Nucleophilic Substitution Organic Substitution versus Inorganic Single Replacement

Nucleophilic Substitution
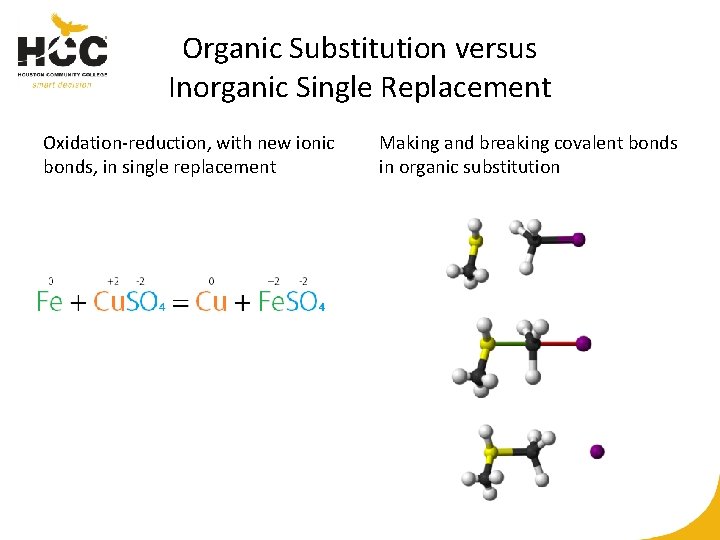
Organic Substitution versus Inorganic Single Replacement Oxidation-reduction, with new ionic bonds, in single replacement Making and breaking covalent bonds in organic substitution
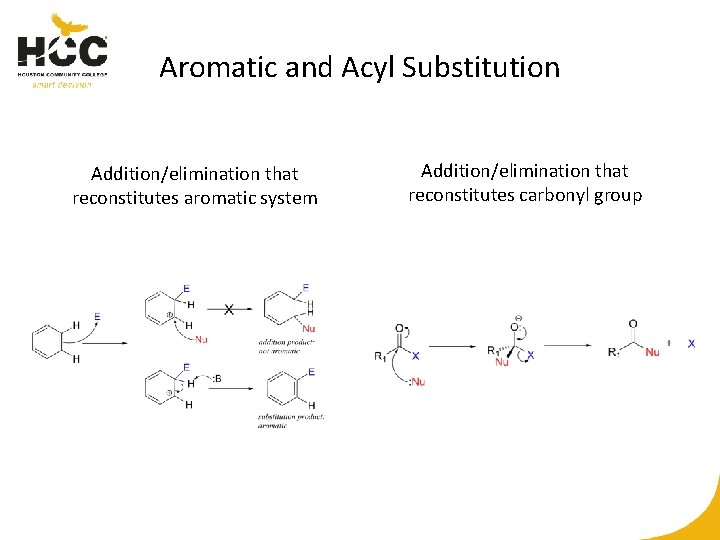
Aromatic and Acyl Substitution Addition/elimination that reconstitutes aromatic system Addition/elimination that reconstitutes carbonyl group
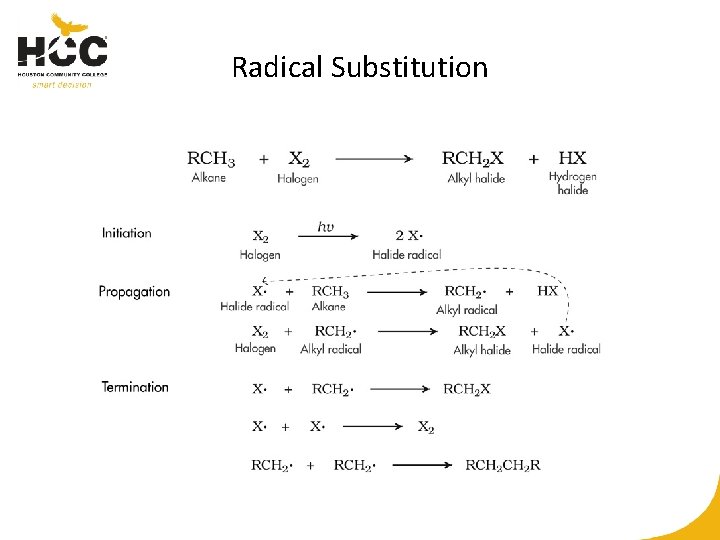
Radical Substitution
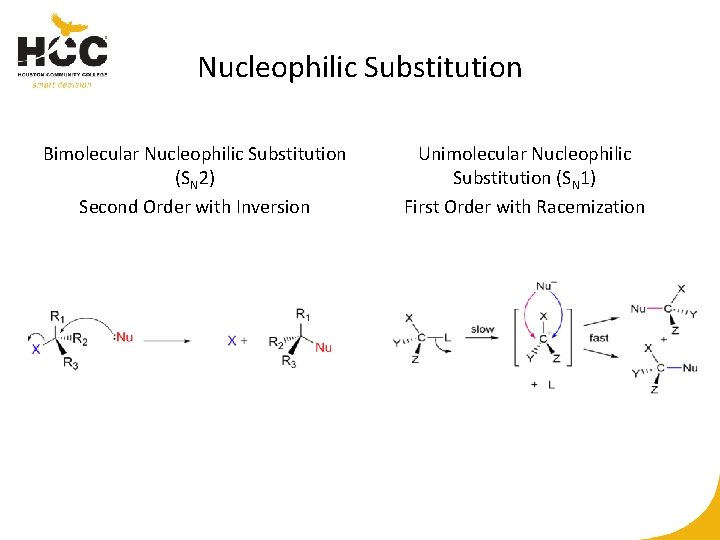
Nucleophilic Substitution Bimolecular Nucleophilic Substitution (SN 2) Second Order with Inversion Unimolecular Nucleophilic Substitution (SN 1) First Order with Racemization
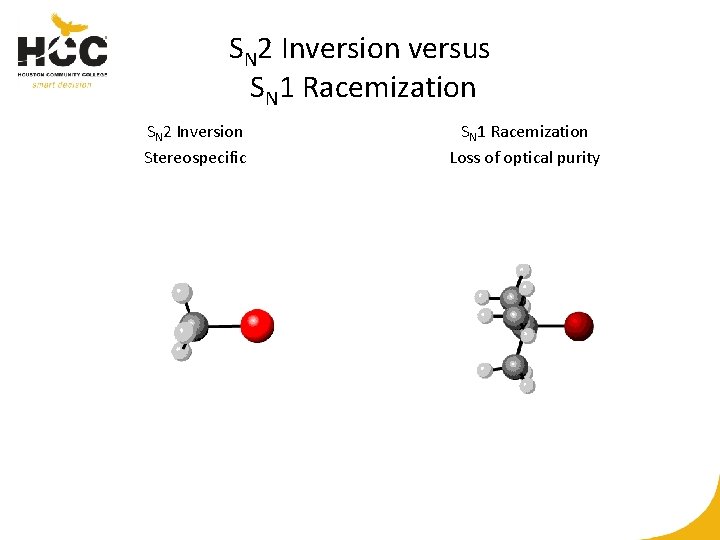
SN 2 Inversion versus SN 1 Racemization SN 2 Inversion Stereospecific SN 1 Racemization Loss of optical purity
![Kinetics of SN 2 versus SN 1 SN 2 • Rate = k[Substrate][Nucleophile] • Kinetics of SN 2 versus SN 1 SN 2 • Rate = k[Substrate][Nucleophile] •](http://slidetodoc.com/presentation_image_h2/bee5d763266bec4a8fb04cca13b68356/image-7.jpg)
Kinetics of SN 2 versus SN 1 SN 2 • Rate = k[Substrate][Nucleophile] • Rate depends on nucleophile conc. • Restrictive collision orientation in RDS SN 1 • Rate = k[Substrate] • Rate independent of nucleophile conc. • Net bond dissociation in RDS
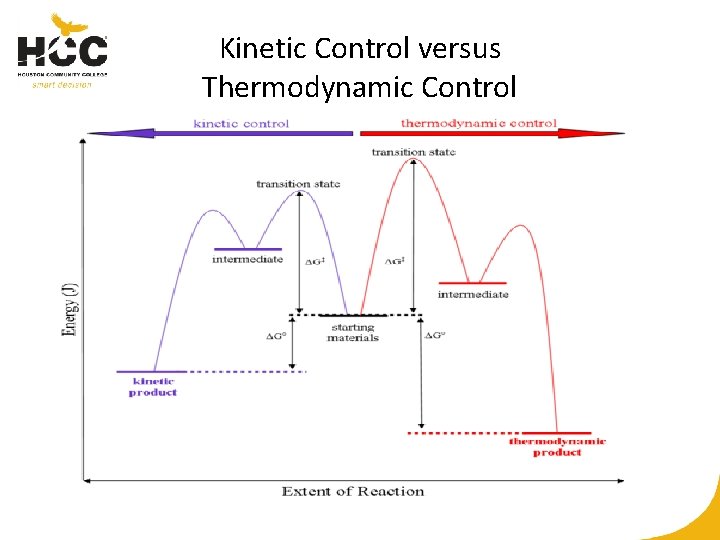
Kinetic Control versus Thermodynamic Control
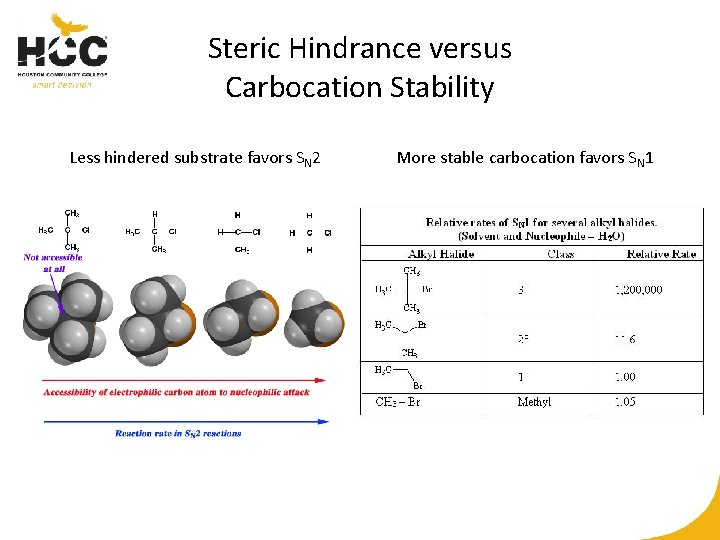
Steric Hindrance versus Carbocation Stability Less hindered substrate favors SN 2 More stable carbocation favors SN 1
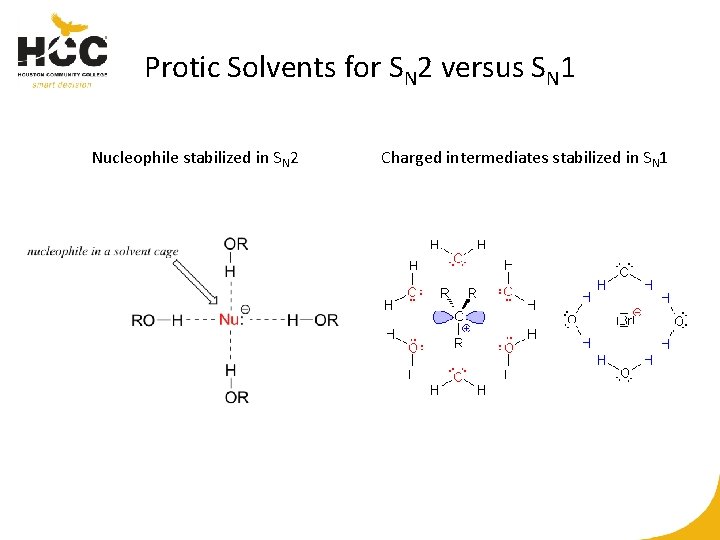
Protic Solvents for SN 2 versus SN 1 Nucleophile stabilized in SN 2 Charged intermediates stabilized in SN 1
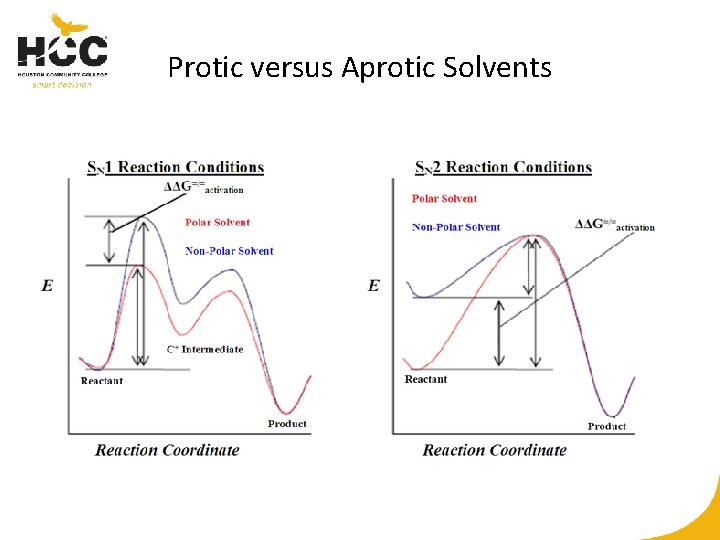
Protic versus Aprotic Solvents
Effect of Nucleophile SN 2 could be favored with superior nucleophiles: • • • Localized, destabilized anion Low electronegativity atom Larger, more polarizable atom High p-character hybrid orbital Low steric hindrance
Factors Favoring SN 2 versus SN 1 • Less hindered substrates favor SN 2; substrates with more stable carbocation intermediates favor SN 1. • Polar aprotic solvents, which avoid sequestration of nucleophiles, are favored for SN 2; protic solvents, which stabilize intermediates, are favored for SN 1. • Exceptional nucleophiles may improve the preference for SN 2 over SN 1.
- Slides: 13