Nucleotides and nucleic acids Lecture 5 Dr Mamoun







- Slides: 36

Nucleotides and nucleic acids Lecture 5 Dr. Mamoun Ahram

Nucleic acids • Monomers • Polymers

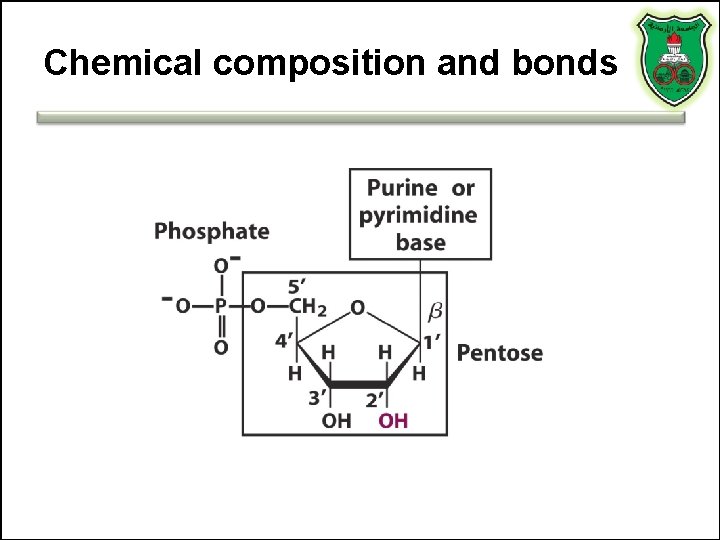
Chemical composition and bonds

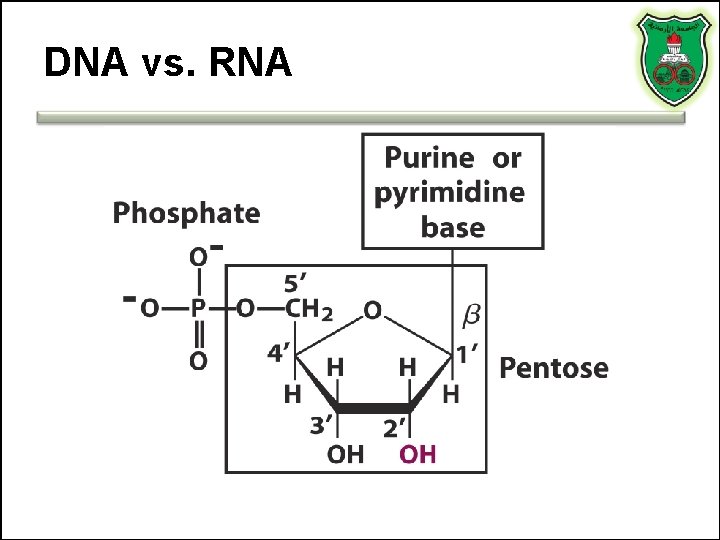
DNA vs. RNA

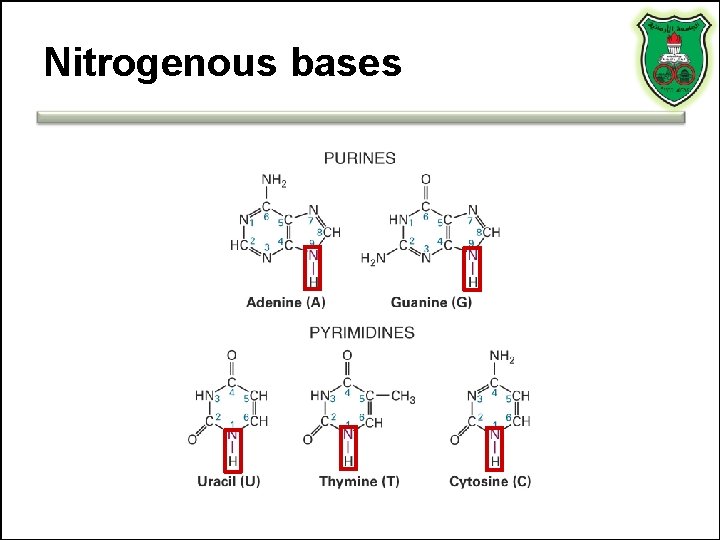
Nitrogenous bases

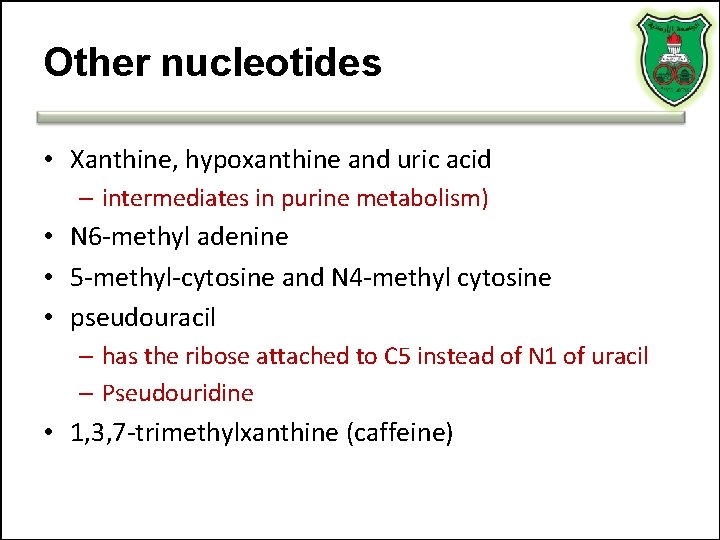
Other nucleotides • Xanthine, hypoxanthine and uric acid – intermediates in purine metabolism) • N 6 -methyl adenine • 5 -methyl-cytosine and N 4 -methyl cytosine • pseudouracil – has the ribose attached to C 5 instead of N 1 of uracil – Pseudouridine • 1, 3, 7 -trimethylxanthine (caffeine)


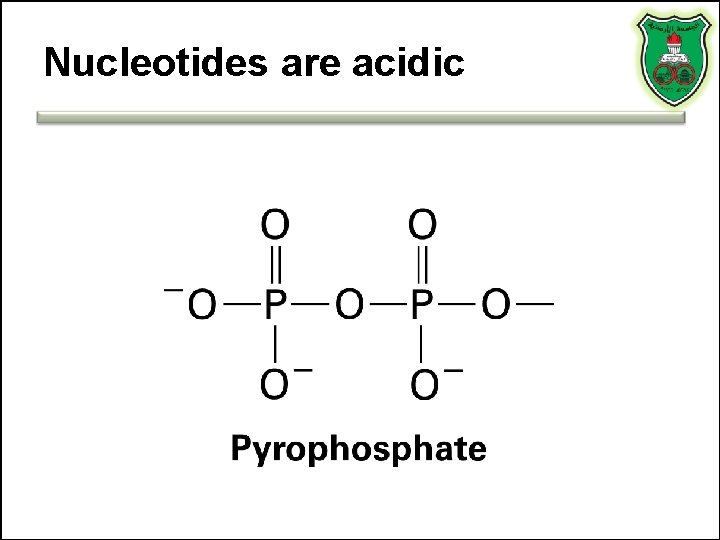
Nucleotides are acidic

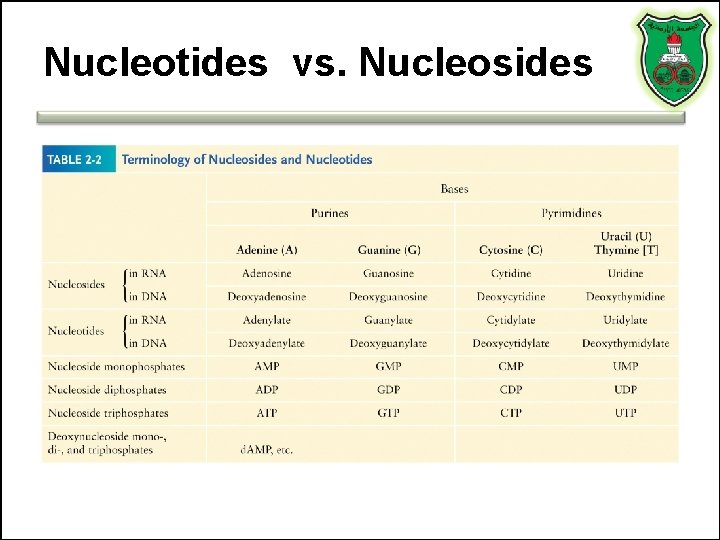
Nucleotides vs. Nucleosides



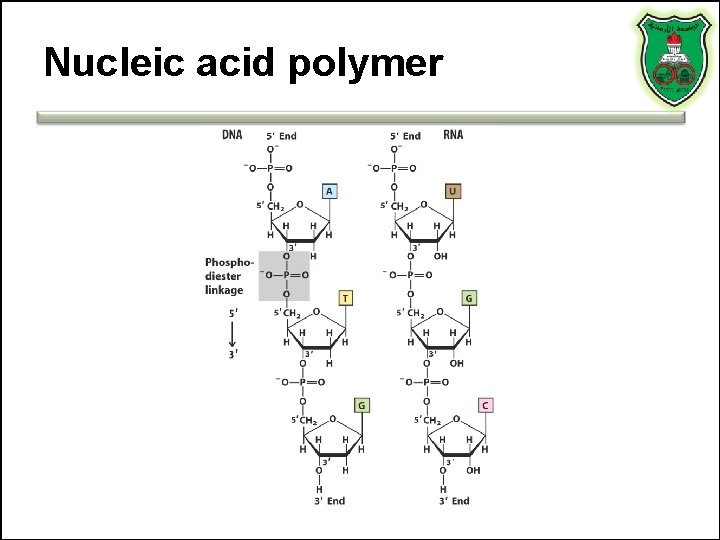
Nucleic acid polymer

A new era

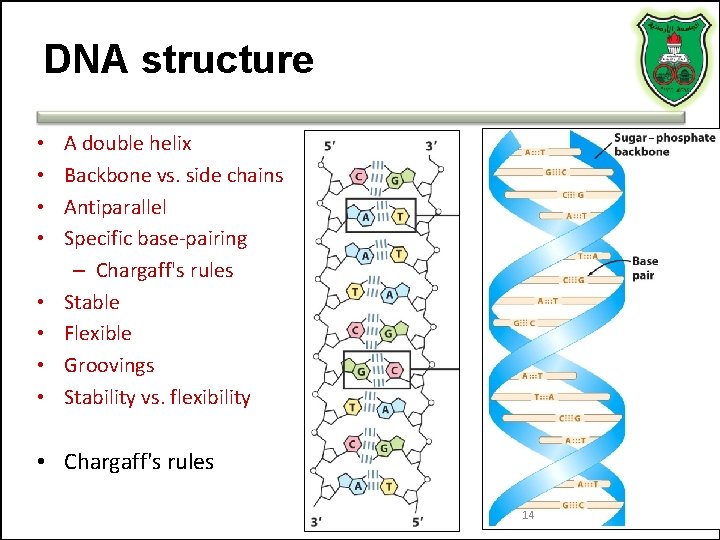
DNA structure • • A double helix Backbone vs. side chains Antiparallel Specific base-pairing – Chargaff's rules Stable Flexible Groovings Stability vs. flexibility • Chargaff's rules 14

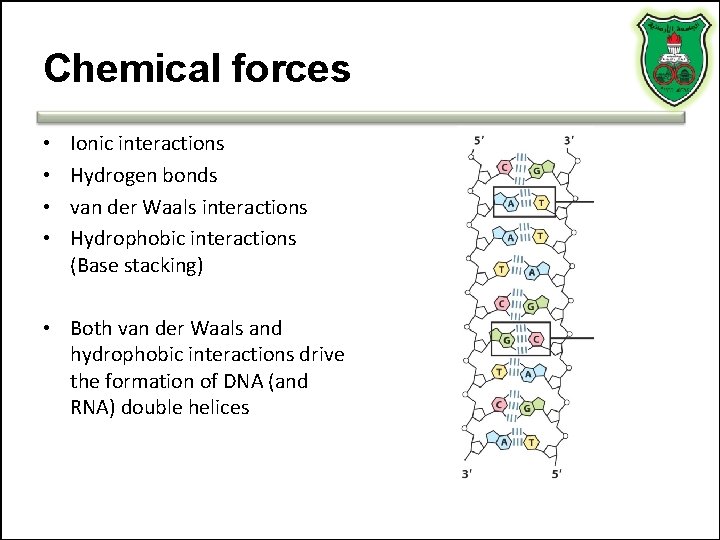
Chemical forces • • Ionic interactions Hydrogen bonds van der Waals interactions Hydrophobic interactions (Base stacking) • Both van der Waals and hydrophobic interactions drive the formation of DNA (and RNA) double helices

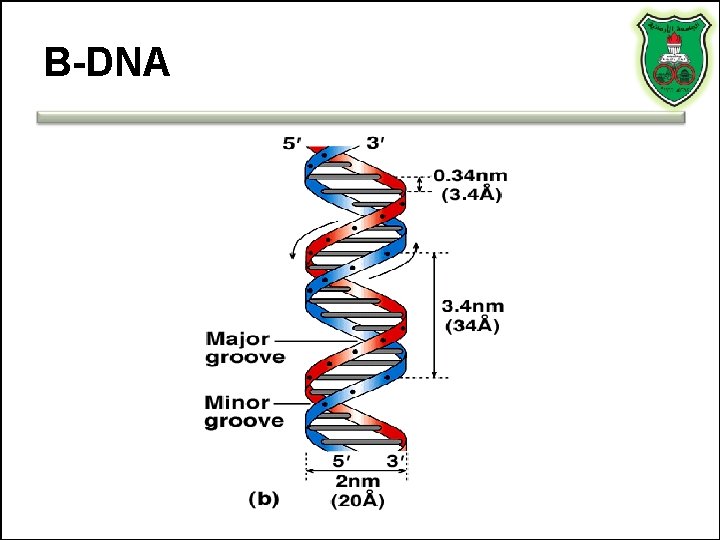
B-DNA

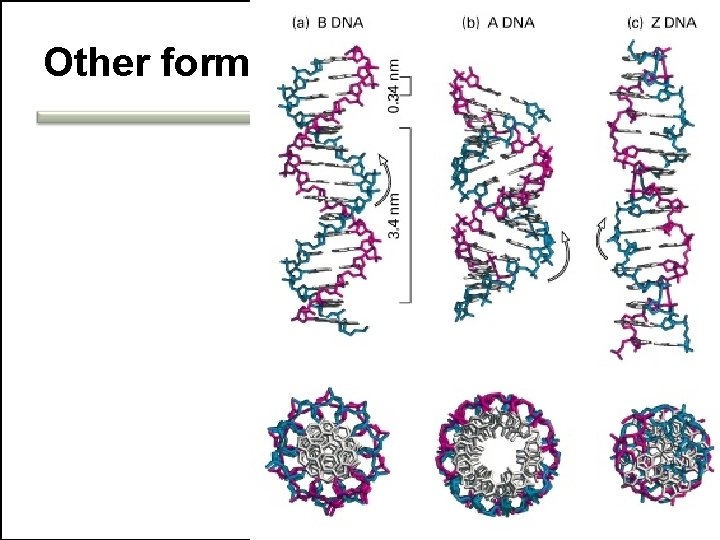
Other forms

Chromosomal packaging • 2 meters of DNA/cell – 40 km of thread in a tennis ball! • How? – Histones •

What is a chromatin? • Chromatin = DNA molecule + proteins • The proteins that bind to the DNA: – histones (H 1, H 2 A, H 2 B, H 3, and H 4) – nonhistone chromosomal proteins

Nucleosomes

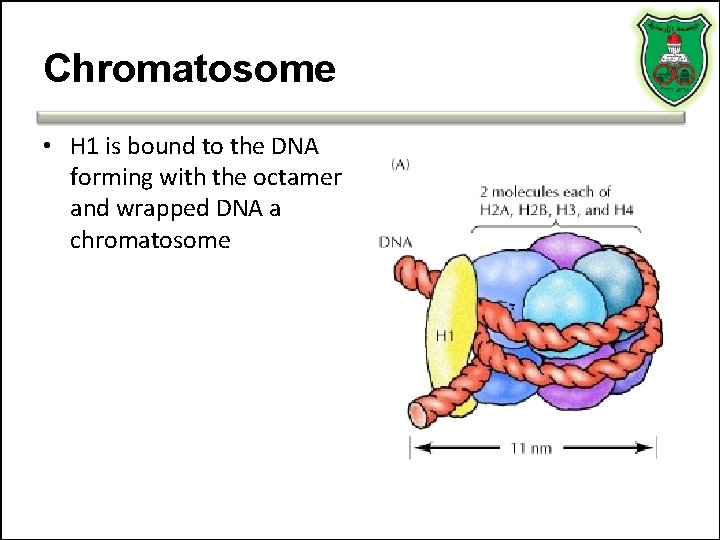
Chromatosome • H 1 is bound to the DNA forming with the octamer and wrapped DNA a chromatosome

DNA-histone interaction • Histones are positively charged – Interaction – Charge neutralization

RNA • Vs. DNA • Secondary structures

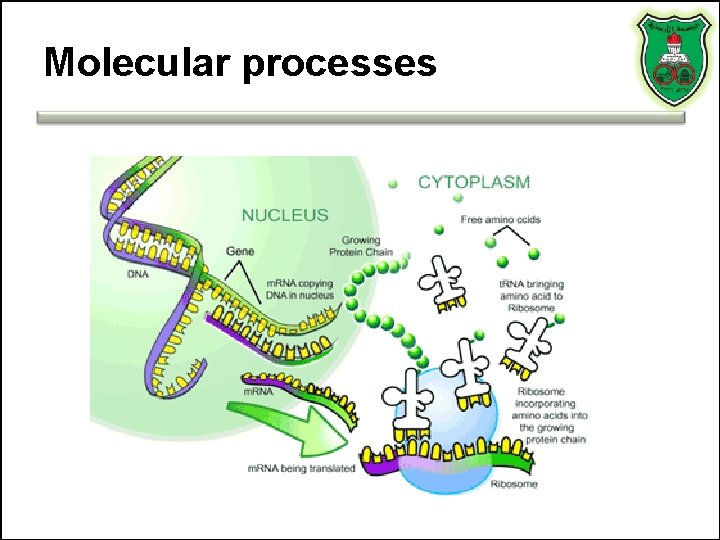
Molecular processes

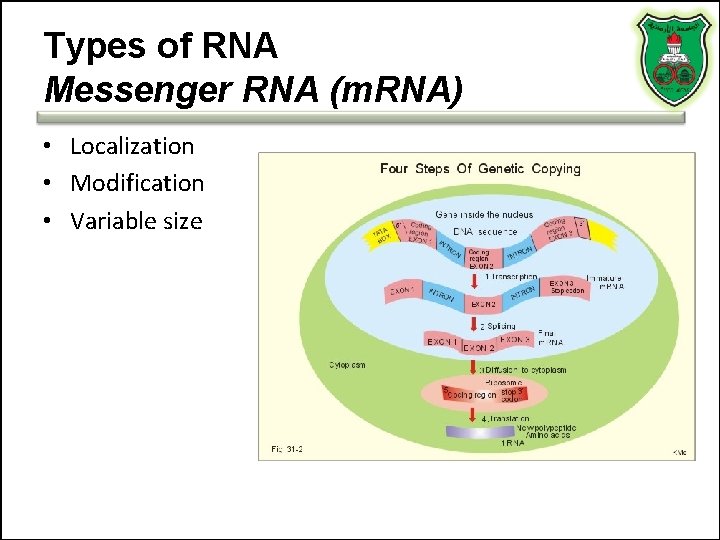
Types of RNA Messenger RNA (m. RNA) • Localization • Modification • Variable size

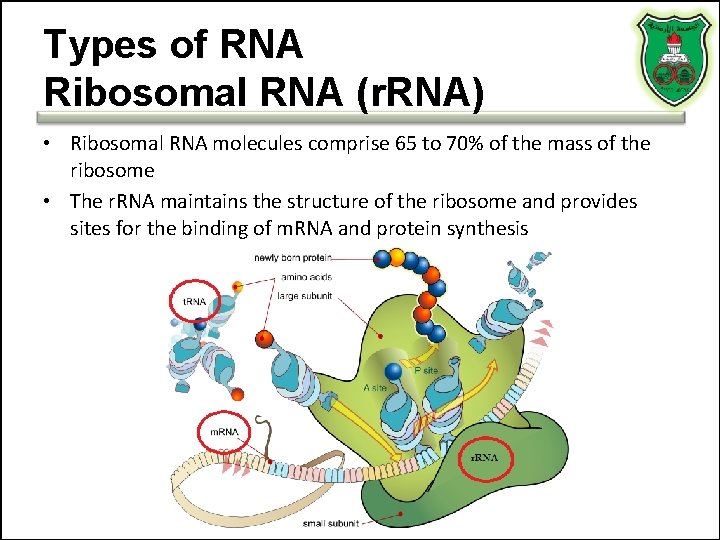
Types of RNA Ribosomal RNA (r. RNA) • Ribosomal RNA molecules comprise 65 to 70% of the mass of the ribosome • The r. RNA maintains the structure of the ribosome and provides sites for the binding of m. RNA and protein synthesis

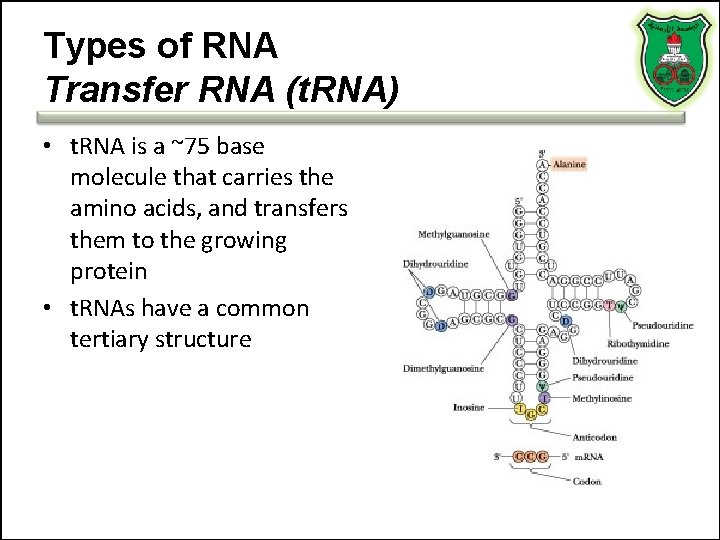
Types of RNA Transfer RNA (t. RNA) • t. RNA is a ~75 base molecule that carries the amino acids, and transfers them to the growing protein • t. RNAs have a common tertiary structure

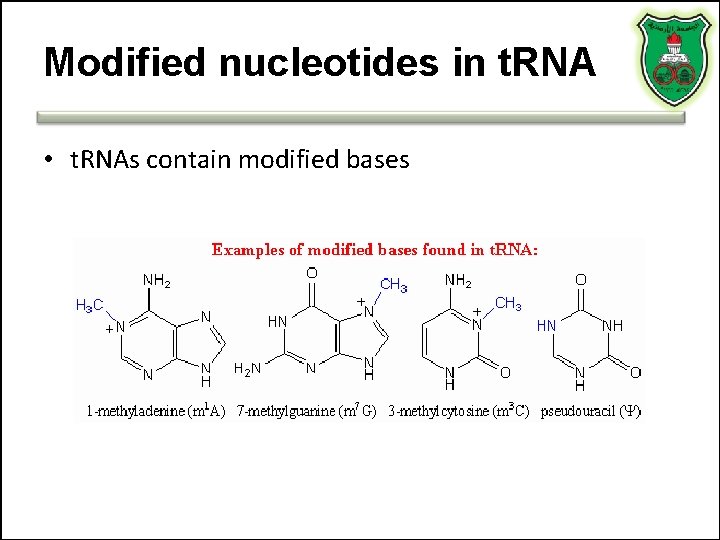
Modified nucleotides in t. RNA • t. RNAs contain modified bases

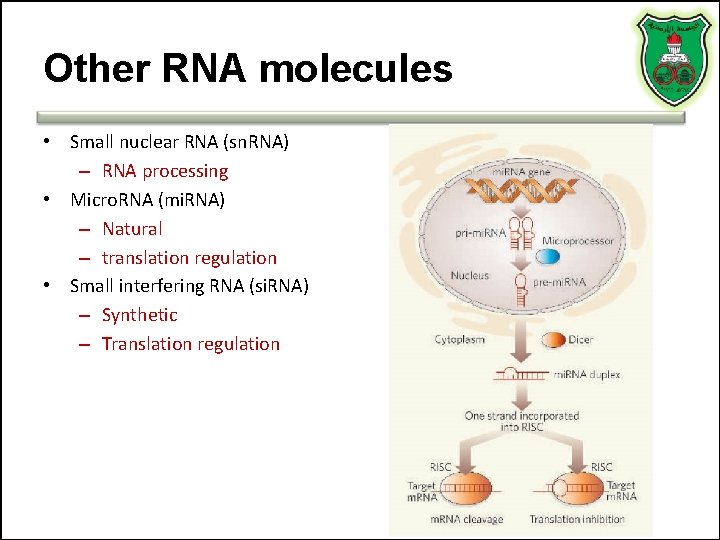
Other RNA molecules • Small nuclear RNA (sn. RNA) – RNA processing • Micro. RNA (mi. RNA) – Natural – translation regulation • Small interfering RNA (si. RNA) – Synthetic – Translation regulation

Light absorbance of nucleic acids • Aromatic pyrimidines and purines can absorb UV light • The peak absorbance is at 260 nm wavelength

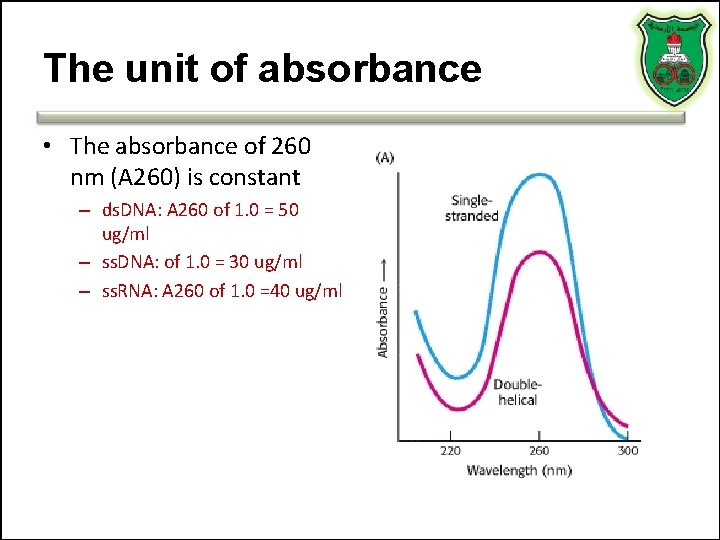
The unit of absorbance • The absorbance of 260 nm (A 260) is constant – ds. DNA: A 260 of 1. 0 = 50 ug/ml – ss. DNA: of 1. 0 = 30 ug/ml – ss. RNA: A 260 of 1. 0 =40 ug/ml

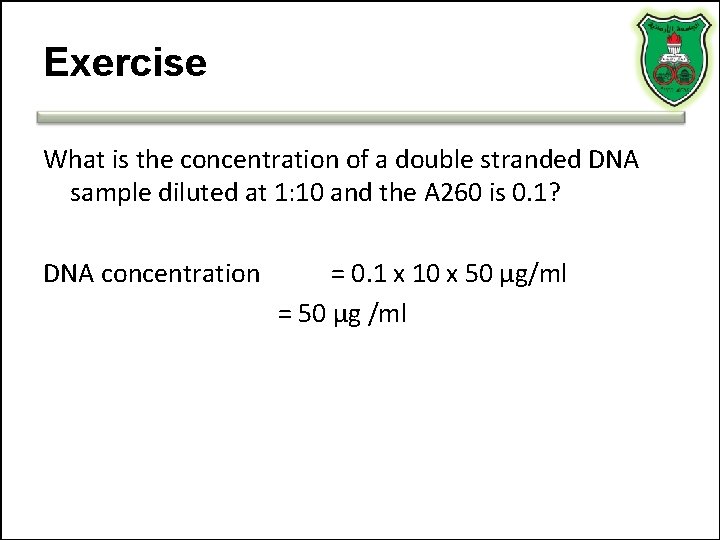
Exercise What is the concentration of a double stranded DNA sample diluted at 1: 10 and the A 260 is 0. 1? DNA concentration = 0. 1 x 10 x 50 µg/ml = 50 µg /ml

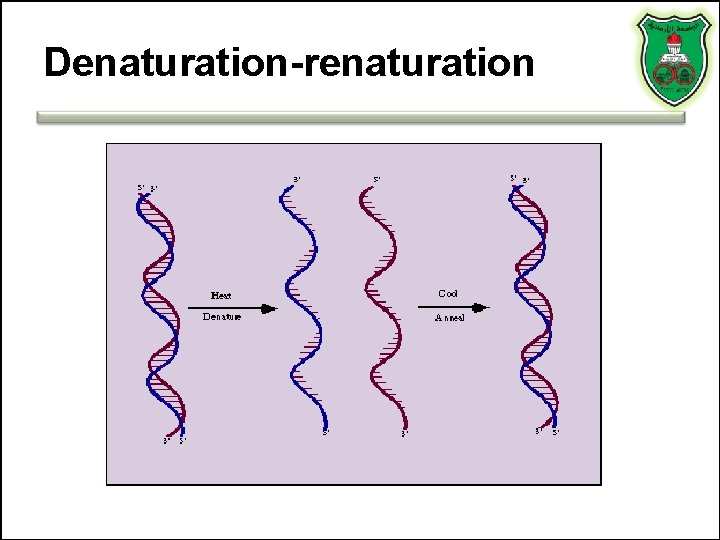
Denaturation-renaturation

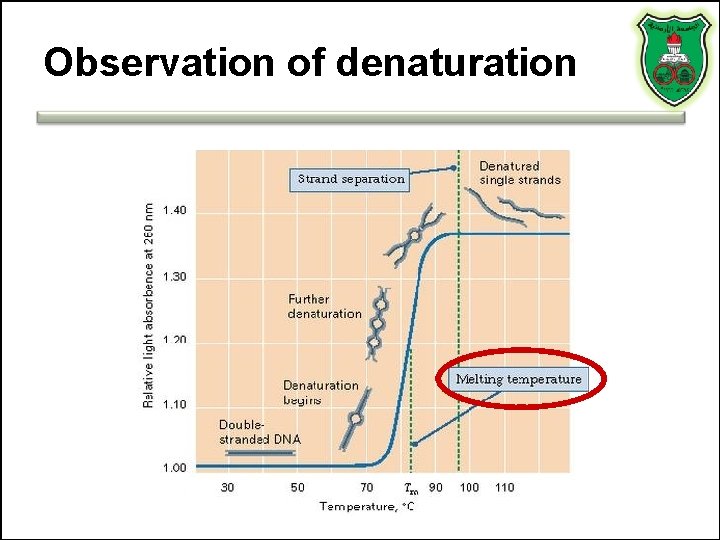
Observation of denaturation

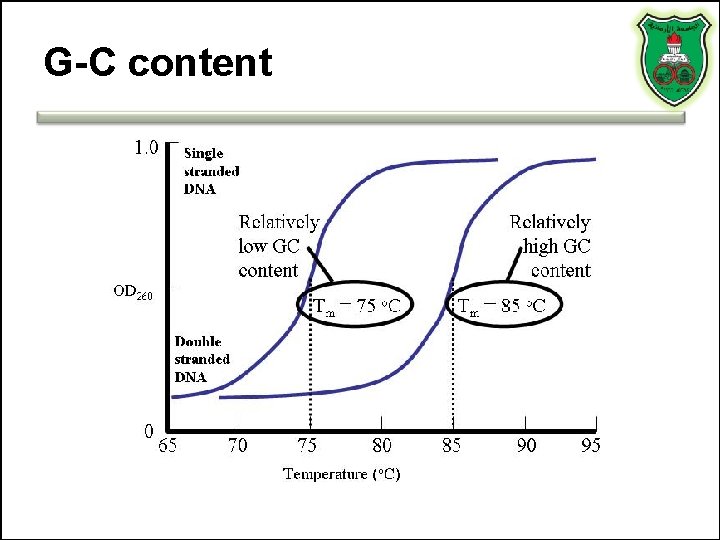
Factors influencing Tm • • G·C pairs p. H Salt and ion concentration Destabilizing agents (alkaline solutions, formamide, urea)

G-C content