Nucleic acids II Nucleic Acids 1 Elements C





- Slides: 47

Nucleic acids

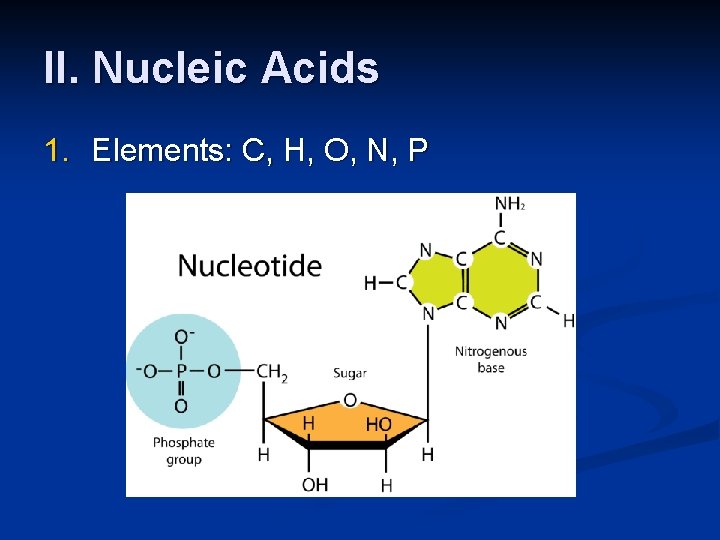
II. Nucleic Acids 1. Elements: C, H, O, N, P

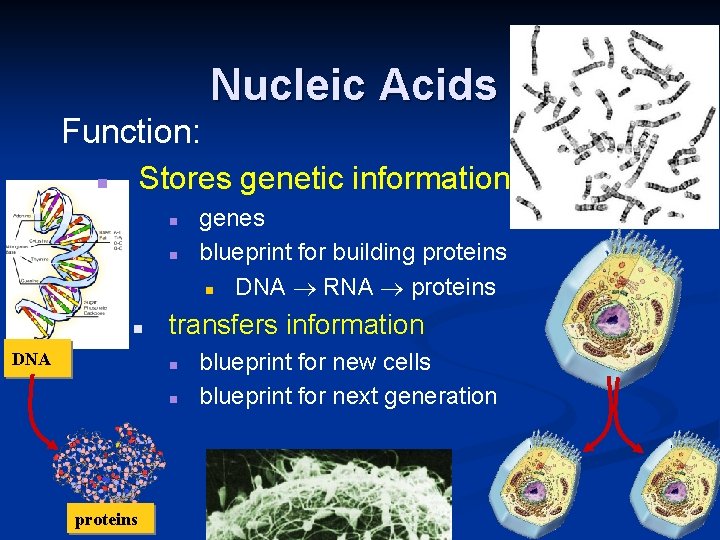
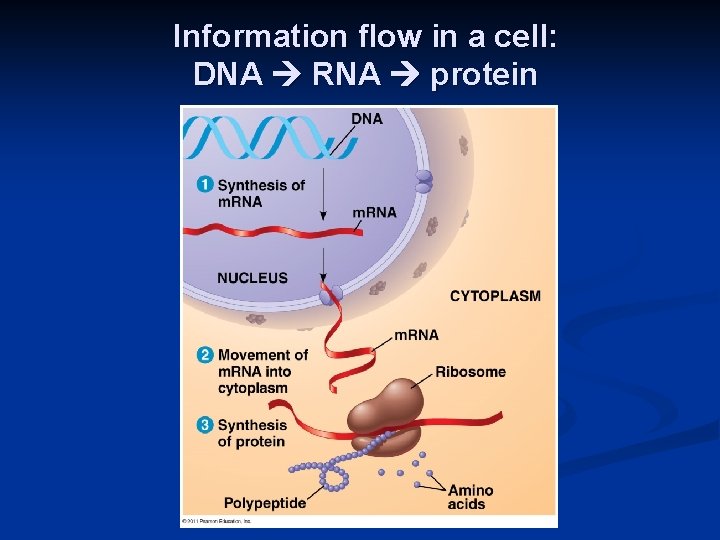
Nucleic Acids Function: Stores genetic information n n DNA transfers information n n proteins genes blueprint for building proteins n DNA RNA proteins blueprint for new cells blueprint for next generation

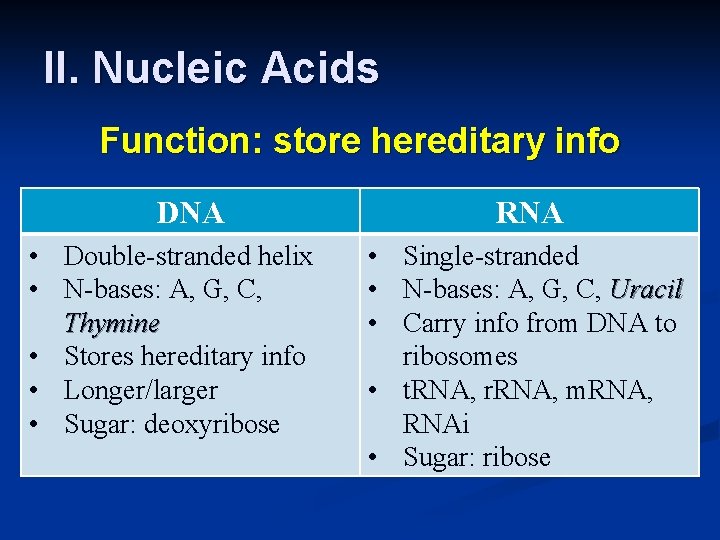
II. Nucleic Acids Function: store hereditary info DNA • Double-stranded helix • N-bases: A, G, C, Thymine • Stores hereditary info • Longer/larger • Sugar: deoxyribose RNA • Single-stranded • N-bases: A, G, C, Uracil • Carry info from DNA to ribosomes • t. RNA, r. RNA, m. RNA, RNAi • Sugar: ribose

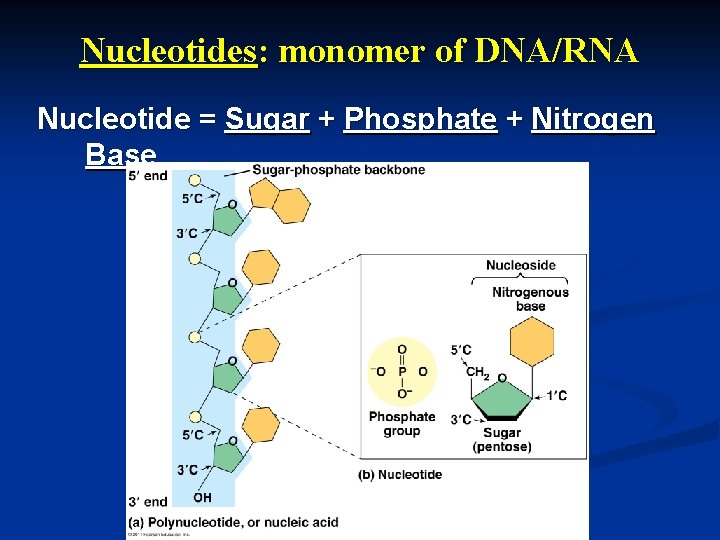
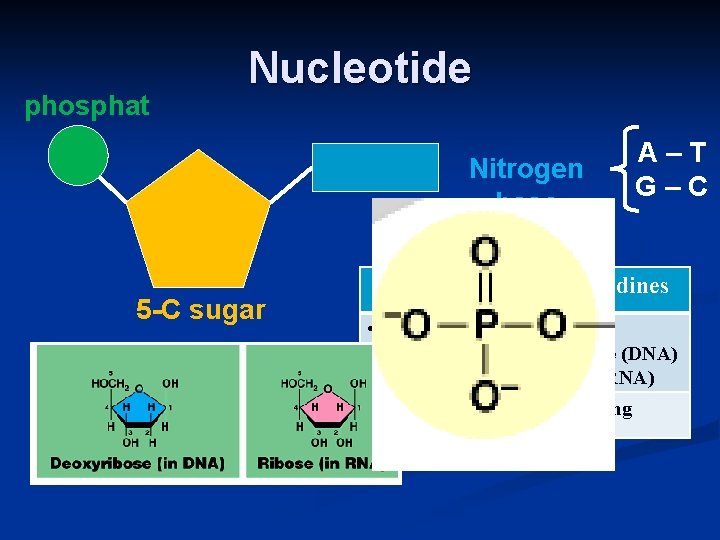
Nucleotides: monomer of DNA/RNA Nucleotide = Sugar + Phosphate + Nitrogen Base

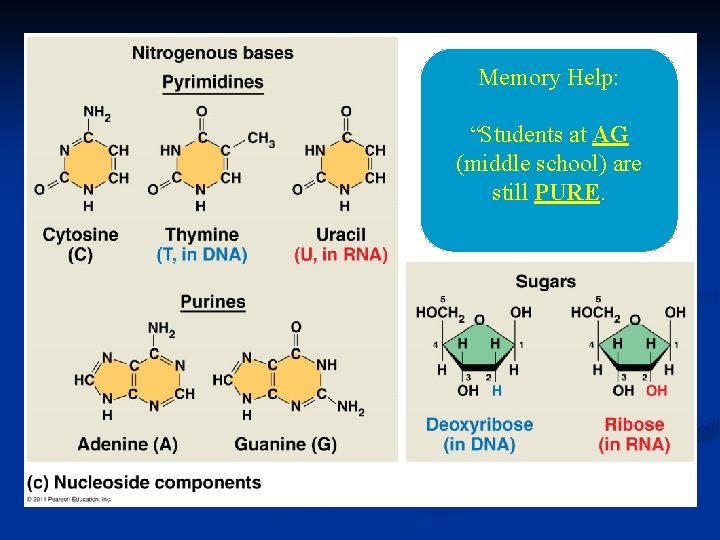
phosphat e Nucleotide 5 -C sugar Nitrogen base Purines A–T G–C Pyrimidines • Adenine • Guanine • Cytosine • Thymine (DNA) • Uracil (RNA) • Double ring • Single ring

Memory Help: “Students at AG (middle school) are still PURE.

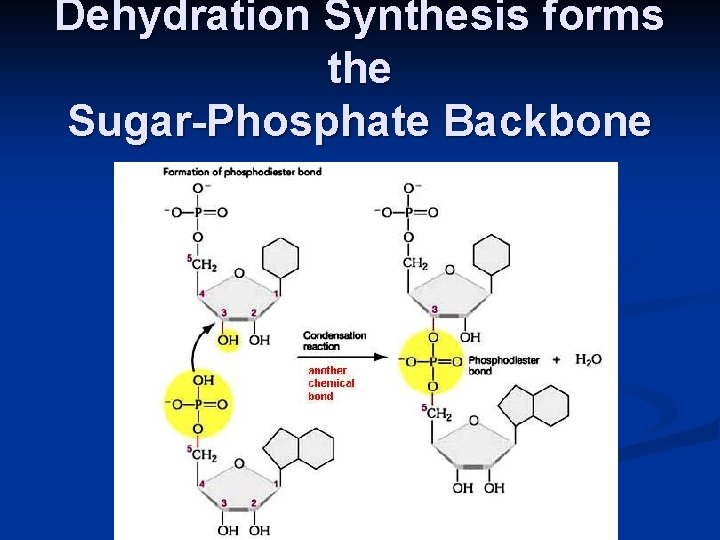
Dehydration Synthesis forms the Sugar-Phosphate Backbone

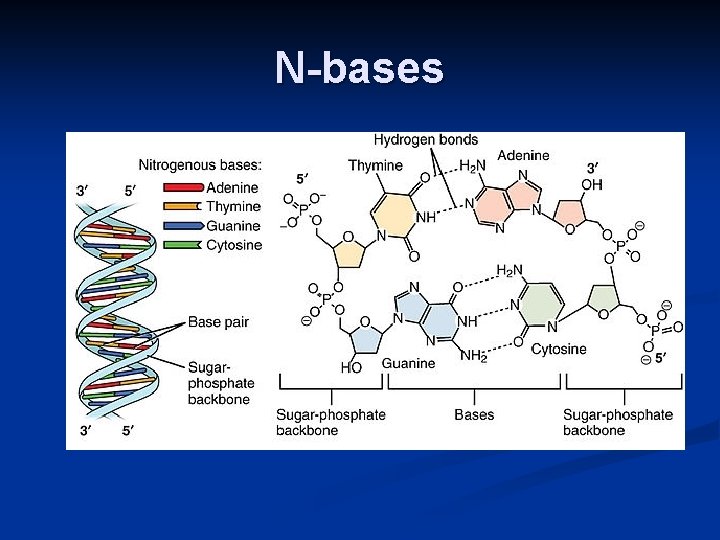
N-bases

Information flow in a cell: DNA RNA protein

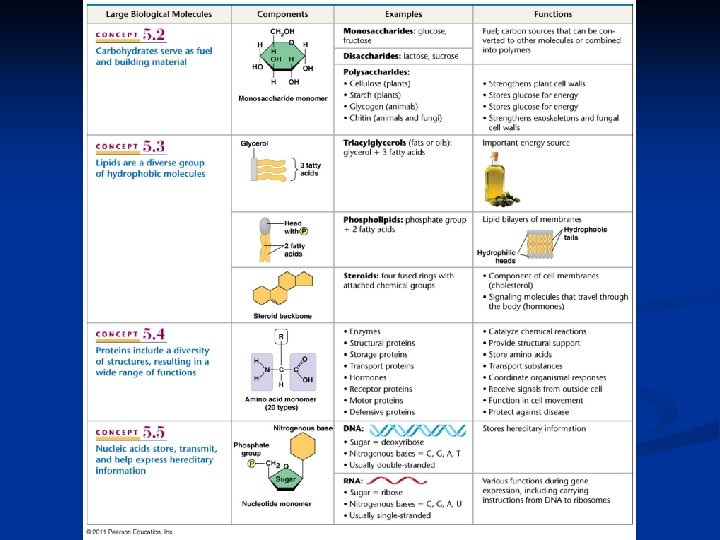
Carbohydrates

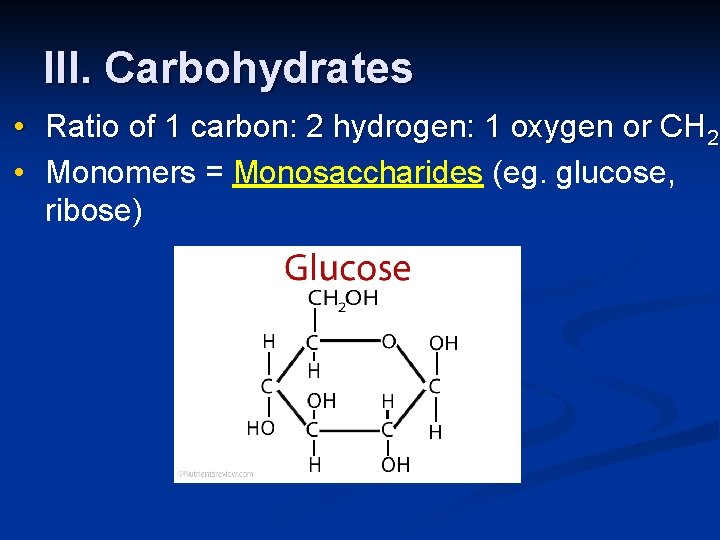
III. Carbohydrates • Ratio of 1 carbon: 2 hydrogen: 1 oxygen or CH 2 O • Monomers = Monosaccharides (eg. glucose, ribose)

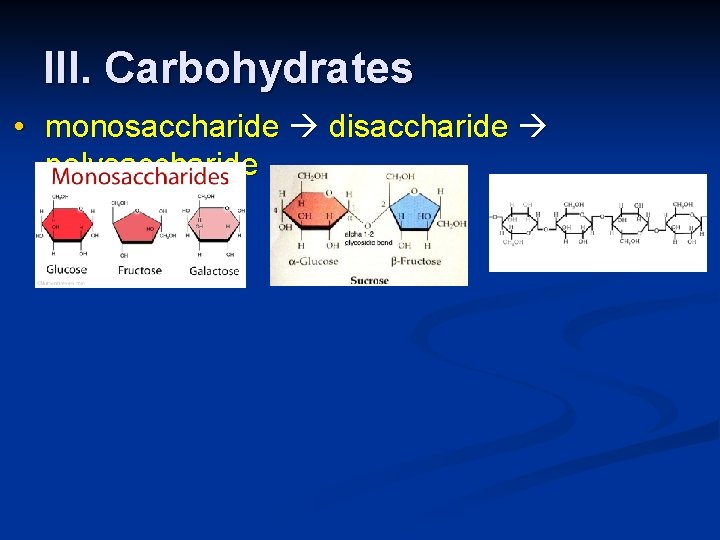
III. Carbohydrates • monosaccharide disaccharide polysaccharide

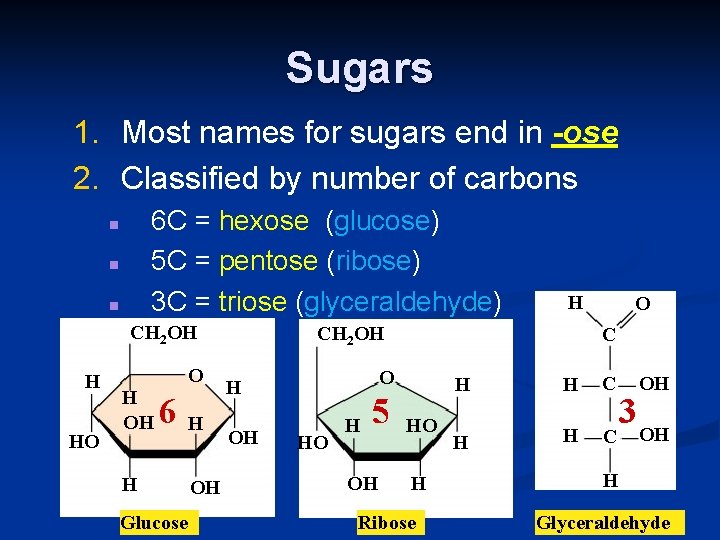
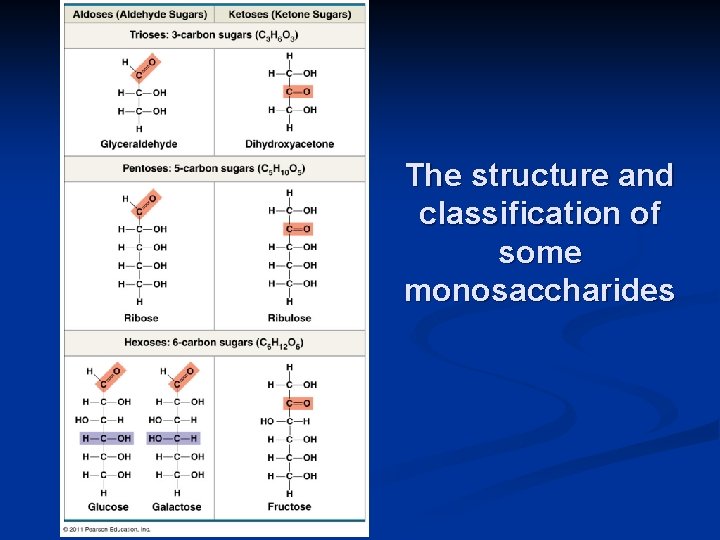
Sugars 1. Most names for sugars end in -ose 2. Classified by number of carbons 6 C = hexose (glucose) 5 C = pentose (ribose) 3 C = triose (glyceraldehyde) n n n CH 2 OH H HO O H OH 6 H Glucose H OH H CH 2 OH OH C O H HO H 5 OH O HO H Ribose H H C OH 3 OH H Glyceraldehyde

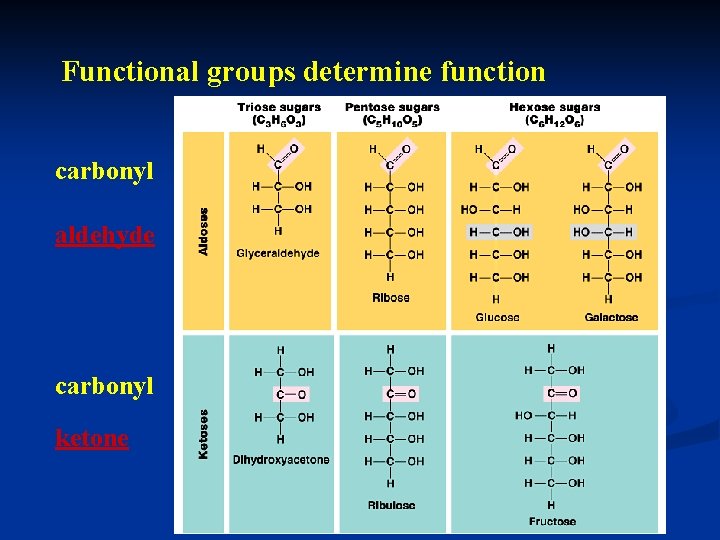
Functional groups determine function carbonyl aldehyde carbonyl ketone

The structure and classification of some monosaccharides

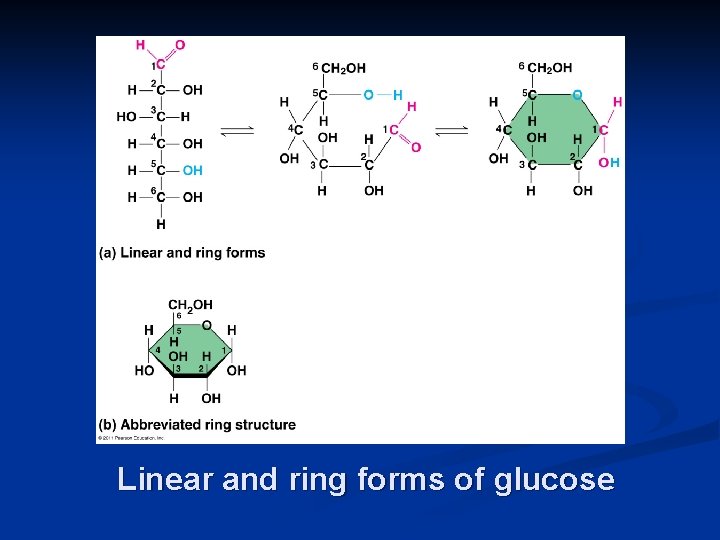
Linear and ring forms of glucose

III. Carbohydrates Examples/Types: • Simple sugars: Fructose, Glucose, Ribose • Complex Polysaccharides: Starch, Cellulose, Glycogen

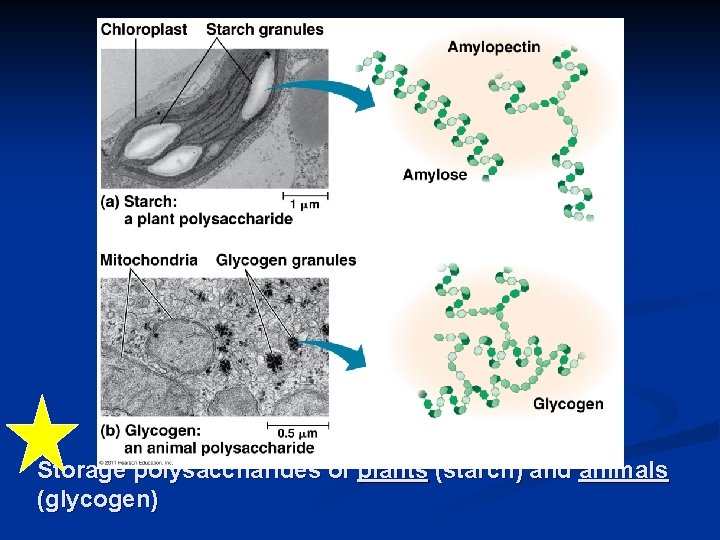
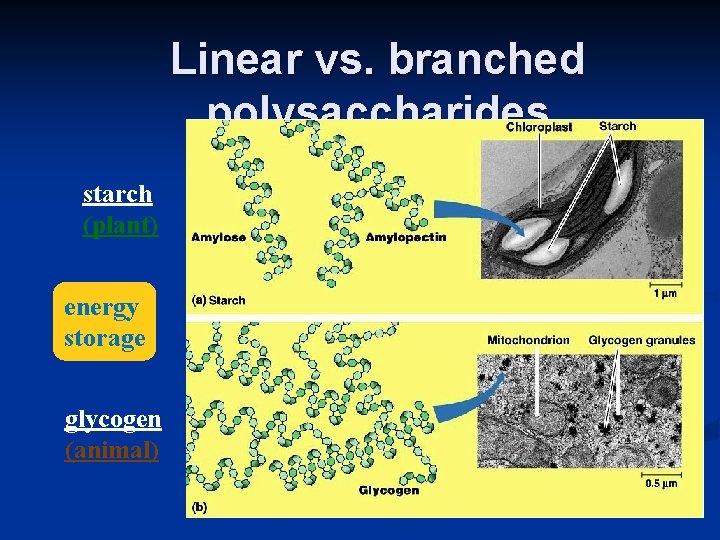
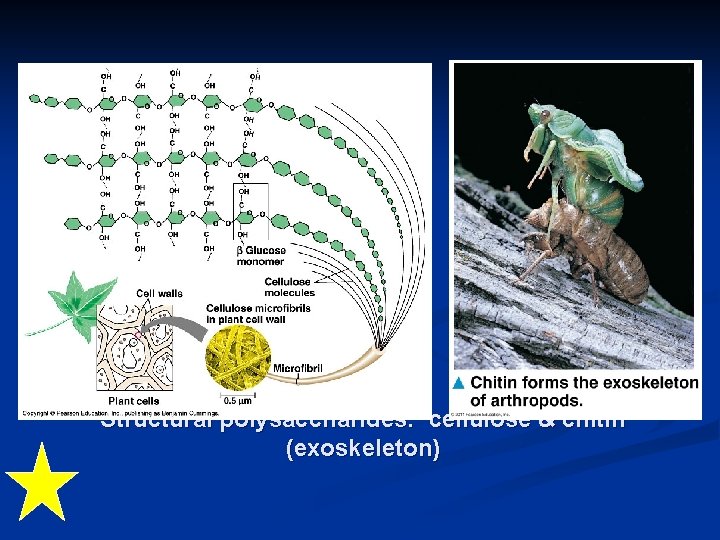
III. Carbohydrates • Functions: • Fuel (energy) and building material § § Differ in Storage (plants-starch, animals-glycogen) position & orientation of Structure (plant-cellulose, arthropod/fungi-chitin ) glycosidic linkage

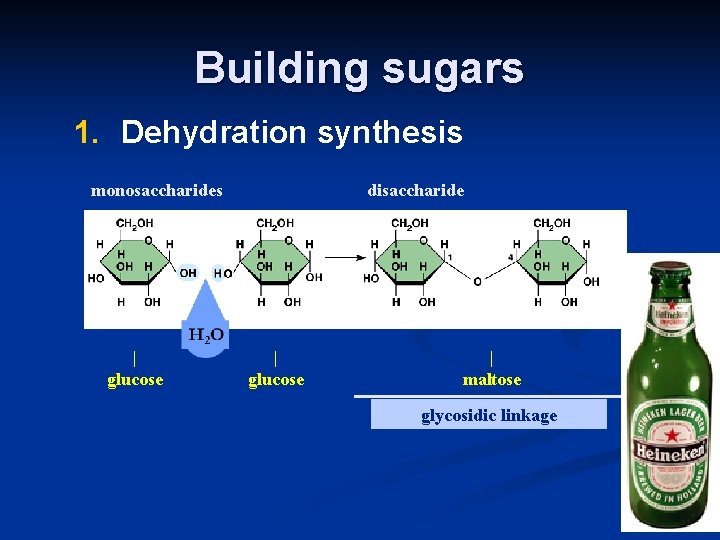
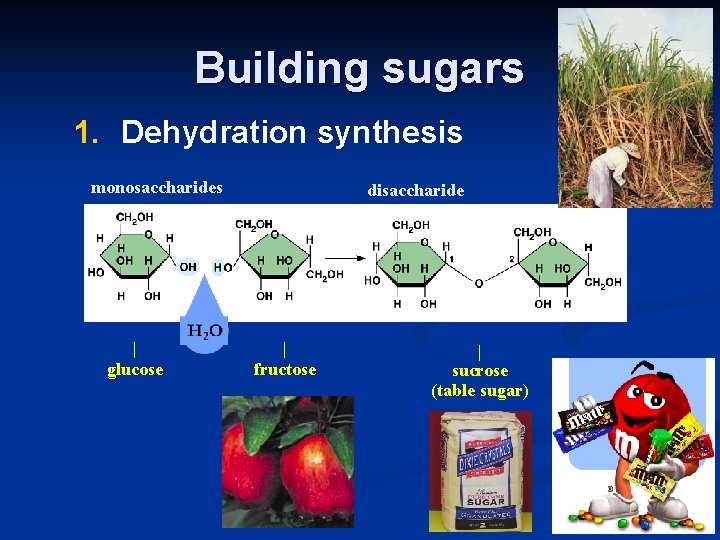
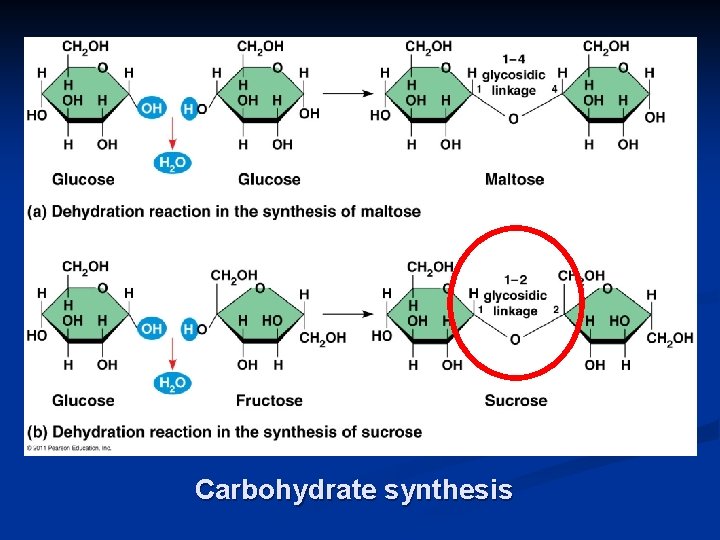
Building sugars 1. Dehydration synthesis monosaccharides | glucose disaccharide | glucose | maltose glycosidic linkage

Building sugars 1. Dehydration synthesis monosaccharides | glucose disaccharide | fructose | sucrose (table sugar)

Carbohydrate synthesis

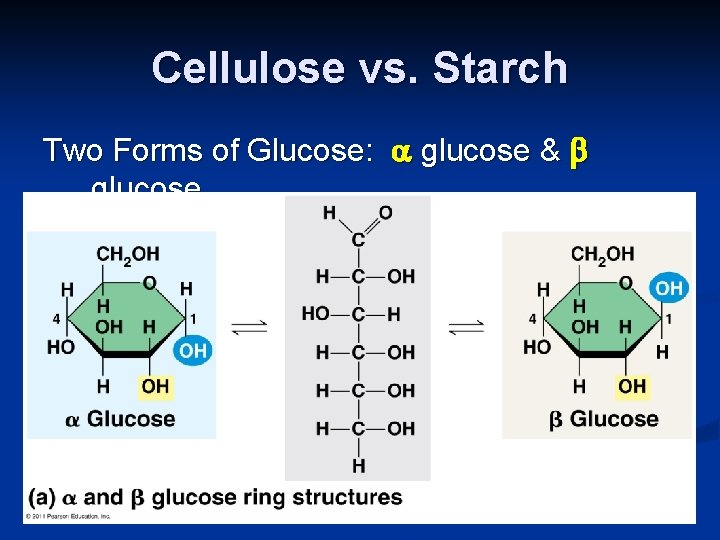
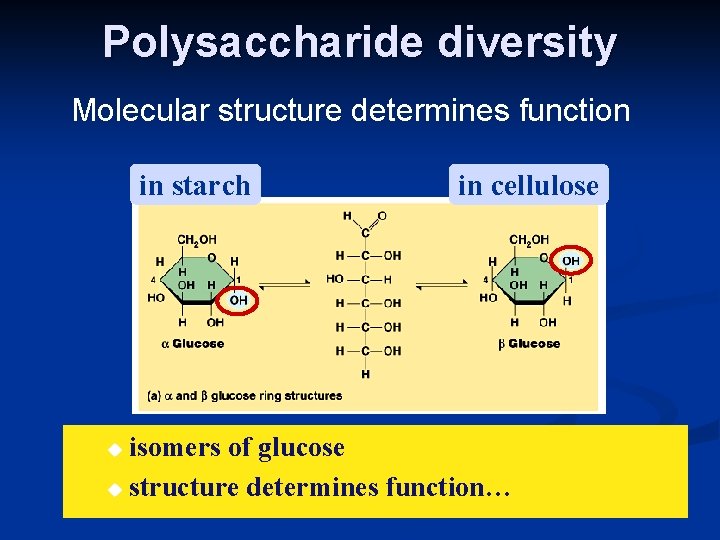
Cellulose vs. Starch Two Forms of Glucose: glucose & glucose

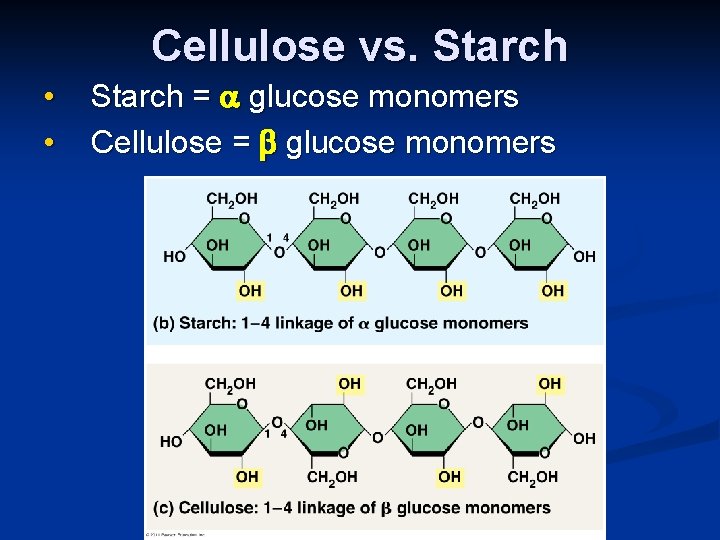
Cellulose vs. Starch • • Starch = glucose monomers Cellulose = glucose monomers

Storage polysaccharides of plants (starch) and animals (glycogen)

Linear vs. branched polysaccharides starch (plant) energy storage glycogen (animal)

Polysaccharide diversity Molecular structure determines function in starch in cellulose isomers of glucose u structure determines function… u

Structural polysaccharides: cellulose & chitin (exoskeleton)

Lipids: Fats & Oils

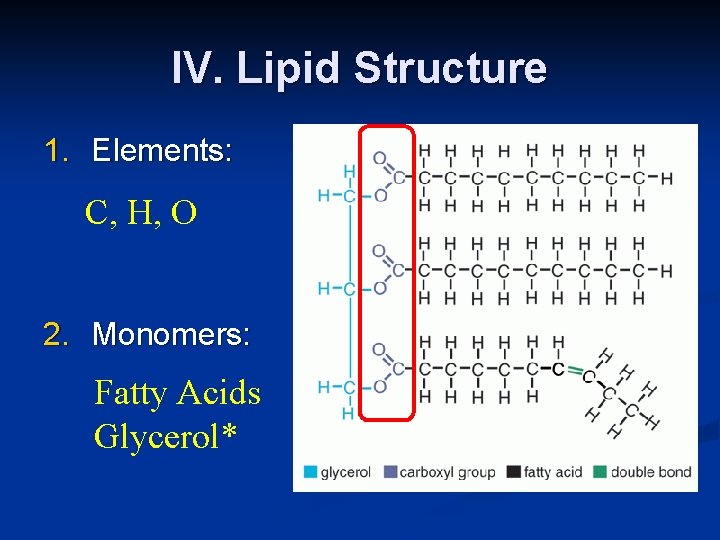
IV. Lipid Structure 1. Elements: C, H, O 2. Monomers: Fatty Acids Glycerol*

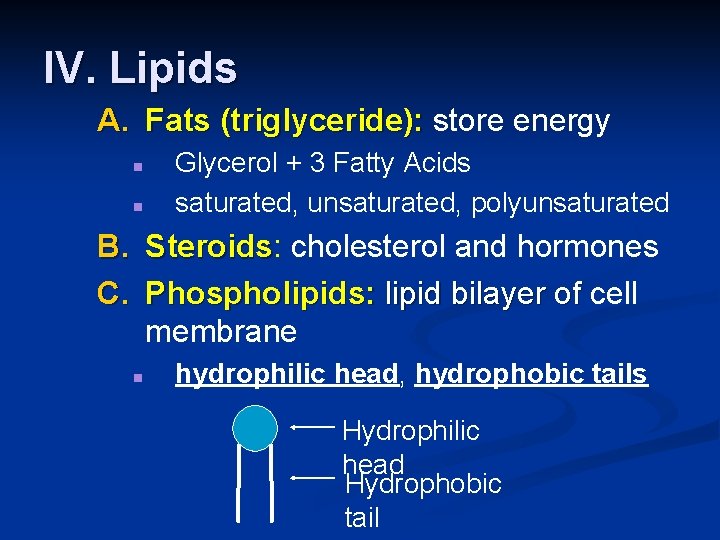
IV. Lipids A. Fats (triglyceride): store energy n n Glycerol + 3 Fatty Acids saturated, unsaturated, polyunsaturated B. Steroids: Steroids cholesterol and hormones C. Phospholipids: lipid bilayer of cell membrane n hydrophilic head, hydrophobic tails Hydrophilic head Hydrophobic tail

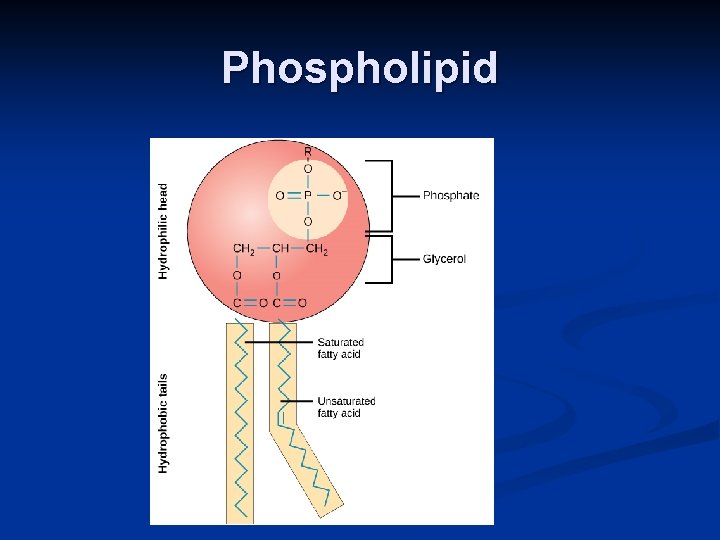
Phospholipid

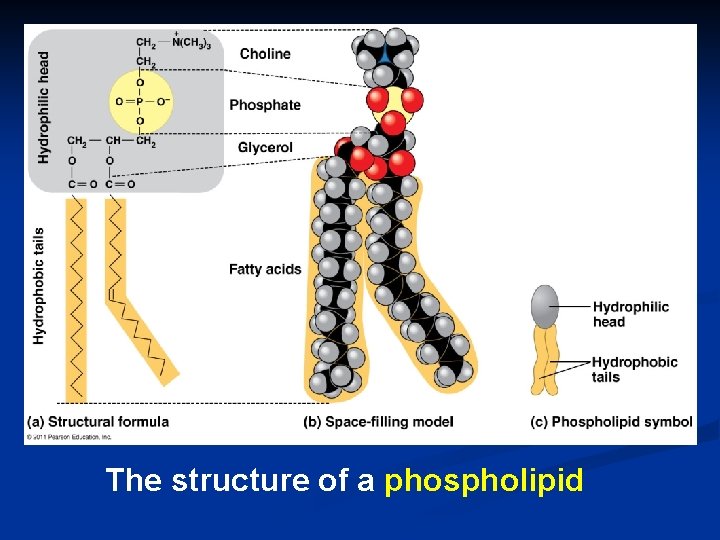
The structure of a phospholipid

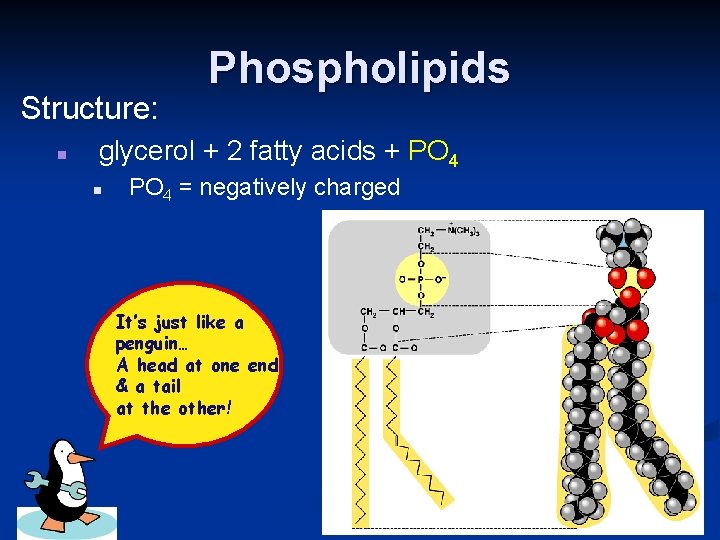
Structure: n Phospholipids glycerol + 2 fatty acids + PO 4 n PO 4 = negatively charged It’s just like a penguin… A head at one end & a tail at the other!

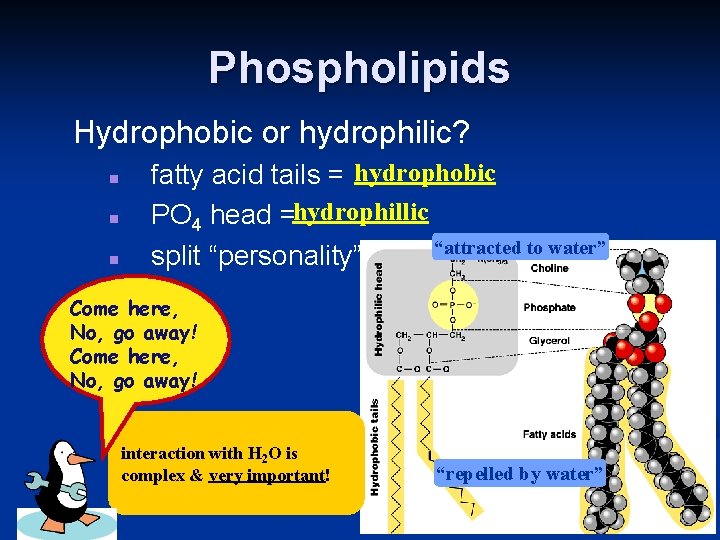
Phospholipids Hydrophobic or hydrophilic? n n n fatty acid tails = hydrophobic PO 4 head =hydrophillic “attracted to water” split “personality” Come here, No, go away! interaction with H 2 O is complex & very important! “repelled by water”

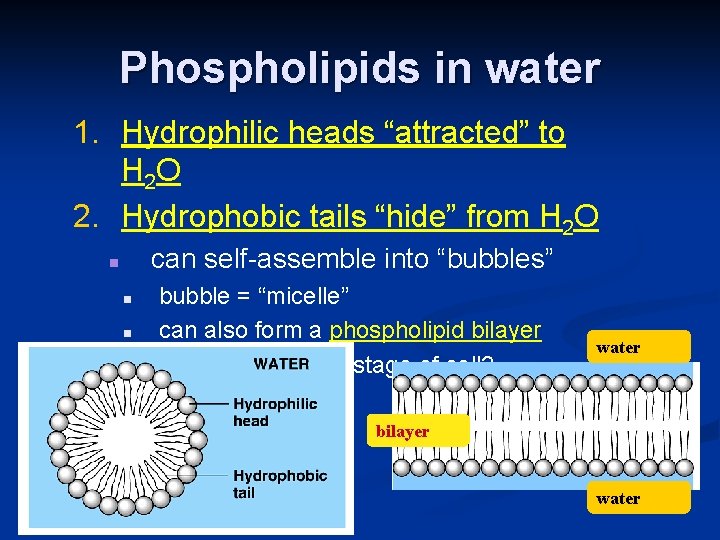
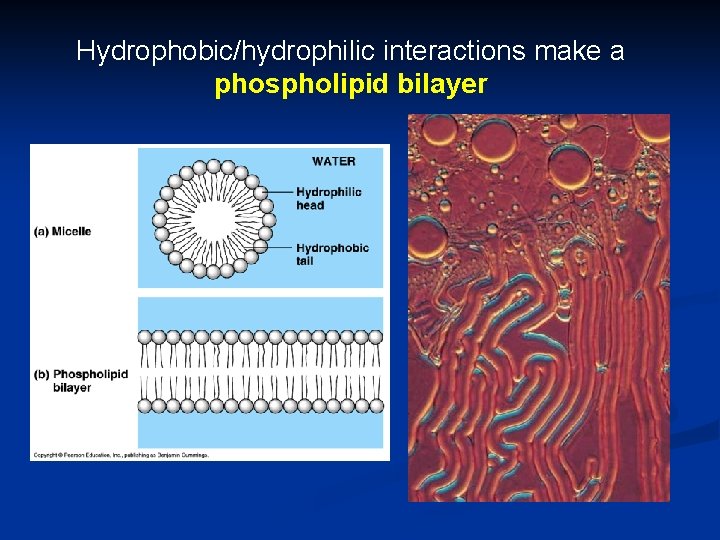
Phospholipids in water 1. Hydrophilic heads “attracted” to H 2 O 2. Hydrophobic tails “hide” from H 2 O can self-assemble into “bubbles” n n bubble = “micelle” can also form a phospholipid bilayer early evolutionary stage of cell? water bilayer water

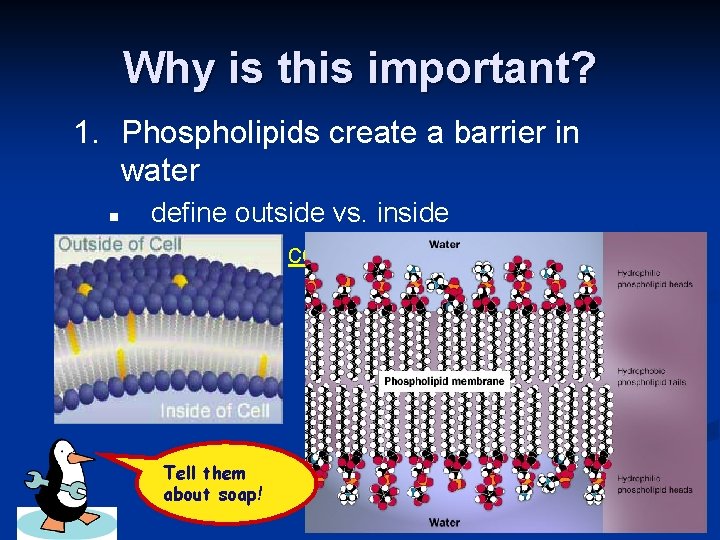
Why is this important? 1. Phospholipids create a barrier in water n n define outside vs. inside they make cell membranes! Tell them about soap!

Hydrophobic/hydrophilic interactions make a phospholipid bilayer

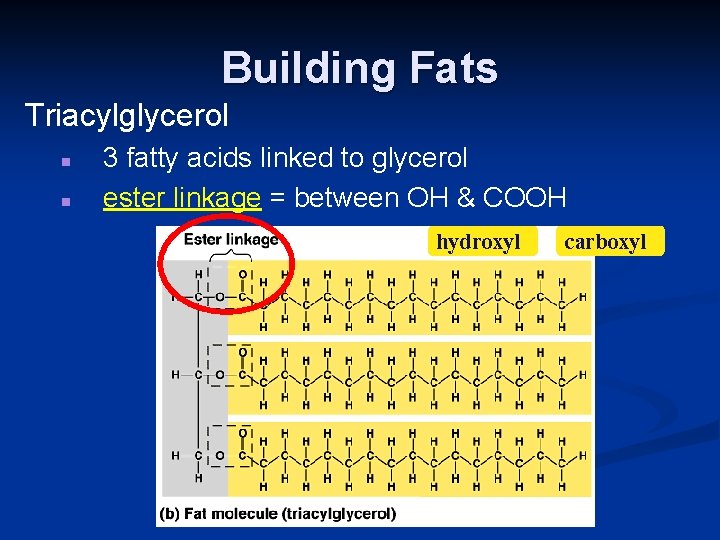
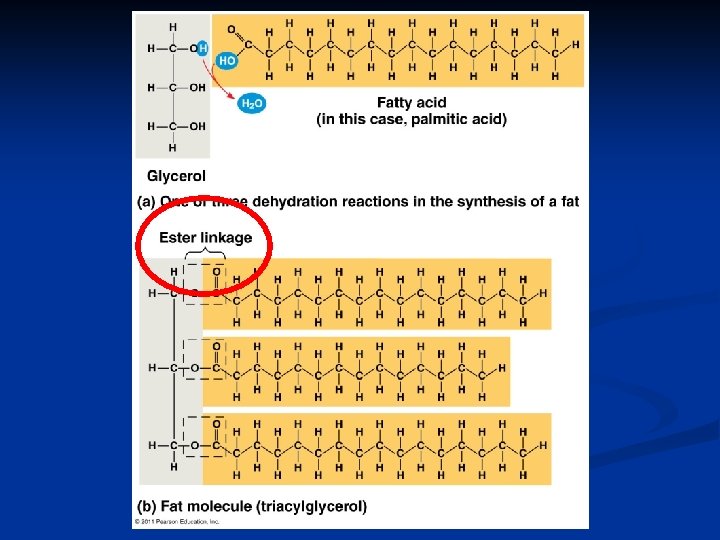
Building Fats Triacylglycerol n n 3 fatty acids linked to glycerol ester linkage = between OH & COOH hydroxyl carboxyl

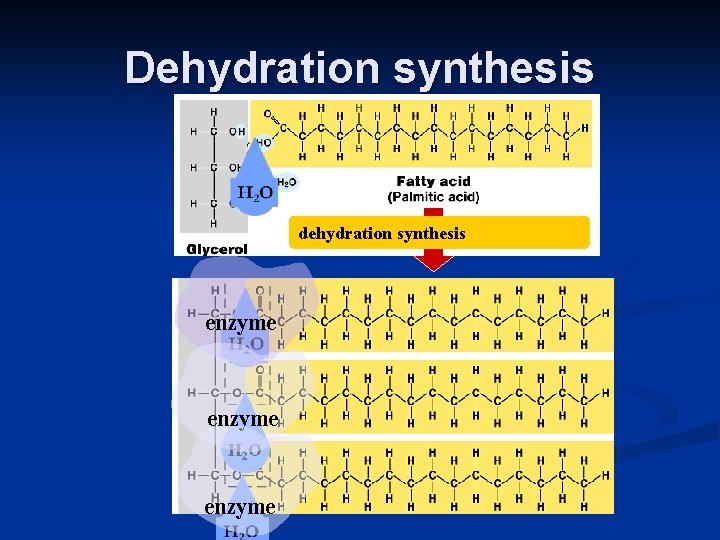
Dehydration synthesis dehydration synthesis enzyme H 2 O enzyme


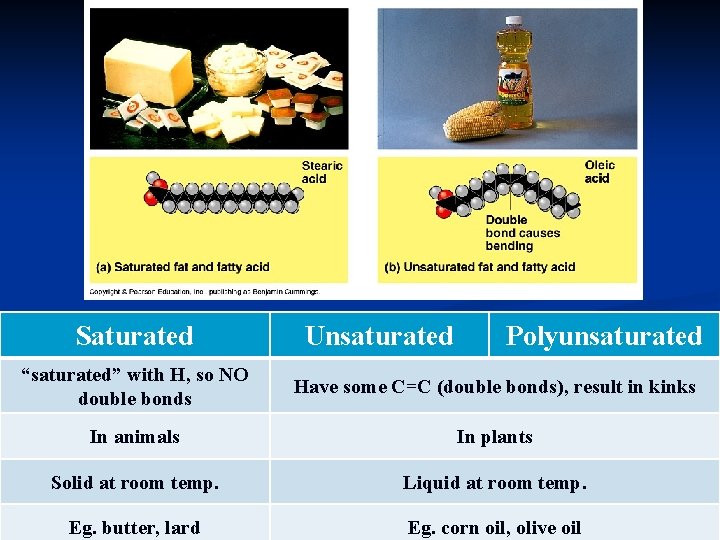
Saturated Unsaturated Polyunsaturated “saturated” with H, so NO double bonds Have some C=C (double bonds), result in kinks In animals In plants Solid at room temp. Liquid at room temp. Eg. butter, lard Eg. corn oil, olive oil

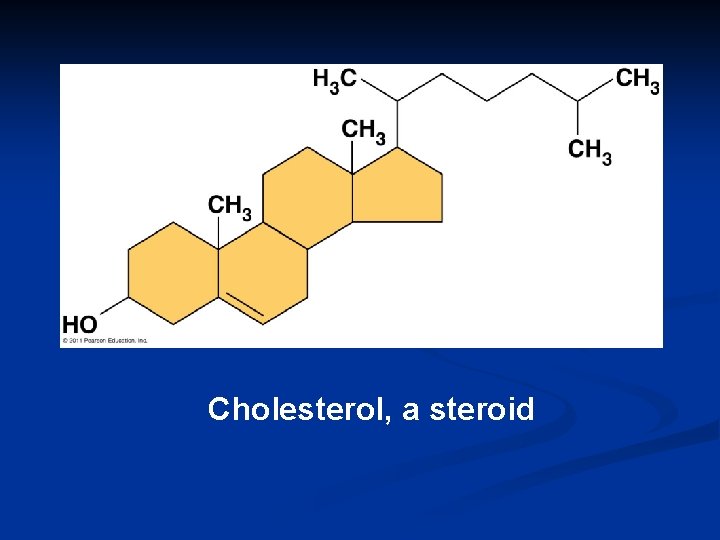
Cholesterol, a steroid

Cholesterol 1. Important cell component n n animal cell membranes precursor of all other steroids n n including vertebrate sex hormones high levels in blood may contribute to cardiovascular disease

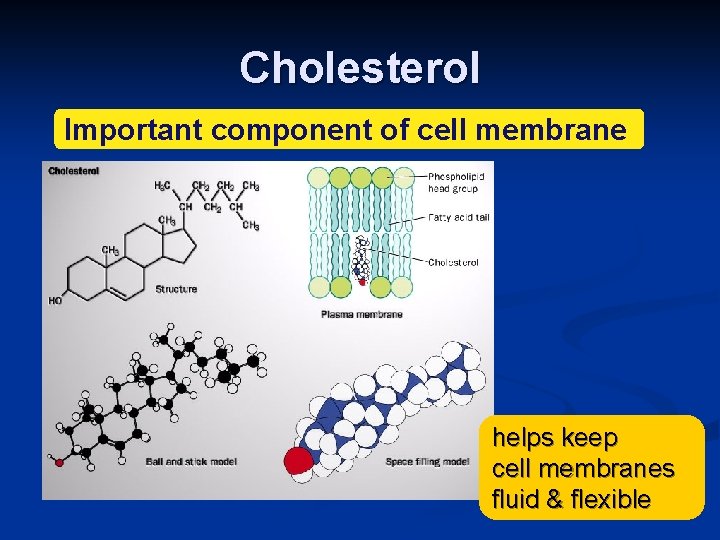
Cholesterol Important component of cell membrane helps keep cell membranes fluid & flexible

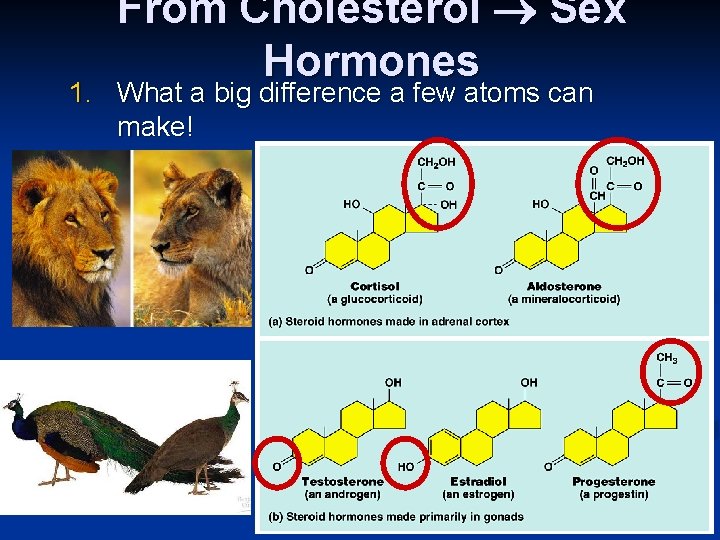
From Cholesterol Sex Hormones 1. What a big difference a few atoms can make!
