Nucleic Acids and their functions Topic 1 5





- Slides: 19

Nucleic Acids and their functions Topic 1. 5

Syllabus content • Check the syllabus content pg 136 make sure you tick this at the end of the topic • Teacher’s notes pg 137 -138

Lesson Objectives • (a) the structure of nucleotides (pentose sugar, phosphate, organic base) • (b) the importance of chemical energy in biological processes • (c) the central role of ATP as an energy carrier and its use in the liberation of energy for cellular activity • (d) the structure of ATP

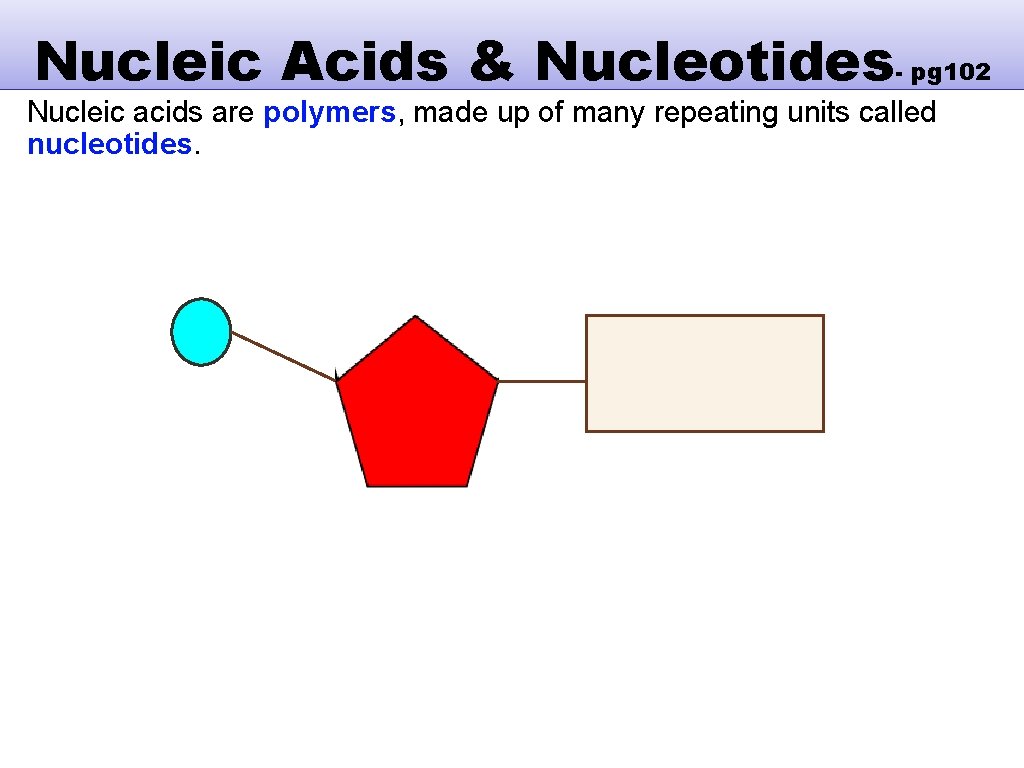
Nucleic Acids & Nucleotides- pg 102 Nucleic acids are polymers, made up of many repeating units called nucleotides. Nucleotides are made up of three components that combine by condensation reaction. These are: One or more phosphate groups (A). A pentose sugar (B). An organic nitrogenous base (contains nitrogen) (C).

Nucleic Acids & Nucleotides- pg 102 Nucleic acids are polymers, made up of many repeating units called nucleotides.

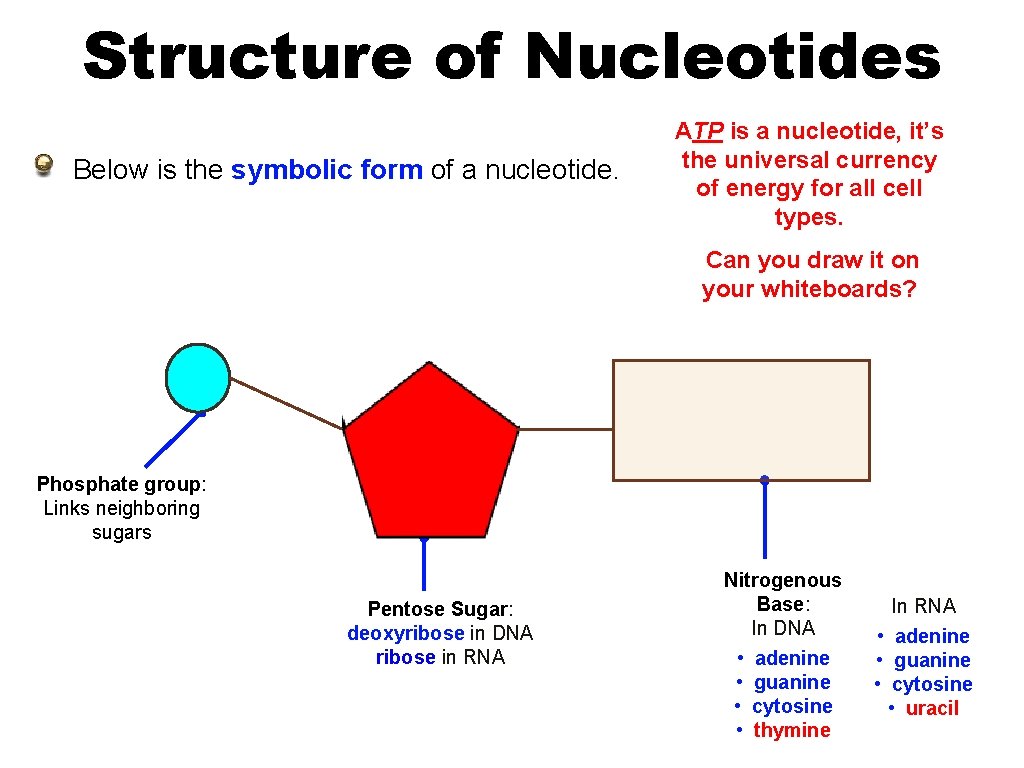
Structure of Nucleotides Below is the symbolic form of a nucleotide. ATP is a nucleotide, it’s the universal currency of energy for all cell types. Can you draw it on your whiteboards? Phosphate group: Links neighboring sugars Pentose Sugar: deoxyribose in DNA ribose in RNA Nitrogenous Base: In DNA • adenine • guanine • cytosine • thymine In RNA • adenine • guanine • cytosine • uracil

ATP pg 139

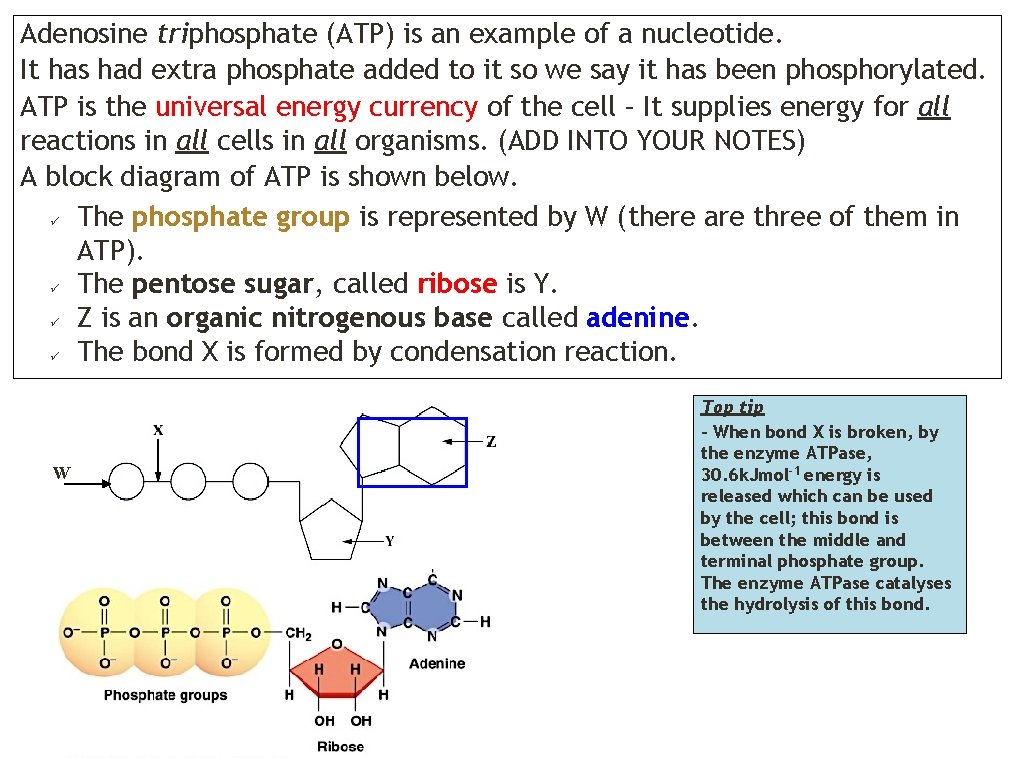
Adenosine triphosphate (ATP) is an example of a nucleotide. It has had extra phosphate added to it so we say it has been phosphorylated. ATP is the universal energy currency of the cell – It supplies energy for all reactions in all cells in all organisms. (ADD INTO YOUR NOTES) A block diagram of ATP is shown below. The phosphate group is represented by W (there are three of them in ATP). The pentose sugar, called ribose is Y. Z is an organic nitrogenous base called adenine. The bond X is formed by condensation reaction. W Top tip – When bond X is broken, by the enzyme ATPase, 30. 6 k. Jmol-1 energy is released which can be used by the cell; this bond is between the middle and terminal phosphate group. The enzyme ATPase catalyses the hydrolysis of this bond.

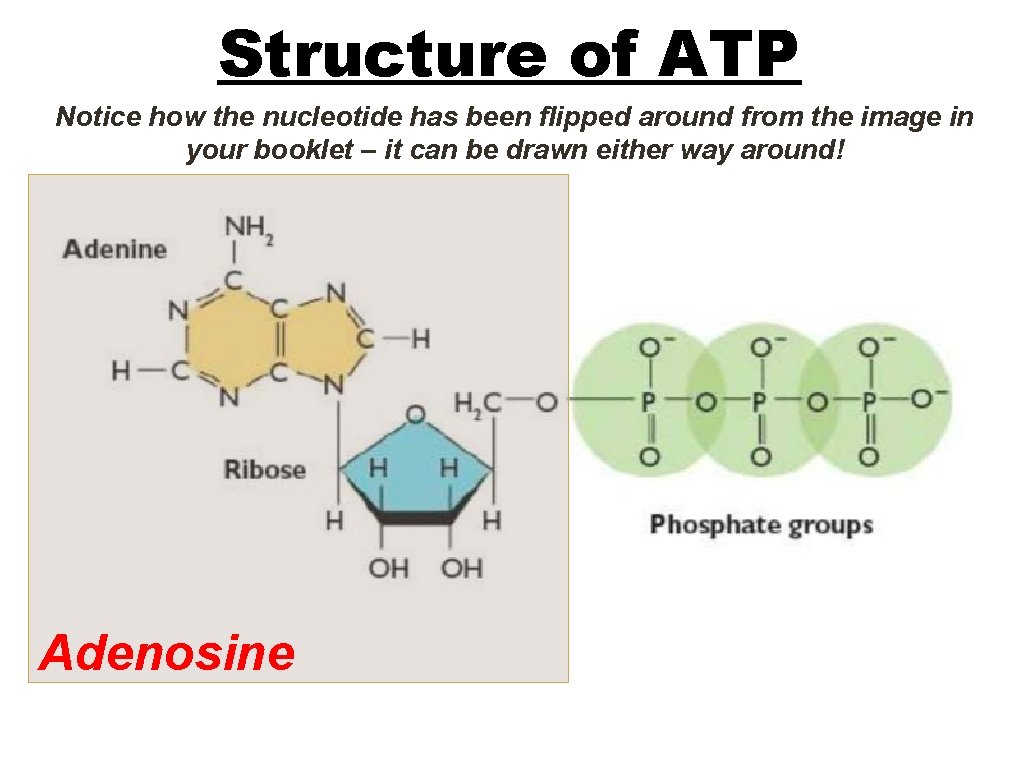
Structure of ATP Notice how the nucleotide has been flipped around from the image in your booklet – it can be drawn either way around! Adenosine

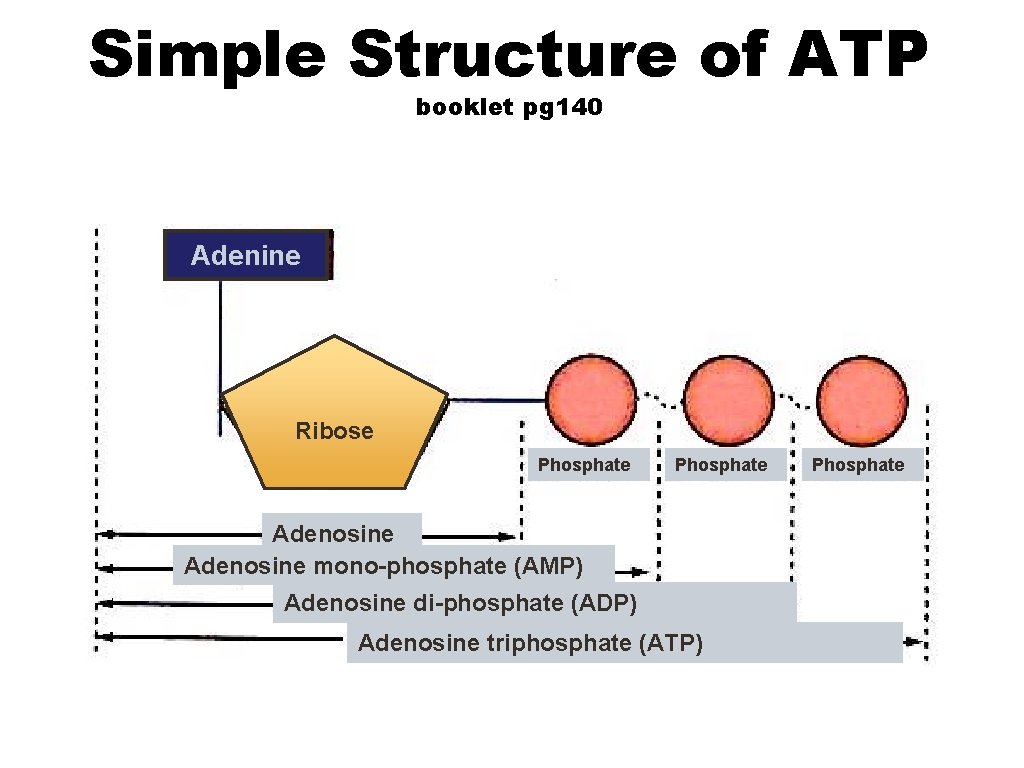
Simple Structure of ATP booklet pg 140 Adenine Ribose Phosphate Adenosine mono-phosphate (AMP) Adenosine di-phosphate (ADP) Adenosine triphosphate (ATP) Phosphate

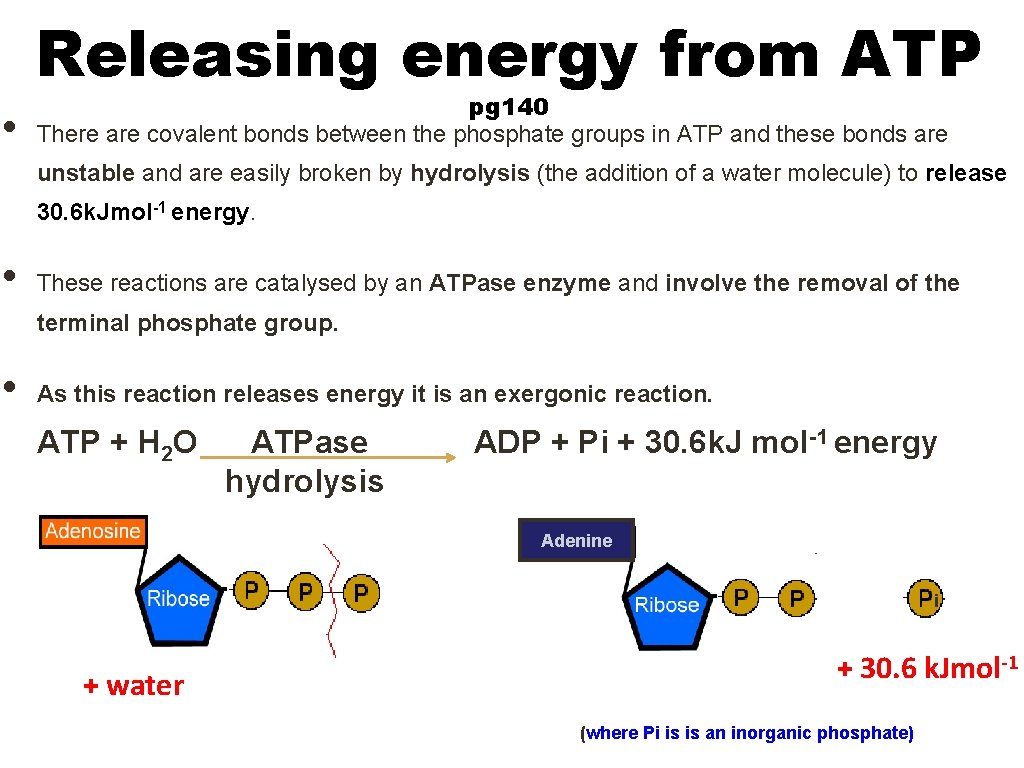
Releasing energy from ATP • pg 140 There are covalent bonds between the phosphate groups in ATP and these bonds are unstable and are easily broken by hydrolysis (the addition of a water molecule) to release 30. 6 k. Jmol-1 energy. • These reactions are catalysed by an ATPase enzyme and involve the removal of the terminal phosphate group. • As this reaction releases energy it is an exergonic reaction. ATP + H 2 O ATPase hydrolysis ADP + Pi + 30. 6 k. J mol -1 energy Adenine i + water + 30. 6 k. Jmol-1 (where Pi is is an inorganic phosphate)

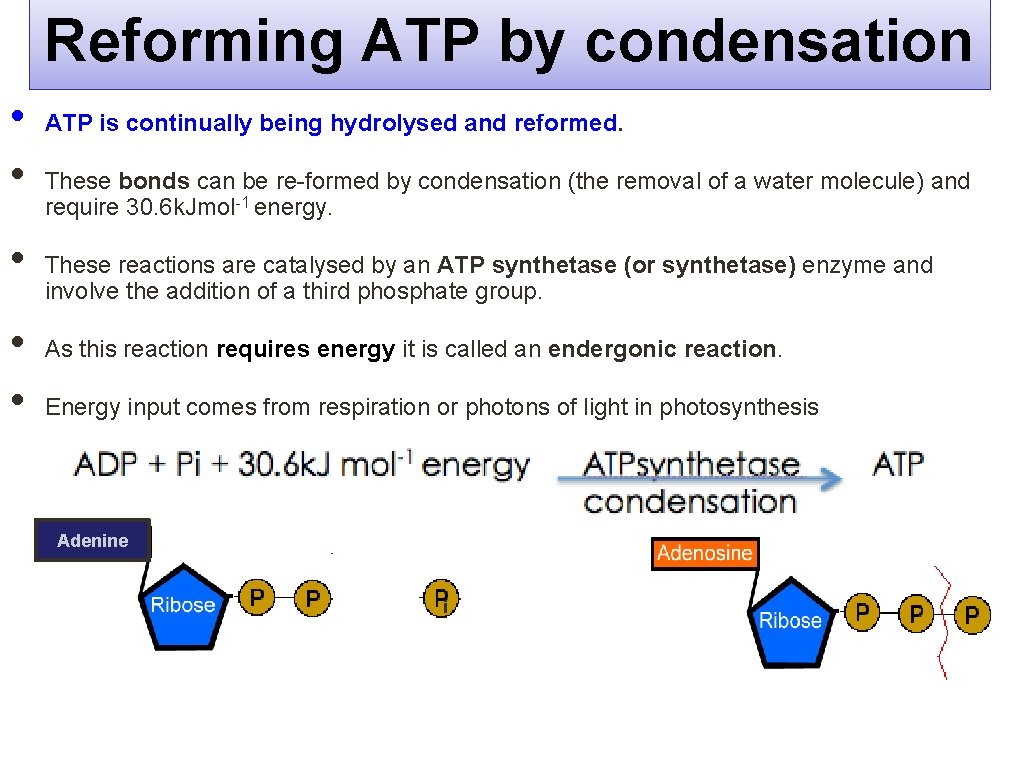
Reforming ATP by condensation • • • ATP is continually being hydrolysed and reformed. These bonds can be re-formed by condensation (the removal of a water molecule) and require 30. 6 k. Jmol-1 energy. These reactions are catalysed by an ATP synthetase (or synthetase) enzyme and involve the addition of a third phosphate group. As this reaction requires energy it is called an endergonic reaction. Energy input comes from respiration or photons of light in photosynthesis Adenine i

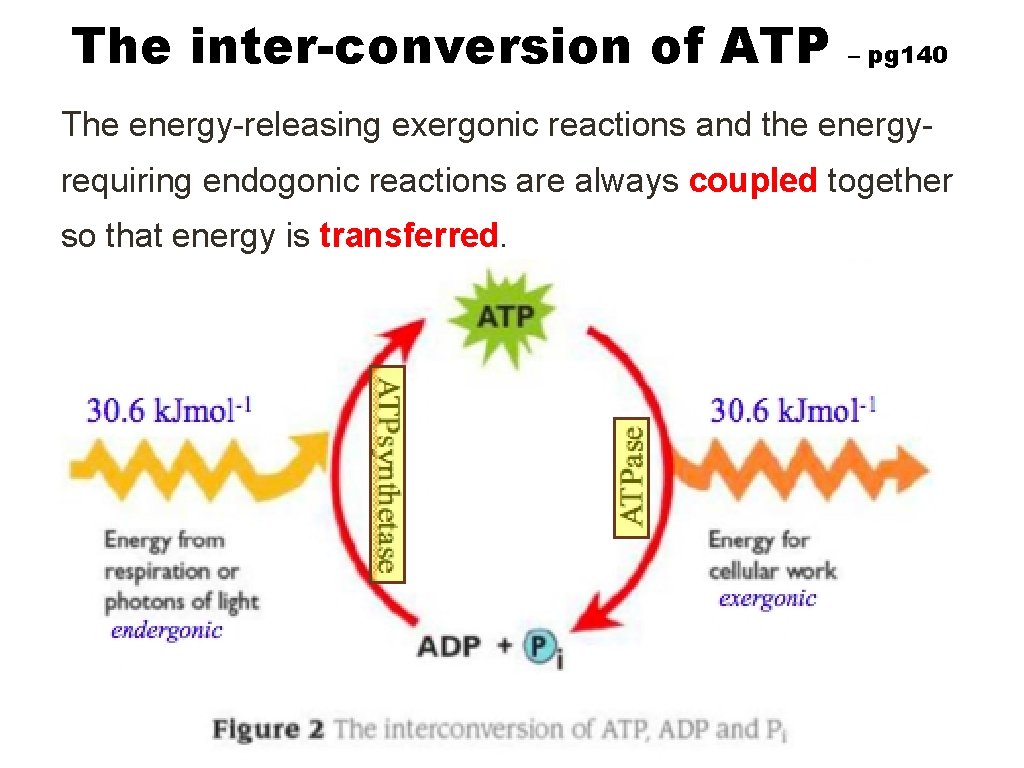
The inter-conversion of ATP – pg 140 The energy-releasing exergonic reactions and the energyrequiring endogonic reactions are always coupled together so that energy is transferred.

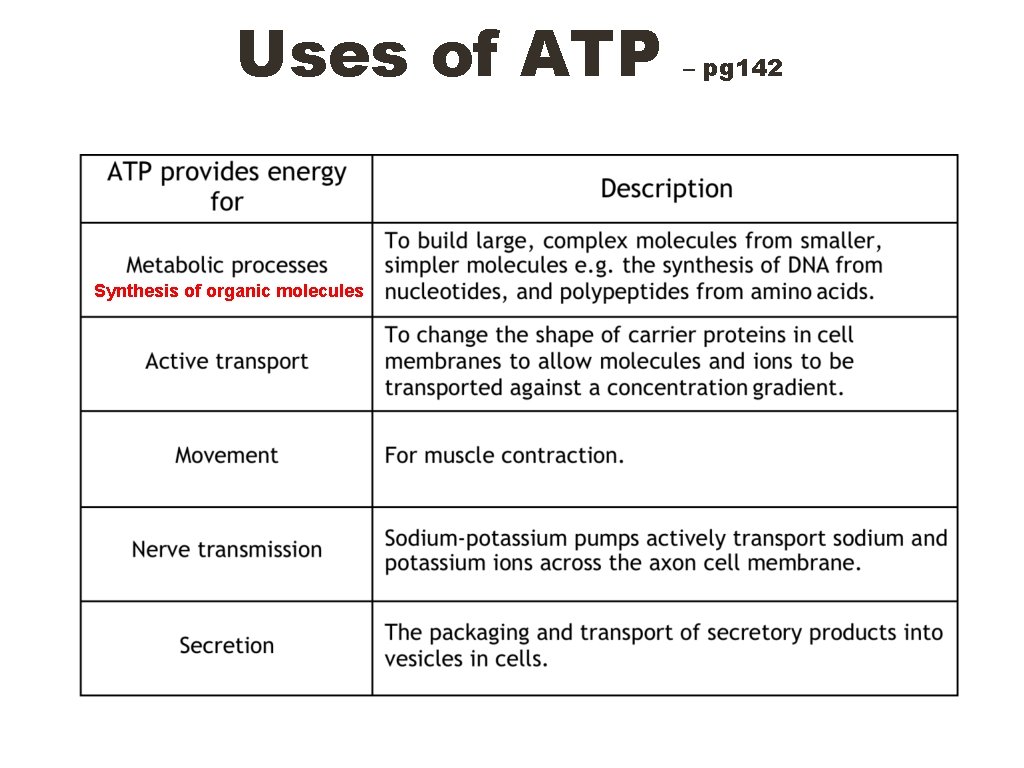
Uses of ATP Synthesis of organic molecules – pg 142

Advantages of using ATP – pg 142 The hydrolysis of ATP to ADP involves a single reaction that releases immediate energy. The breakdown of glucose involves a number of intermediates and it takes much longer for the energy to be released. Only one enzyme (ATPase) is needed to release energy from ATP, while many are needed in the case of glucose. ATP releases energy in small useable amounts when and where needed, whereas glucose contains large amounts of energy that may not be needed immediately. ATP is soluble and easily transported e. g. from companion cell to sieve element in phloem. ATP provides a common source of energy for many different chemical reactions, increasing efficiency and control by the cell. ATP is the universal intermediary molecule between energy-yielding and energy-requiring reactions in the cell.

Remember Energy Facts • The ‘Law of conservation of energy’ states that energy: • Cannot be created or destroyed • Can be converted from one form to another • When we refer to ATP and energy we must state that ATP is hydrolysed to release energy.

Think!! Summary questions 1) What type of base is adenine? 2) ATP is a nucleotide • Is it deoxyribose or ribose sugar? • Give reasons for your answer 3) Give 3 examples of cellular activities that require ATP. 4) Describe three advantages of ATP for its function as the universal source of energy

1) What type of base is adenine? Nitrogenous organic base 2) ATP is a nucleic acid / nucleotide derivative. • • Is it derived from DNA or RNA nucleotides? RNA nucleotides because it contains ribose sugar not deoxyribose sugar. 3) Give 3 examples of cellular activities that require ATP – active transport, muscle contraction, synthesis of organic molecules e. g. proteins, mitosis/meiosis.

4) Describe three advantages of ATP for its function as the universal source of energy. • ATP is soluble so it diffuses easily across cell membranes which means it can be easily transported around the cell. • ATP is an immediate source of energy as only one type of enzyme is needed to hydrolyse it • ATP releases energy in useable amounts that are matched closely to the energy required in a coupled reaction.