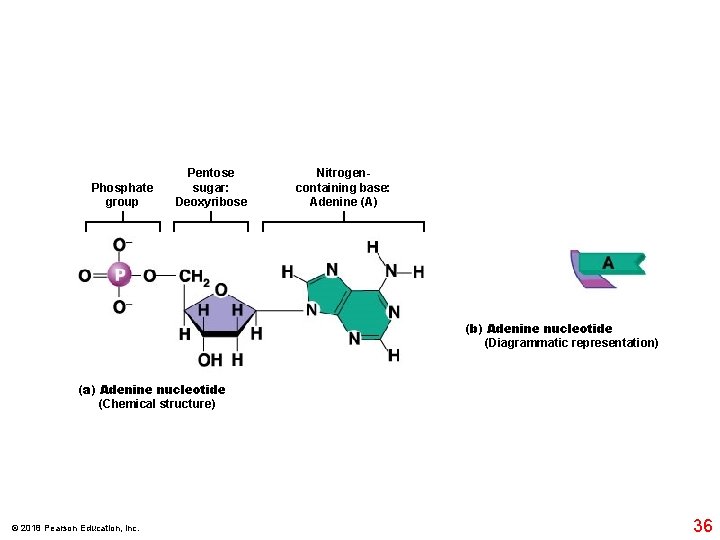
Phosphate group Pentose sugar: Deoxyribose Nitrogencontaining base: Adenine (A) (b) Adenine nucleotide (Diagrammatic representation) (a) Adenine nucleotide (Chemical structure) © 2018 Pearson Education, Inc. 36

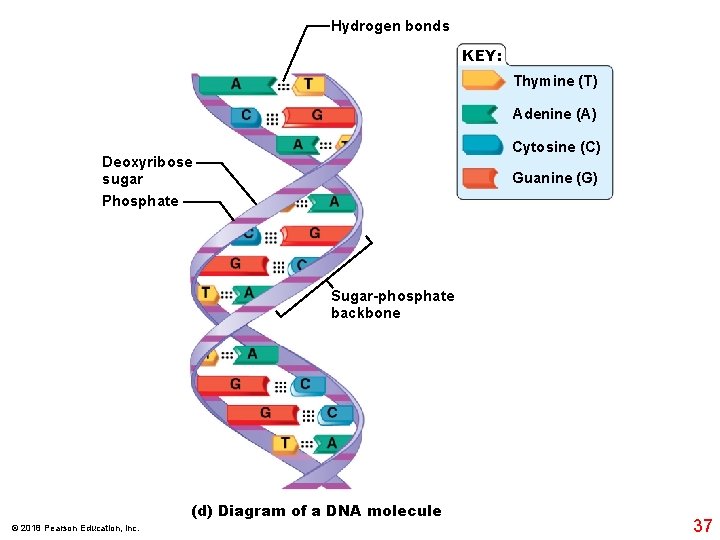
Hydrogen bonds KEY: Thymine (T) Adenine (A) Cytosine (C) Deoxyribose sugar Phosphate Guanine (G) Sugar-phosphate backbone (d) Diagram of a DNA molecule © 2018 Pearson Education, Inc. 37

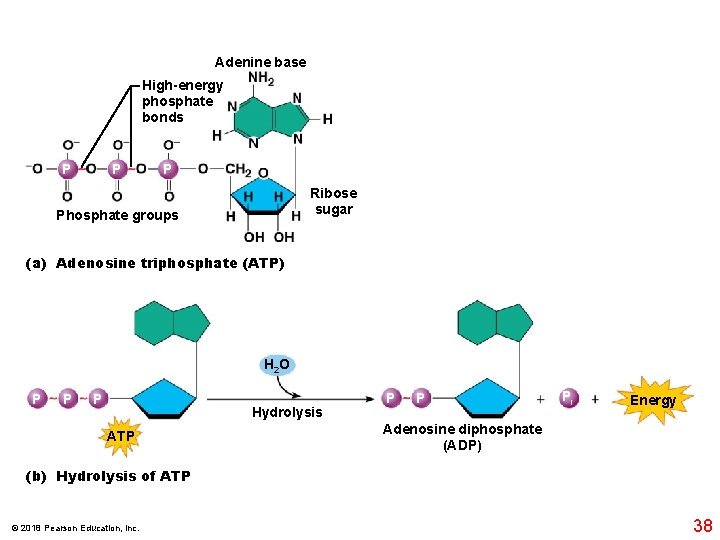
Adenine base High-energy phosphate bonds P P P Ribose sugar Phosphate groups (a) Adenosine triphosphate (ATP) H 2 O P P P Hydrolysis ATP P P Pi Energy Adenosine diphosphate (ADP) (b) Hydrolysis of ATP © 2018 Pearson Education, Inc. 38

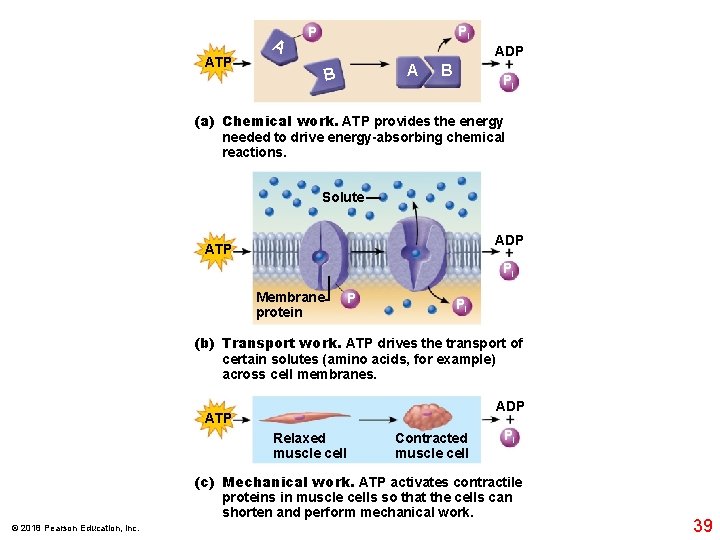
A Pi P ATP A B ADP + Pi B (a) Chemical work. ATP provides the energy needed to drive energy-absorbing chemical reactions. Solute ADP + Pi ATP Membrane protein P Pi (b) Transport work. ATP drives the transport of certain solutes (amino acids, for example) across cell membranes. ATP Relaxed muscle cell Contracted muscle cell ADP + Pi (c) Mechanical work. ATP activates contractile proteins in muscle cells so that the cells can shorten and perform mechanical work. © 2018 Pearson Education, Inc. 39