Nucleic Acid Amplification Tests for the Diagnosis of




- Slides: 15

Nucleic Acid Amplification Tests for the Diagnosis of Chlamydia trachomatis Rectal Infections Bachmann LH 1, 2, Johnson R 3, Cheng H 1, Markowitz L 3, Papp JR 3, Burkett T 1, Hook III EW 1 1 The University of Alabama at Birmingham, AL; 2 Birmingham VA Medical Center, Birmingham, AL; 3 Centers for Disease Control and Prevention, Atlanta, GA

Background • Routine testing for C. trachomatis at exposed extra-genital sites is recommended • Optimal methods for the diagnosis of rectal C. trachomatis infection are unclear • Nucleic acid amplification tests (NAATS) hold promise, though performance at the rectal site has not been well defined

Objectives • To study the performance of NAATs currently marketed in the U. S. for the diagnosis of rectal chlamydial infection • To develop a database which can be used with multiple analytic approaches

Methods • Males and females >15 years old who acknowledged receptive anal sex within the prior 2 months • Females >15 years old presenting as a contact to gonorrhea, chlamydia or NGU or with untreated gonococcal or chlamydial infection

Exclusion criteria • History of antibiotic agents active against N. gonorrhoeae or C. trachomatis within prior 30 days • Inability or unwillingness to provide informed consent

Methods • Tests performed included: – C. trachomatis culture – Gen-Probe APTIMA Combo 2® Assay (TMA; Gen-Probe Inc. , San Diego, CA) – Roche COBAS AMPLICOR™ (PCR; Roche Diagnostics Systems Inc. , Pleasanton, CA) – BDProbe. Tec™ ET Amplified DNA Assay (SDA; Becton, Dickinson and Co. , Sparks, MD)

Methods • 4 swabs were collected from the rectum – Separate swabs for GP, BD, GC culture and CT culture. – PCR performed from CT culture transport media • Swab order rotated every 3 months

Inclusion Rules: Current Analysis • Initial visit • Subsequent visit > 30 days after last visit if initial visit test results – or > 30 days after treatment for positive test result • Set must be complete • Result must be positive or negative

Current Analysis: Definition of Infection • Rotating • Any 2 of 3 positive tests was considered a “true positive”

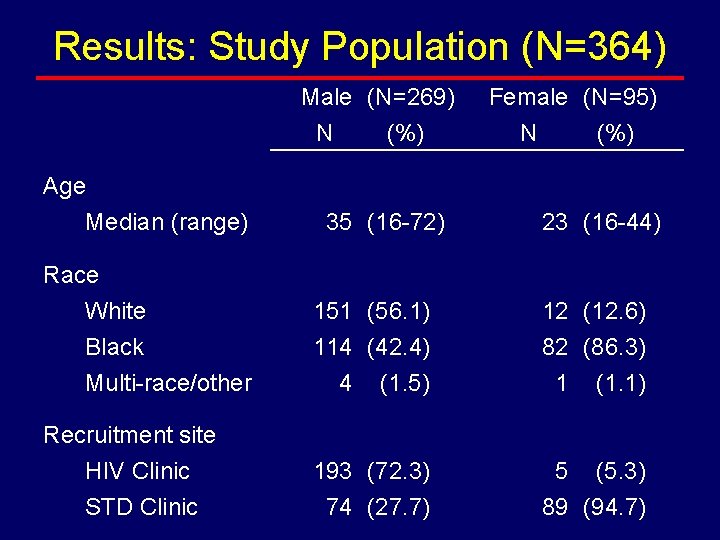
Results: Study Population (N=364) Male (N=269) N (%) Age Median (range) 35 (16 -72) Female (N=95) N (%) 23 (16 -44) Race White Black Multi-race/other 151 (56. 1) 114 (42. 4) 4 (1. 5) 12 (12. 6) 82 (86. 3) 1 (1. 1) Recruitment site HIV Clinic STD Clinic 193 (72. 3) 74 (27. 7) 5 (5. 3) 89 (94. 7)

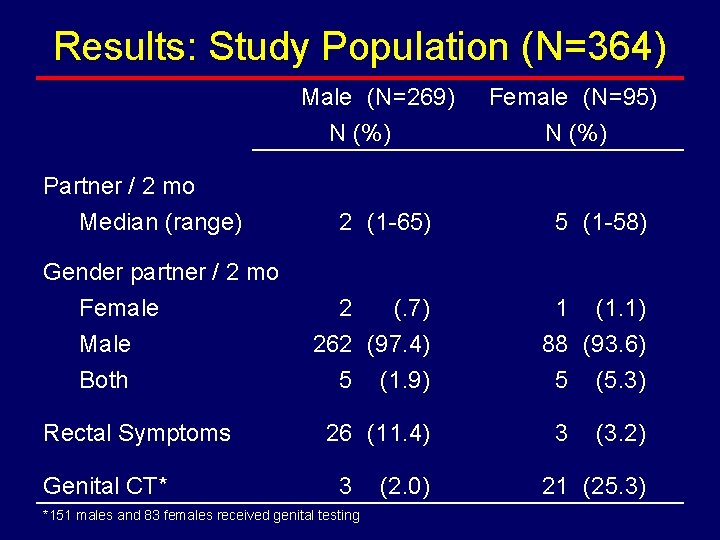
Results: Study Population (N=364) Male (N=269) N (%) Partner / 2 mo Median (range) Gender partner / 2 mo Female Male Both Rectal Symptoms Genital CT* Female (N=95) N (%) 2 (1 -65) 5 (1 -58) 2 (. 7) 262 (97. 4) 5 (1. 9) 1 (1. 1) 88 (93. 6) 5 (5. 3) 26 (11. 4) 3 *151 males and 83 females received genital testing (2. 0) 3 (3. 2) 21 (25. 3)

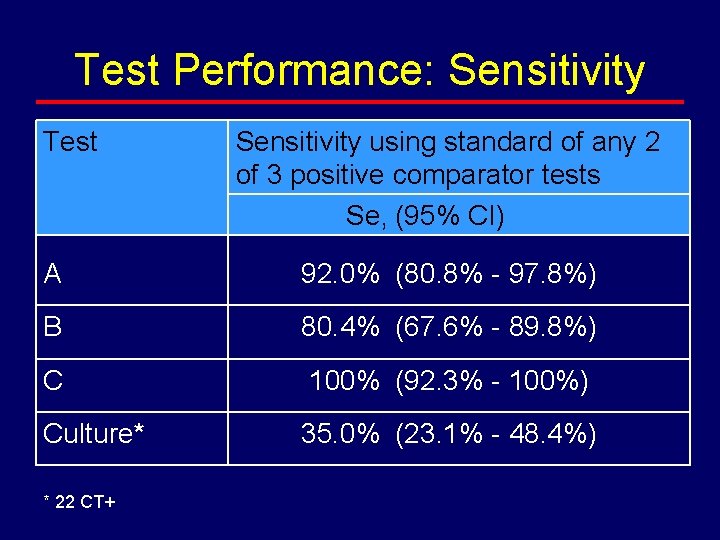
Test Performance: Sensitivity Test Sensitivity using standard of any 2 of 3 positive comparator tests Se, (95% CI) A 92. 0% (80. 8% - 97. 8%) B 80. 4% (67. 6% - 89. 8%) C 100% (92. 3% - 100%) Culture* 35. 0% (23. 1% - 48. 4%) * 22 CT+

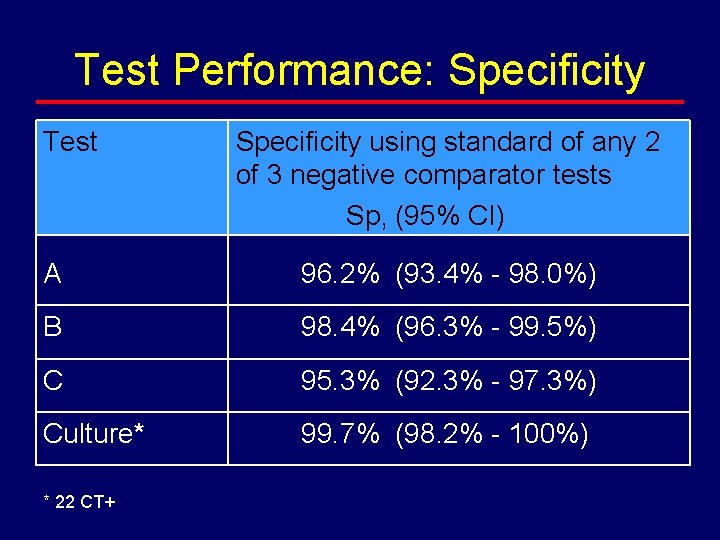
Test Performance: Specificity Test Specificity using standard of any 2 of 3 negative comparator tests Sp, (95% CI) A 96. 2% (93. 4% - 98. 0%) B 98. 4% (96. 3% - 99. 5%) C 95. 3% (92. 3% - 97. 3%) Culture* 99. 7% (98. 2% - 100%) * 22 CT+

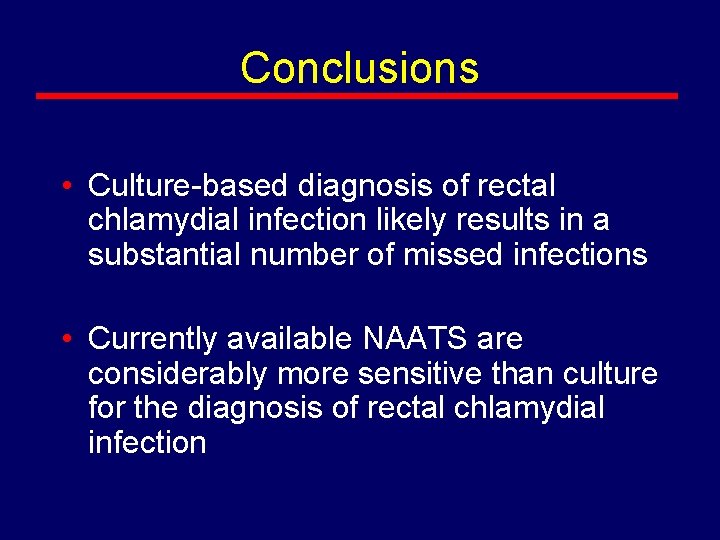
Conclusions • Culture-based diagnosis of rectal chlamydial infection likely results in a substantial number of missed infections • Currently available NAATS are considerably more sensitive than culture for the diagnosis of rectal chlamydial infection

Conclusions (cont) • The specificity of NAATS for the diagnosis of rectal chlamydial infection, while less than culture, is acceptable • NAATS hold promise as a public health tool for the diagnosis and control of rectal chlamydial infection