Nucleation Growth All phase transformations can be described

Nucleation & Growth • All phase transformations can be described as nucleation & growth processes. • In order to understand, predict and engineer phase transformations, we must understand the driving force, the kinetics and the mechanisms of transformations. Driving Force • Example: solidification: as you cool a liquid below the liquidus, so the driving force for solidification increases. This driving force is often called undercooling or Supercooling. • Example: phase change: for a pure substance with more than one allotrope, as you cool it below the phase transformation temperature, so the driving force for the phase change increases.
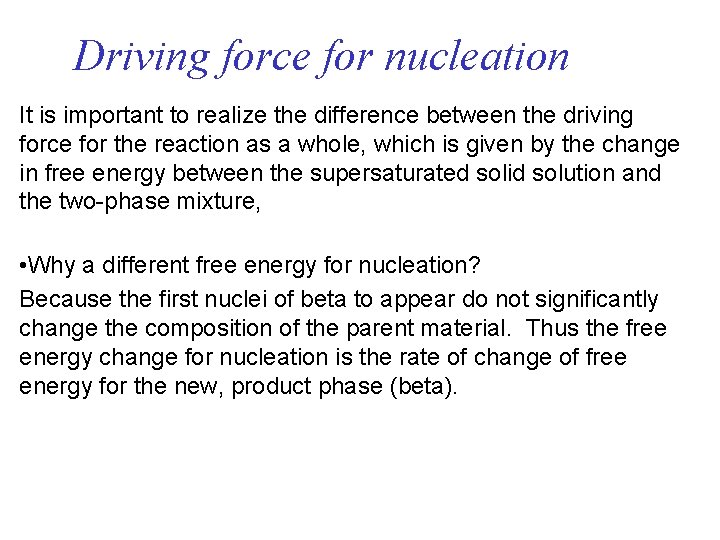
Driving force for nucleation It is important to realize the difference between the driving force for the reaction as a whole, which is given by the change in free energy between the supersaturated solid solution and the two-phase mixture, • Why a different free energy for nucleation? Because the first nuclei of beta to appear do not significantly change the composition of the parent material. Thus the free energy change for nucleation is the rate of change of free energy for the new, product phase (beta).
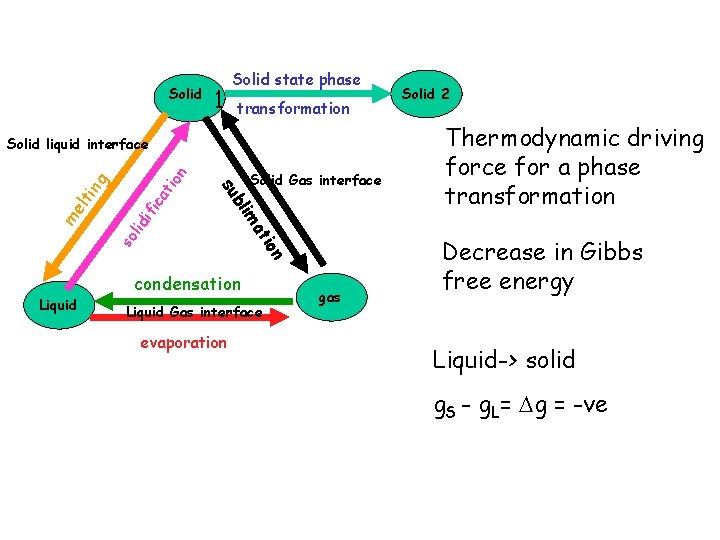
Solid state phase 1 transformation so lid ifi ca t g lti n n tio ma me Solid Gas interface bli su ion Solid liquid interface Liquid condensation Liquid Gas interface evaporation gas Solid 2 Thermodynamic driving force for a phase transformation Decrease in Gibbs free energy Liquid-> solid g. S - g. L= g = -ve
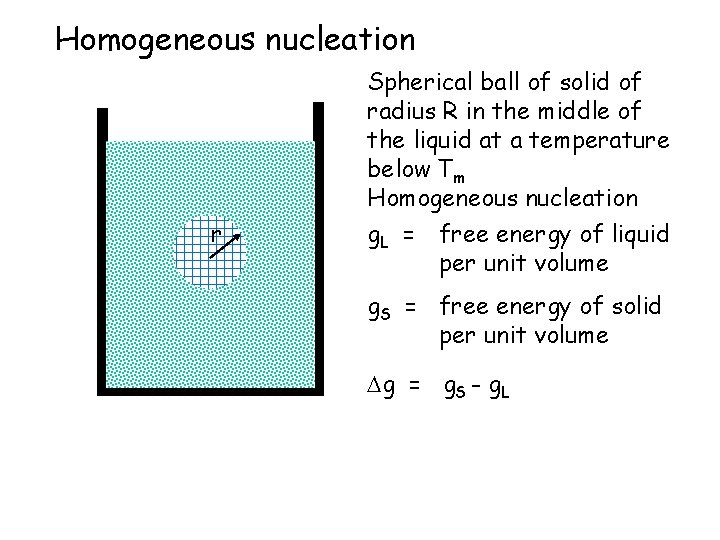
Homogeneous nucleation r Spherical ball of solid of radius R in the middle of the liquid at a temperature below Tm Homogeneous nucleation g. L = free energy of liquid per unit volume g. S = free energy of solid per unit volume g = g. S - g. L
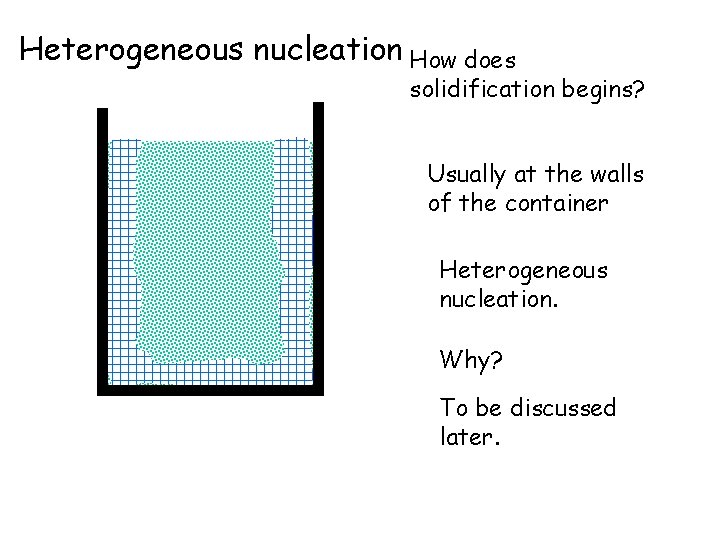
Heterogeneous nucleation How does solidification begins? Usually at the walls of the container Heterogeneous nucleation. Why? To be discussed later.
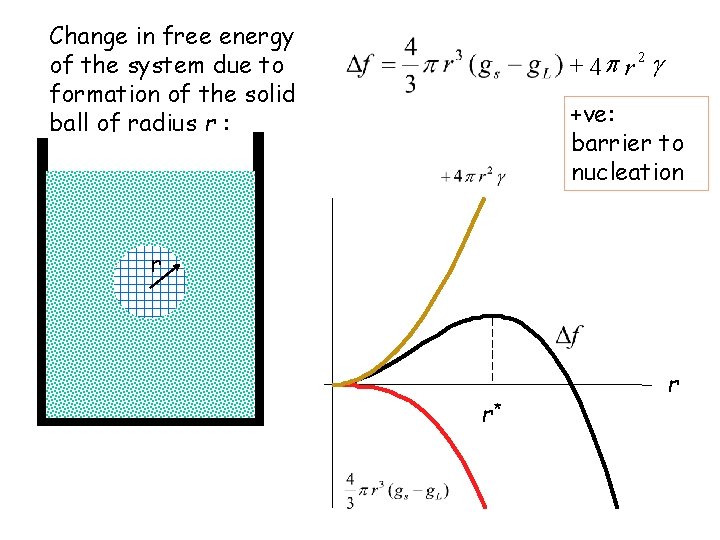
Change in free energy of the system due to formation of the solid ball of radius r : + 4 p r 2 g +ve: barrier to nucleation r r* r
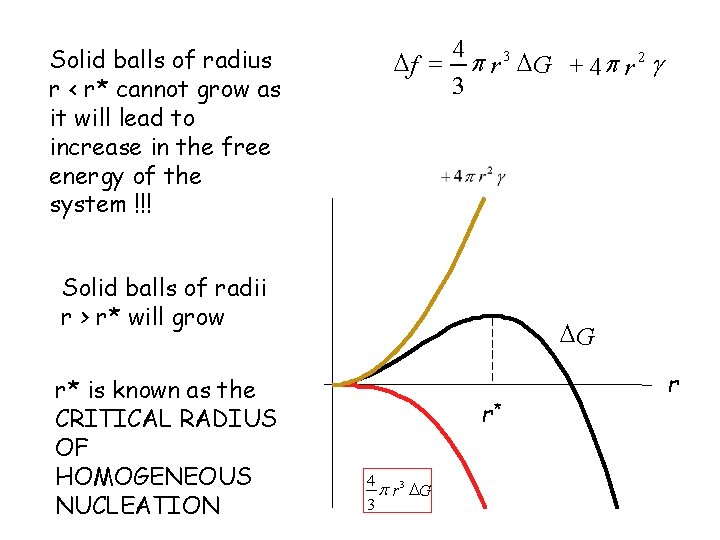
Solid balls of radius r < r* cannot grow as it will lead to increase in the free energy of the system !!! 4 f = p r 3 G + 4 p r 2 g 3 Solid balls of radii r > r* will grow r* is known as the CRITICAL RADIUS OF HOMOGENEOUS NUCLEATION G r* 4 3 p r G 3 r
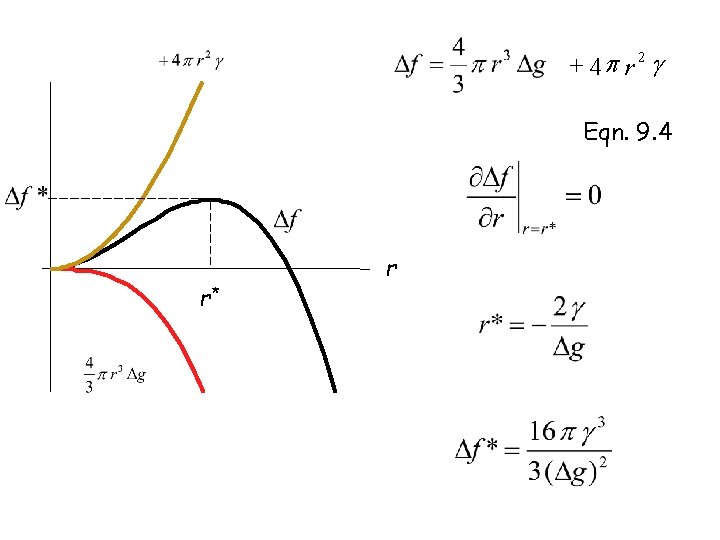
+ 4 p r 2 g Eqn. 9. 4 r* r
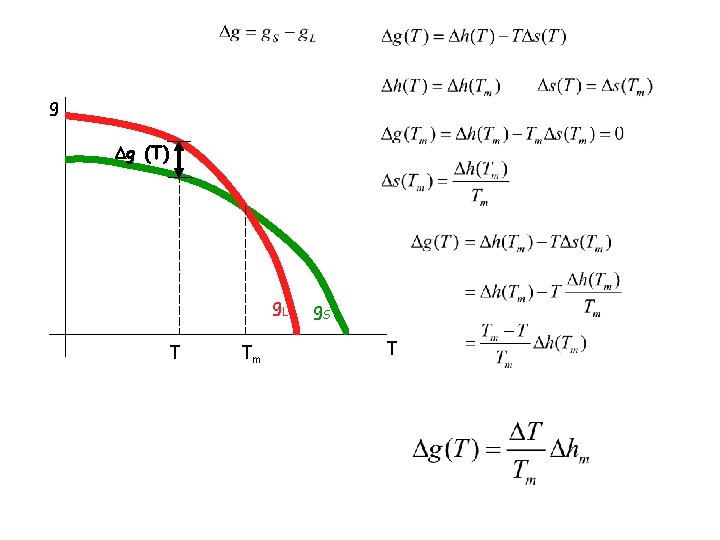
g g (T) g. L T Tm g. S T
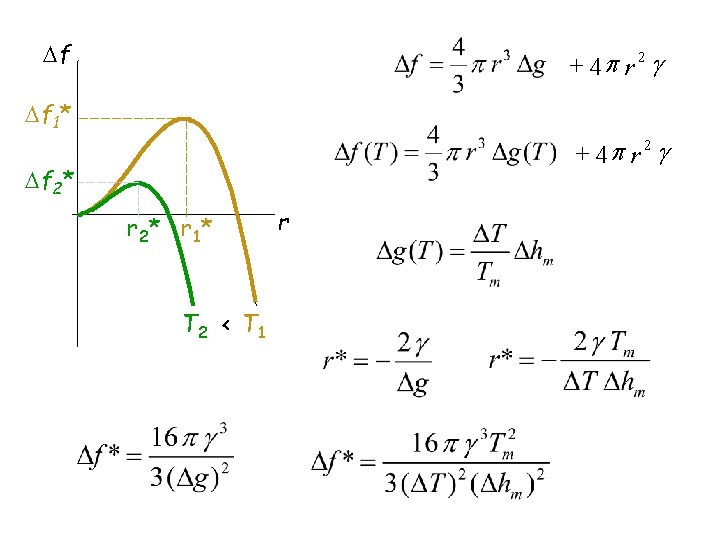
f + 4 p r 2 g f 1* + 4 p r 2 g f 2* r 2 * r 1* T 2 < T 1 r
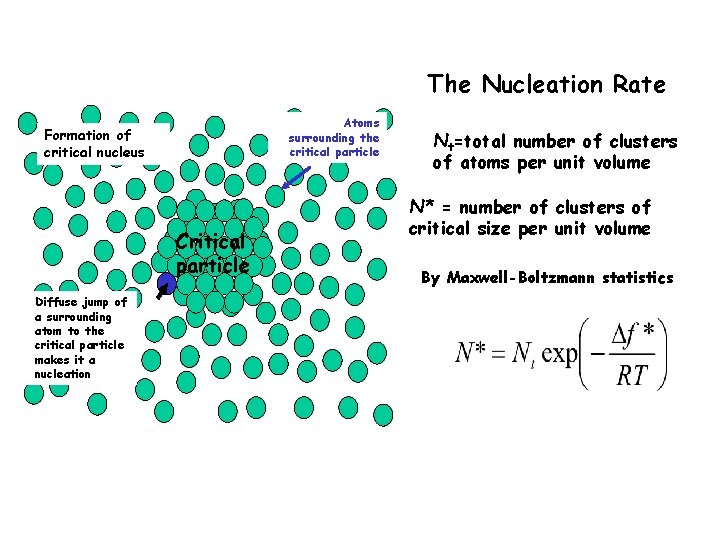
The Nucleation Rate Atoms surrounding the critical particle Formation of critical nucleus Critical particle Diffuse jump of a surrounding atom to the critical particle makes it a nucleation Nt=total number of clusters of atoms per unit volume N* = number of clusters of critical size per unit volume By Maxwell-Boltzmann statistics
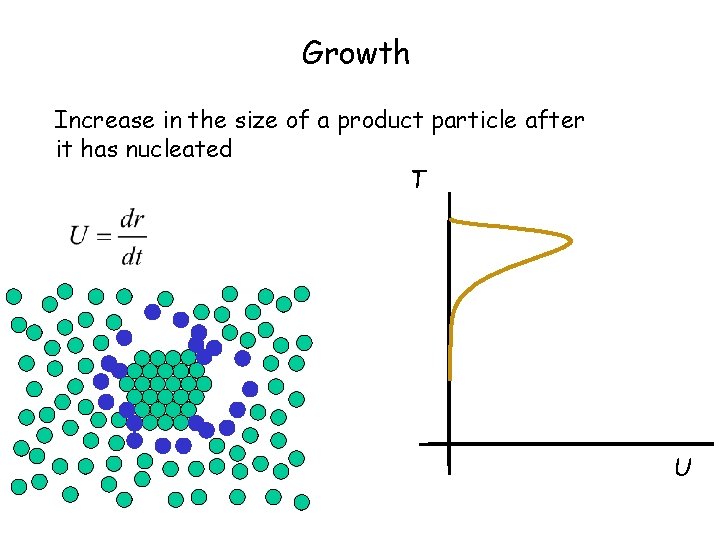
Growth Increase in the size of a product particle after it has nucleated T U
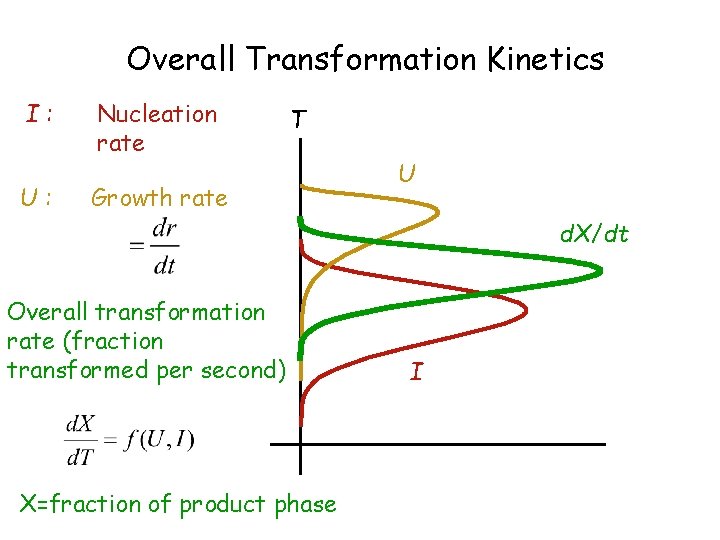
Overall Transformation Kinetics I: U: Nucleation rate T Growth rate U d. X/dt Overall transformation rate (fraction transformed per second) X=fraction of product phase I
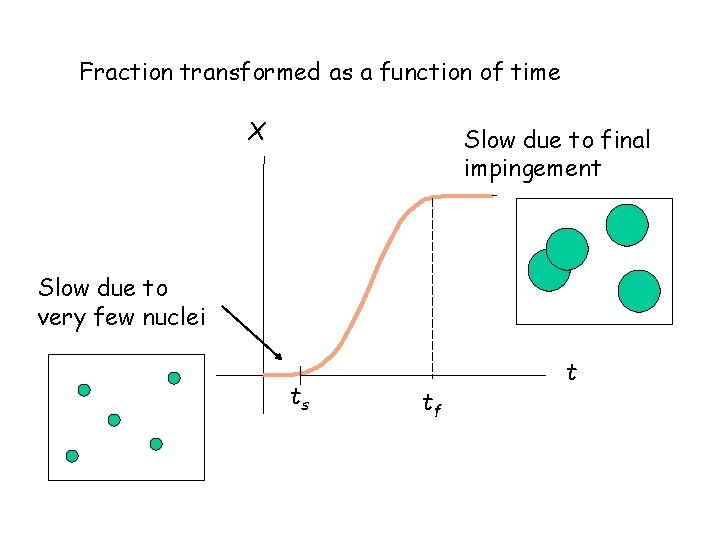
Fraction transformed as a function of time X Slow due to final impingement Slow due to very few nuclei ts tf t
- Slides: 14