Nuclear Stability Radioactive Decay Notation for a nuclide

Nuclear Stability & Radioactive Decay
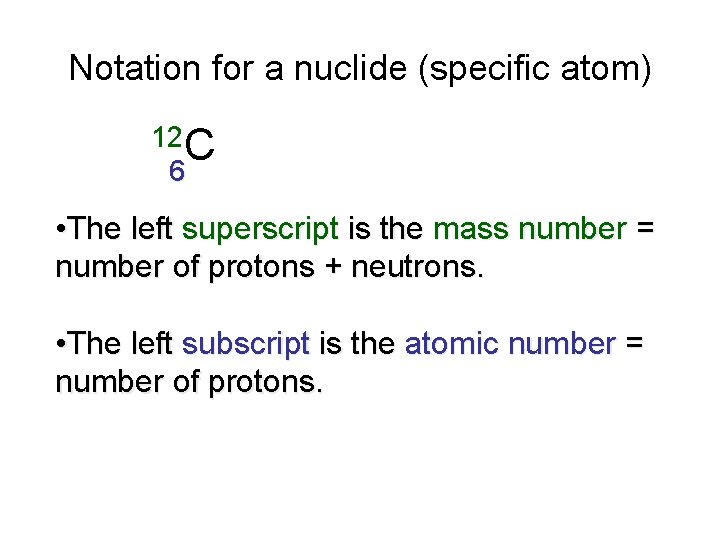
Notation for a nuclide (specific atom) 12 C 6 • The left superscript is the mass number = number of protons + neutrons. • The left subscript is the atomic number = number of protons.
Isotopes • Atoms with identical atomic numbers but different mass numbers. • (Two nuclides can have different atomic numbers and different mass numbers. )

Nuclear Stability • Determined by neutron/proton ratio. – All nuclides with 84 or more protons are unstable. – Light elements (up to atomic number 20): like a neutron/proton ratio of 1. – For heavier elements, the neutron/proton ratio required for stability > 1, and increases as atomic number increases. – Of 2000 known nuclides, only 279 are stable with respect to radioactive decay.
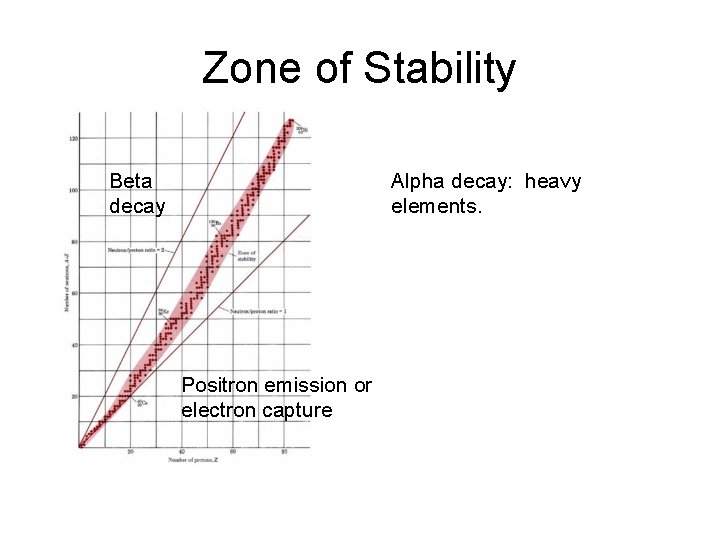
Zone of Stability Beta decay Alpha decay: heavy elements. Positron emission or electron capture
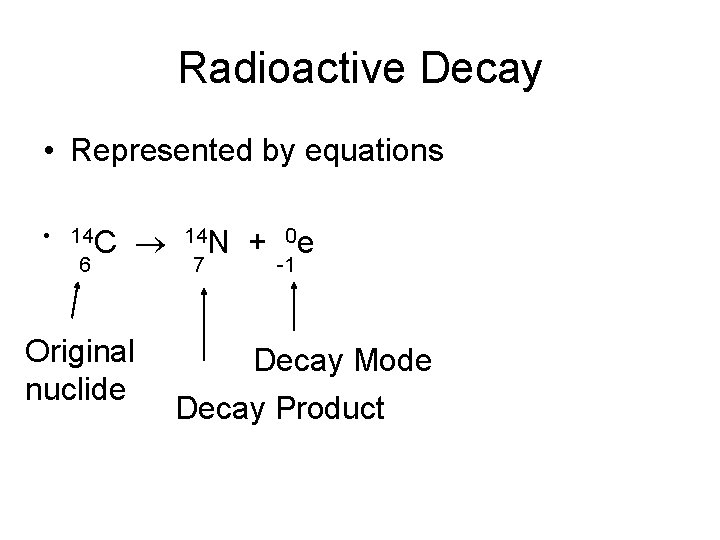
Radioactive Decay • Represented by equations • 14 C 6 Original nuclide 14 N 7 + 0 e -1 Decay Mode Decay Product
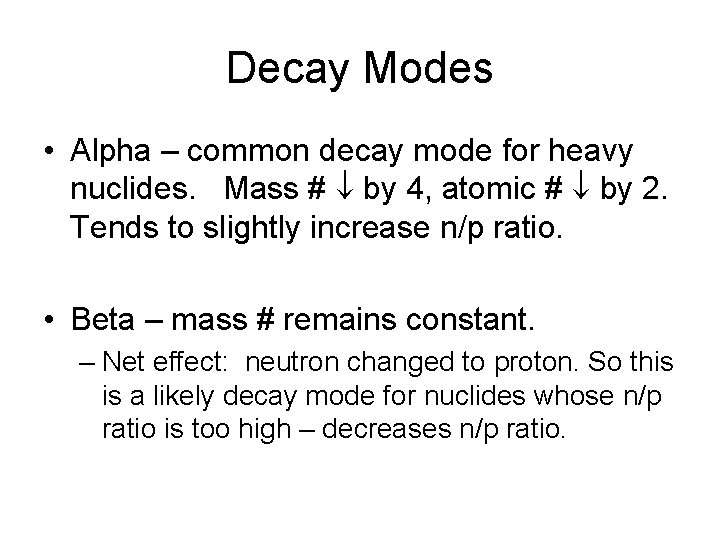
Decay Modes • Alpha – common decay mode for heavy nuclides. Mass # by 4, atomic # by 2. Tends to slightly increase n/p ratio. • Beta – mass # remains constant. – Net effect: neutron changed to proton. So this is a likely decay mode for nuclides whose n/p ratio is too high – decreases n/p ratio.
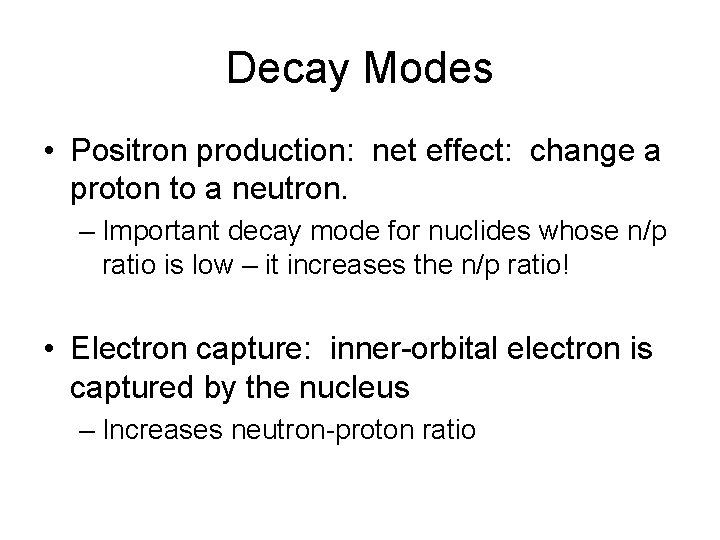
Decay Modes • Positron production: net effect: change a proton to a neutron. – Important decay mode for nuclides whose n/p ratio is low – it increases the n/p ratio! • Electron capture: inner-orbital electron is captured by the nucleus – Increases neutron-proton ratio
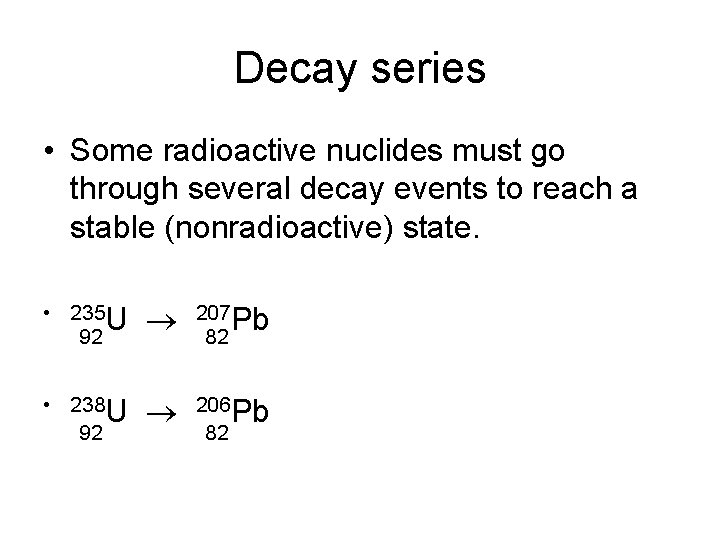
Decay series • Some radioactive nuclides must go through several decay events to reach a stable (nonradioactive) state. • 235 U 92 207 Pb 82 • 238 U 92 206 Pb 82
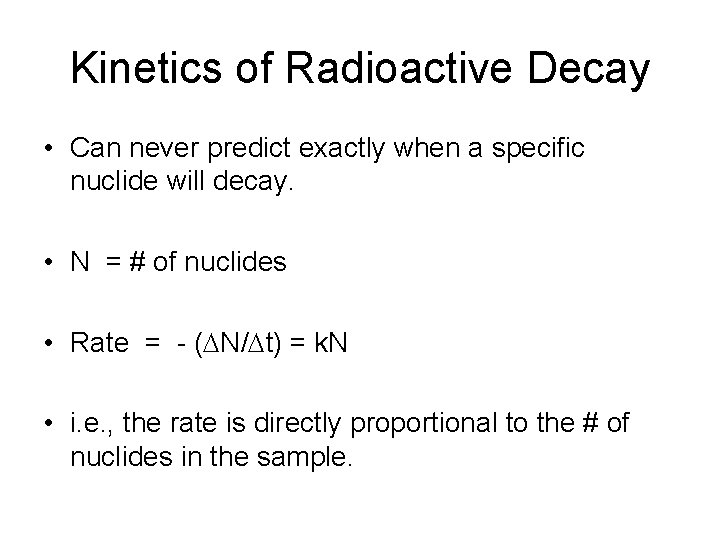
Kinetics of Radioactive Decay • Can never predict exactly when a specific nuclide will decay. • N = # of nuclides • Rate = - ( N/ t) = k. N • i. e. , the rate is directly proportional to the # of nuclides in the sample.
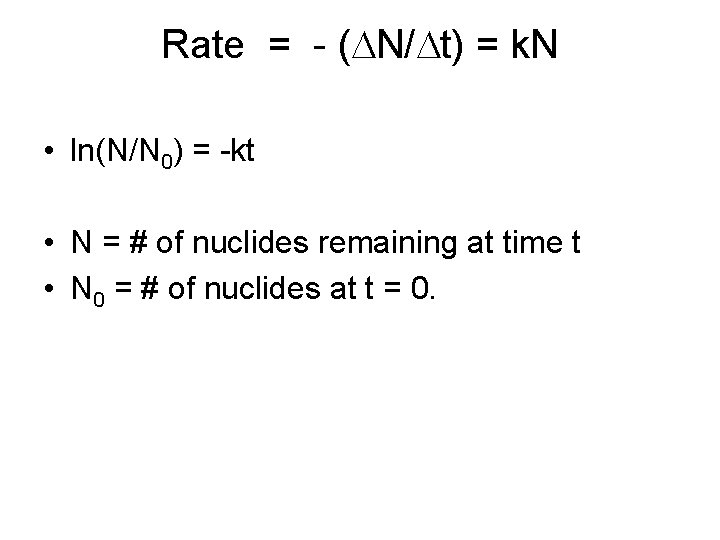
Rate = - ( N/ t) = k. N • ln(N/N 0) = -kt • N = # of nuclides remaining at time t • N 0 = # of nuclides at t = 0.
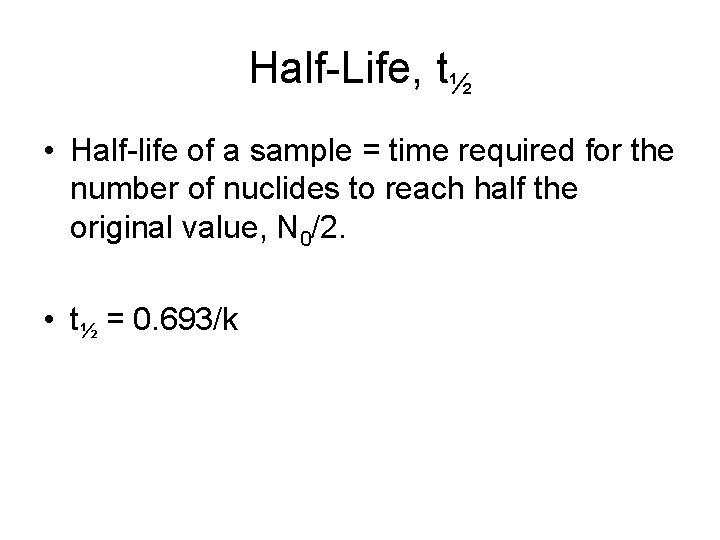
Half-Life, t½ • Half-life of a sample = time required for the number of nuclides to reach half the original value, N 0/2. • t½ = 0. 693/k
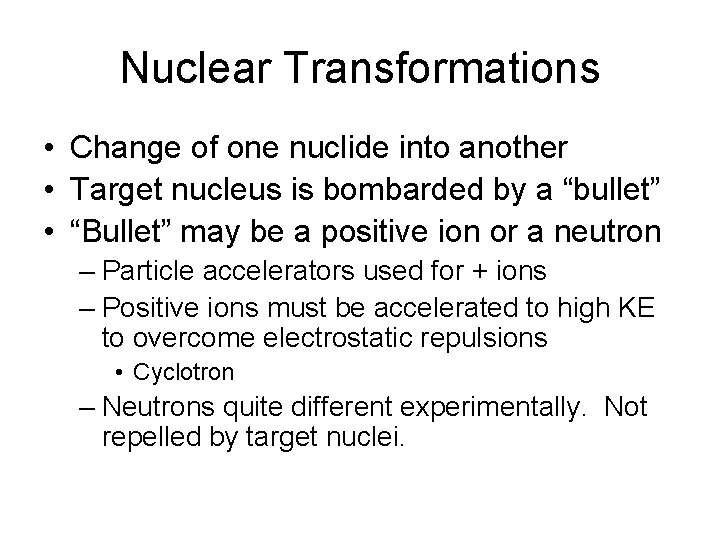
Nuclear Transformations • Change of one nuclide into another • Target nucleus is bombarded by a “bullet” • “Bullet” may be a positive ion or a neutron – Particle accelerators used for + ions – Positive ions must be accelerated to high KE to overcome electrostatic repulsions • Cyclotron – Neutrons quite different experimentally. Not repelled by target nuclei.
Transuranium Elements • Elements 93 – 1** have been synthesized.
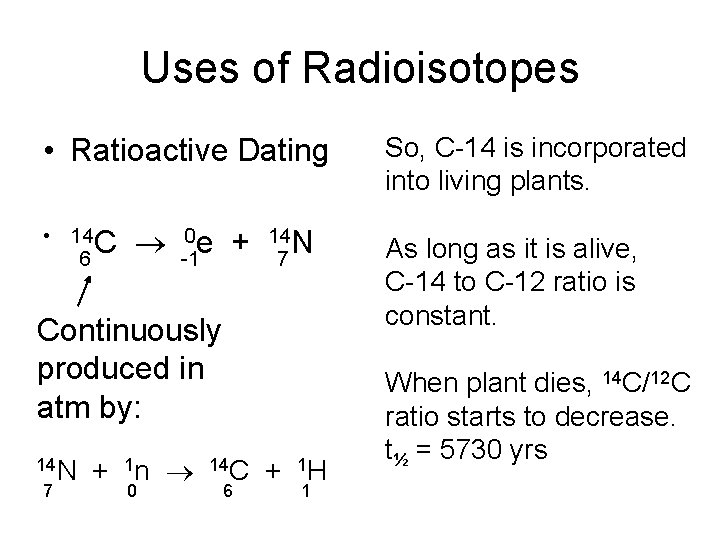
Uses of Radioisotopes • Ratioactive Dating • 14 C 6 0 e + -1 14 N 7 Continuously produced in atm by: 14 N 7 + 1 n 0 14 C 6 + 1 H 1 So, C-14 is incorporated into living plants. As long as it is alive, C-14 to C-12 ratio is constant. When plant dies, 14 C/12 C ratio starts to decrease. t½ = 5730 yrs
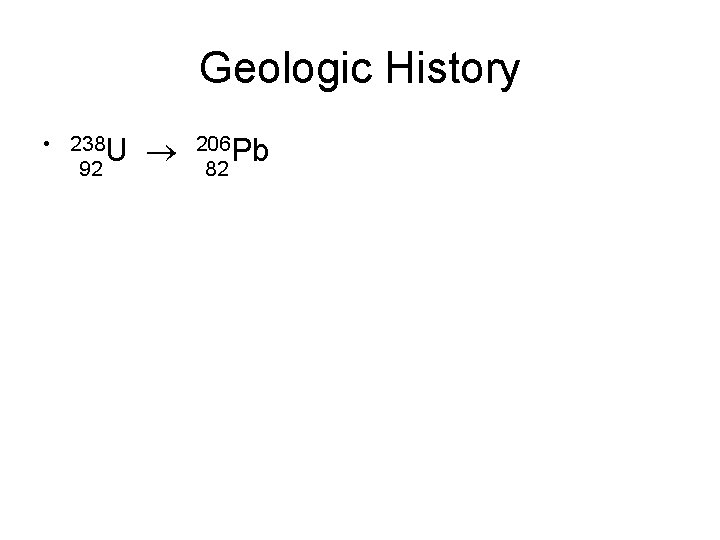
Geologic History • 238 U 92 206 Pb 82

Medical Applications • Radiotracers/Diagnosis – Radioactive nuclide whose pathway in an organism can be traced by monitoring its radioactivity. – I-131 thyroid – Th-201 heart • Treatment
Thermodynamic Stability of Nucleus • Mass of a nucleus is always less than the sum of the masses of the protons and neutrons that make up the nucleus. • This difference is a measure of the binding energy • Binding energy = energy released when nucleus is formed.
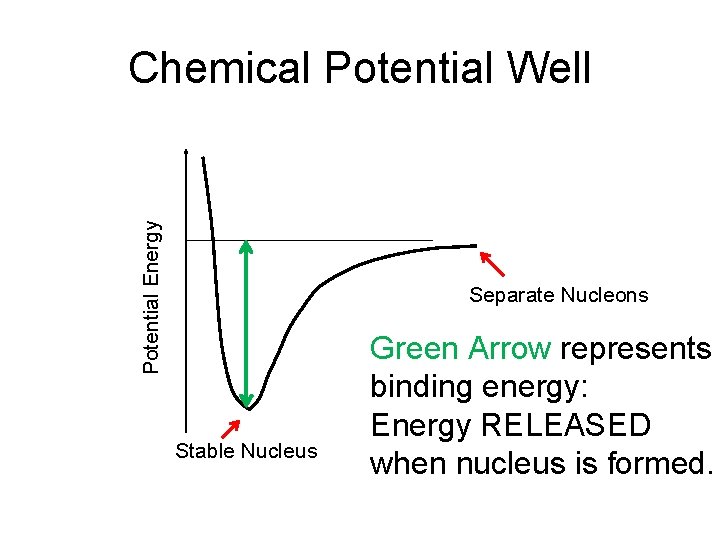
Potential Energy Chemical Potential Well Separate Nucleons Stable Nucleus Green Arrow represents binding energy: Energy RELEASED when nucleus is formed.

Mass Defect for O-16 • 8 p: (8 X 1. 007276 amu) = 8. 058208 amu • 8 n: (8 X 1. 008665 amu) = 8. 06932 amu • 8 e: (8 X 0. 0005486 amu) = 0. 004389 amu • • Total combined mass = 16. 125789 Atomic mass of 0 -16 = 15. 994915 amu m = 0. 130874 amu Use 1 amu = 1. 66054 X 10 -27 kg
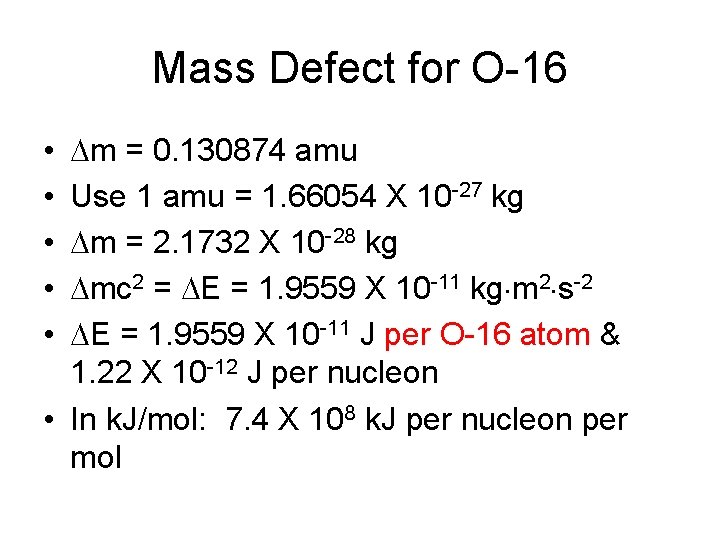
Mass Defect for O-16 m = 0. 130874 amu Use 1 amu = 1. 66054 X 10 -27 kg m = 2. 1732 X 10 -28 kg mc 2 = E = 1. 9559 X 10 -11 kg m 2 s-2 E = 1. 9559 X 10 -11 J per O-16 atom & 1. 22 X 10 -12 J per nucleon • In k. J/mol: 7. 4 X 108 k. J per nucleon per mol • • •
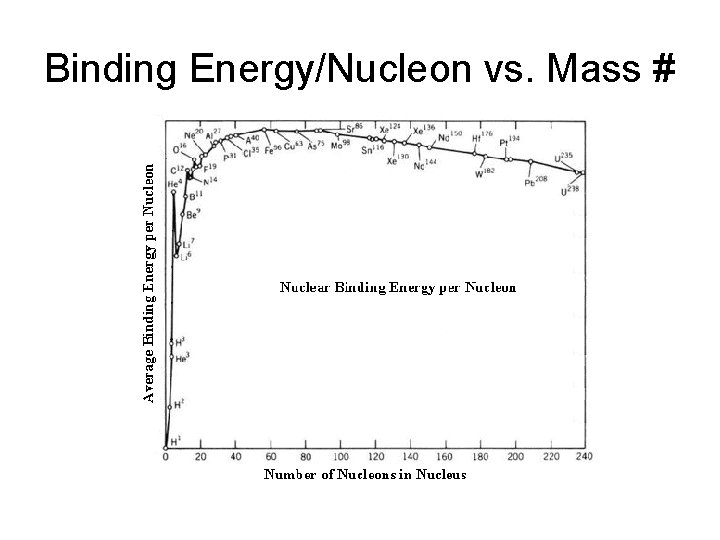
Binding Energy/Nucleon vs. Mass #

Units of binding energy • Chemists use k. J/(mol nucleon) • Physics uses a different unit: Mev

Nuclear Fission • Splitting a heavy nucleus into two smaller nuclei. • U-235 and Pu-239 are fissionable fuels • Reaction initiated by a neutron • Many, many possible products

Nuclear Fusion • Two light nuclei combine to form a heavier, more stable nucleus. • Occurs in the stars. • Sun: 73% H, 26% He, and 1% other • Protons fuse to form He
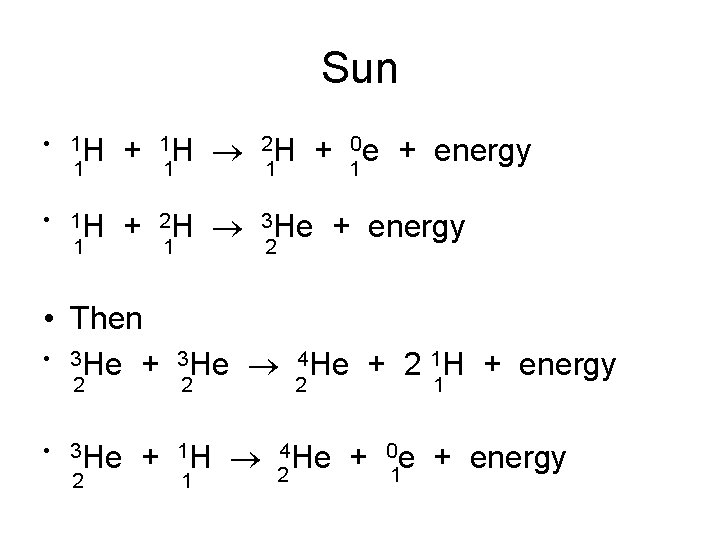
Sun • 1 H 1 + 11 H 21 H + 10 e + energy • 1 H 1 + 2 H 3 He + energy 1 2 • Then • 3 He + 3 He 4 He + 2 1 H + energy 2 • 3 He 2 2 2 1 + 1 H 24 He + 01 e + energy 1

Fusion vs. Fission as Energy Source

Nuclear vs. Ordinary Chemical & Physical Change • Nuclear transformations involve much larger energy changes than ordinary chemical & physical changes – Orders of magnitude larger
Risks of radionuclides • Somatic damage = damage to the organism itself, resulting in sickness or death • Genetic damage = damage to genes
- Slides: 29