Nuclear Reactions Natural Transmutation 1 term on reactant


















- Slides: 35

Nuclear Reactions

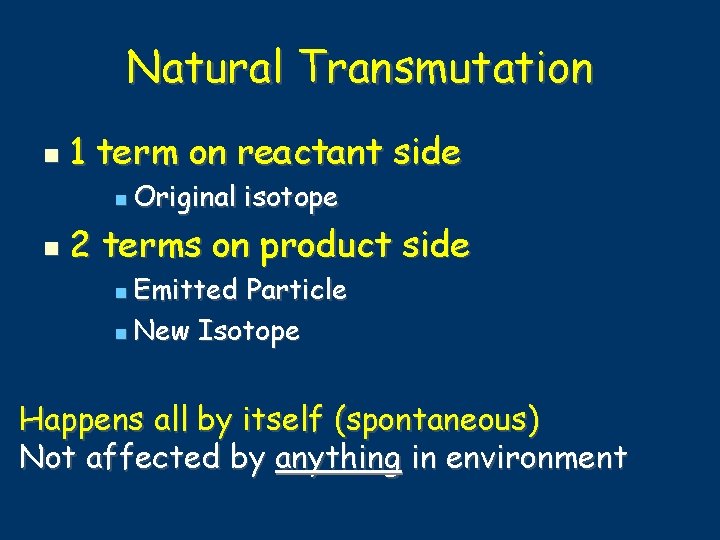
Natural Transmutation 1 term on reactant side Original isotope 2 terms on product side Emitted Particle New Isotope Happens all by itself (spontaneous) Not affected by anything in environment

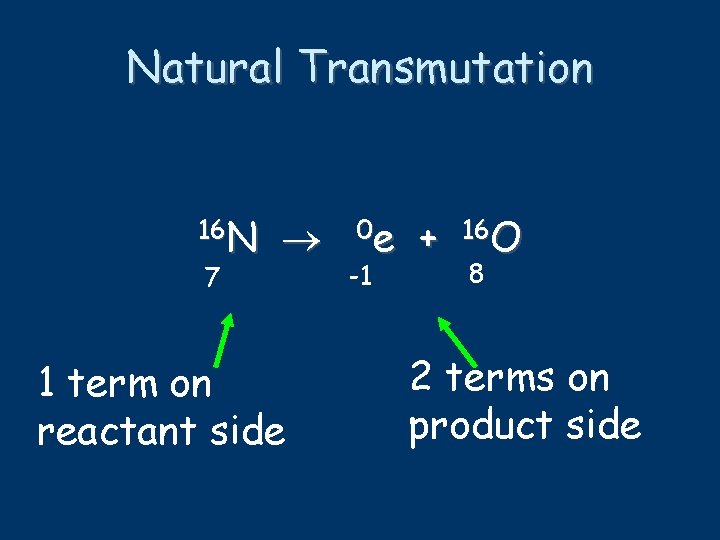
Natural Transmutation 16 N 7 1 term on reactant side 0 e -1 + 16 O 8 2 terms on product side

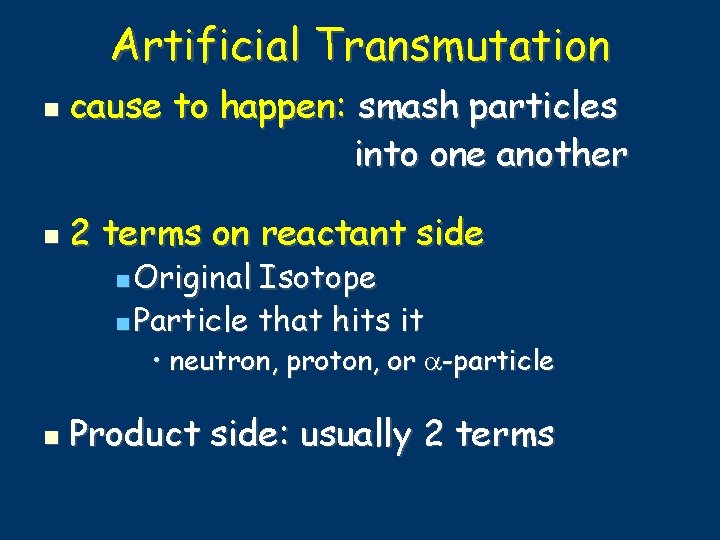
Artificial Transmutation cause to happen: smash particles into one another 2 terms on reactant side Original Isotope Particle that hits it • neutron, proton, or -particle Product side: usually 2 terms

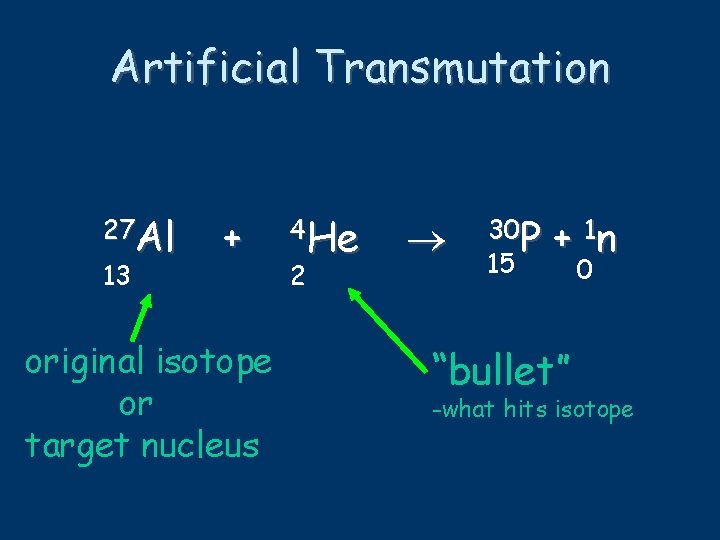
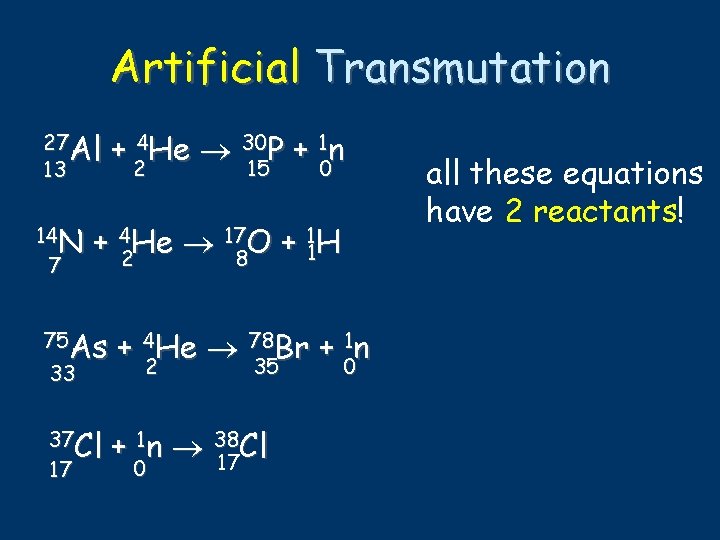
Artificial Transmutation 27 Al 13 + original isotope or target nucleus 4 He 2 30 P 15 + 1 n “bullet” 0 -what hits isotope

Artificial Transmutation 27 Al 13 14 N 7 + 4 He 30 P + 1 n 2 0 15 + 42 He 178 O + 11 H 75 As 33 + 4 He 78 Br + 1 n 2 0 35 37 Cl 17 + 1 n 38 Cl 17 0 all these equations have 2 reactants!

Bombarding with protons or protons & -particles have positive charge and mass • do some damage when hit target nucleus • must be accelerated to high speeds to overcome repulsive forces between nucleus & particle (both are +)

What is an accelerator? vacuum chamber (usually long pipe) • surrounded by vacuum pumps, magnets, radiofrequency cavities, high voltage instruments & electronic circuits inside pipe particles are accelerated to very high speeds then smashed into each other

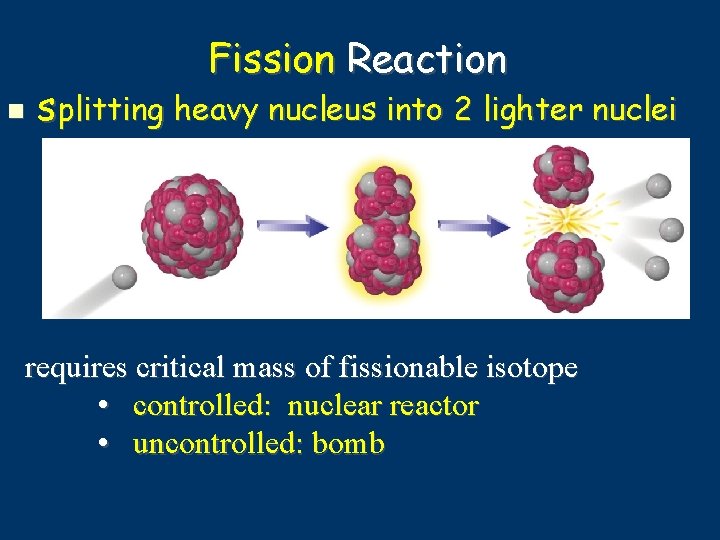
Fission Reaction splitting heavy nucleus into 2 lighter nuclei requires critical mass of fissionable isotope • controlled: nuclear reactor • uncontrolled: bomb

Fission q reactant side: 2 terms 1 heavy isotope (examples: U-235 or Pu-239) bombarding particle – usually a neutron product side: at least 2 terms 2 medium-weight isotopes 1 or more neutrons huge amount energy released Fission = Division

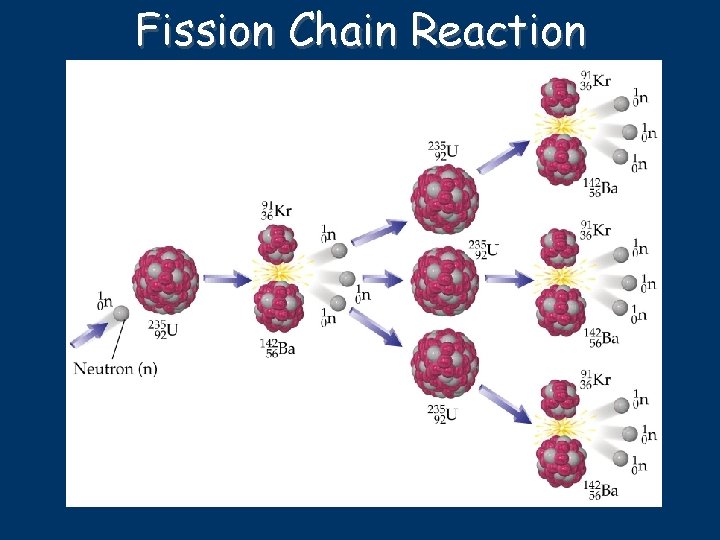
Fission Chain Reaction

Fission 235 U 92 + 1 n 91 Kr + 142 Ba + 31 n + energy 0 56 235 U 92 1 n + energy + 1 n 72 Zn + 160 Sm + 4 62 0 0 36 30 0 more than 200 different product isotopes identified from fission of U-235 small amount of mass is converted to energy according to E = mc 2

Fusion reactant side has 2 small nuclei: • H + H; H + He; He + He product side: • 1 nucleus (slightly larger; still small) and maybe a particle source of sun’s energy 2 nuclei unite 2 H 1 + 13 H 24 He + 10 n + energy

CERN 27 kilometer ring • particles travel just below speed of light • 10 hrs: particles make 400 million revolutions of ring

Fermi. Lab 4 miles in circumference!


Balancing Nuclear Equations

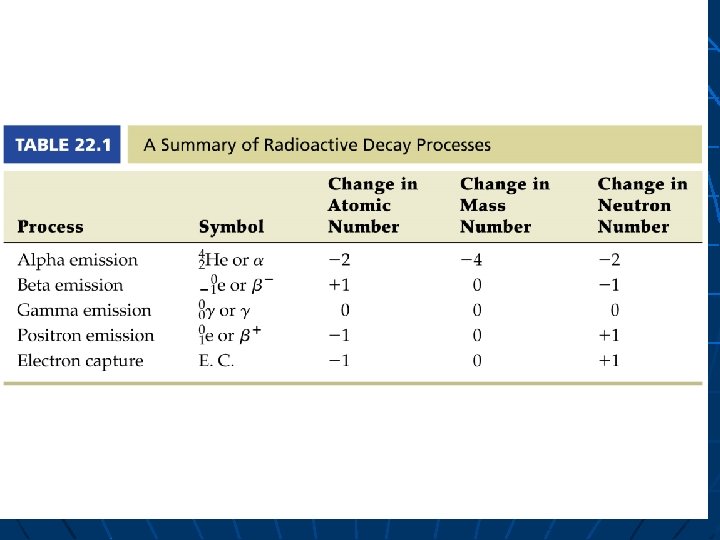
Nuclear Equations - tasks identify type (4 types) balance to find unknown term

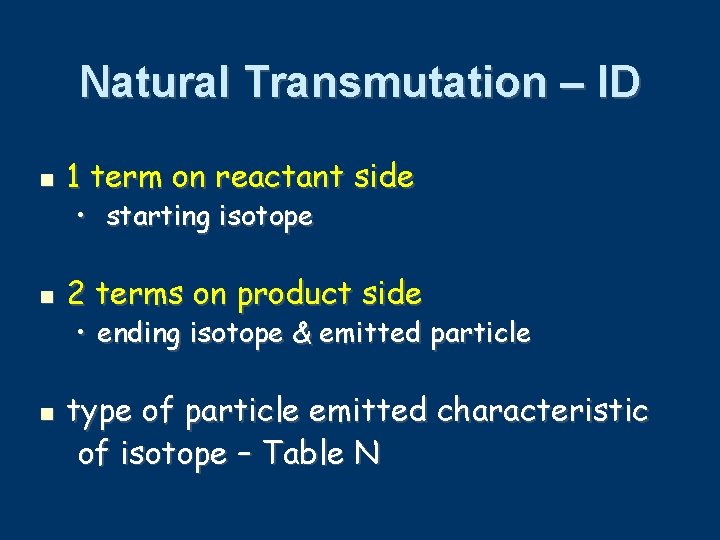
Natural Transmutation – ID 1 term on reactant side • starting isotope 2 terms on product side • ending isotope & emitted particle type of particle emitted characteristic of isotope – Table N

Nuclear Equations to balance: use conservation of both atomic number & mass number • mass number = left superscript • atomic number = left subscript

Balancing Nuclear Equations 16 N 7 0 e -1 + 16 O 8 conservation of mass number: 16 = 0 + 16 conservation of atomic number: 7 = -1 + 8

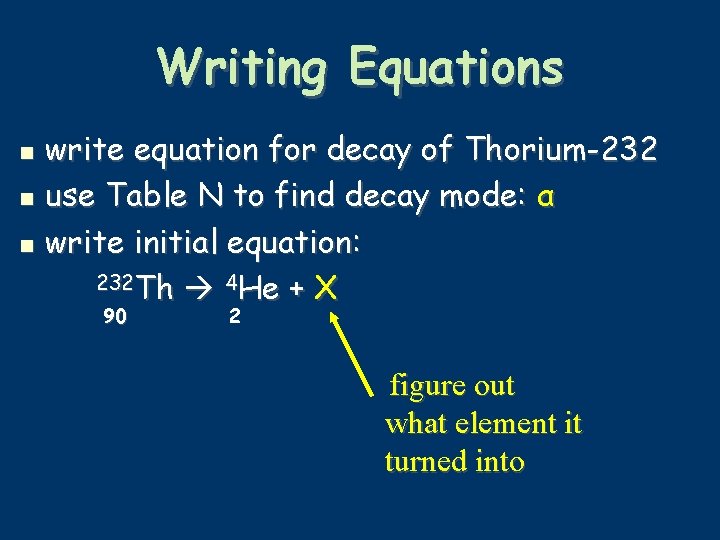
Writing Equations write equation for decay of Thorium-232 use Table N to find decay mode: α write initial equation: 232 Th 4 He + X 90 2 figure out what element it turned into

What’s under the hat? Little cats X, Y, & Z!

Write an equation for the α decay of Th-232 95 Th 4 He + YX what’s X? 2 Z

so Y = 228 232 = 4 + Y 232 Th 90 4 He 2 + Y Z X conservation of mass number: sum mass numbers on left side must = sum mass numbers on right side

232 Th 90 4 He + 228 X 2 90 = 2 + Z Z so Z = 88 conservation of atomic number: sum of atomic numbers on left side must = sum of atomic numbers on right side

232 Th 90 4 He + 228 X 2 88 X = Ra use PT to find X: 232 Th 90 4 He + 228 Ra 2 88

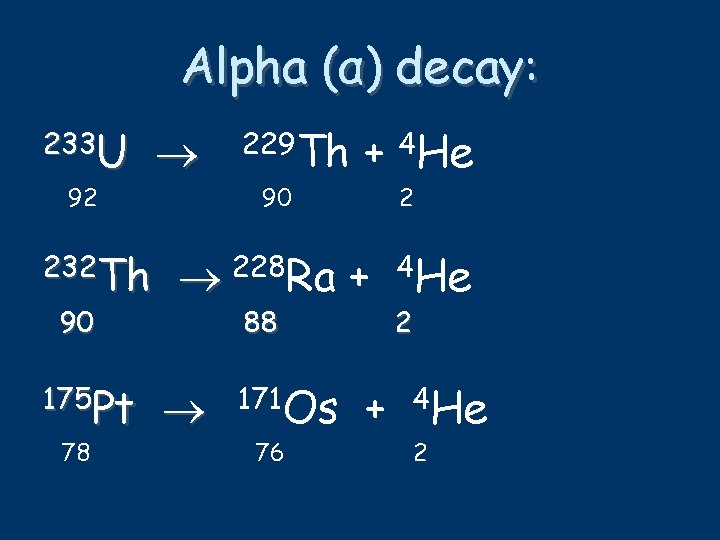
Alpha (α) decay: 233 U 92 232 Th 90 175 Pt 78 229 Th 90 + 4 He 2 228 Ra + 88 171 Os 76 + 4 He 2

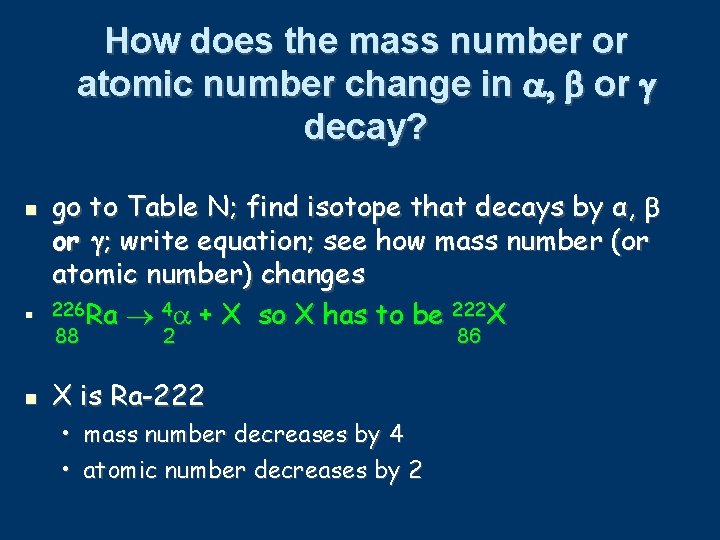
How does the mass number or atomic number change in α, β or γ decay? go to Table N: • • • find isotope that decays by α or β decay write equation see how mass number (or atomic number) changes 226 Ra 88 42 + X so X has to be 22286 X α decay of Ra-226: • mass number decreases by 4 • atomic number decreases by 2

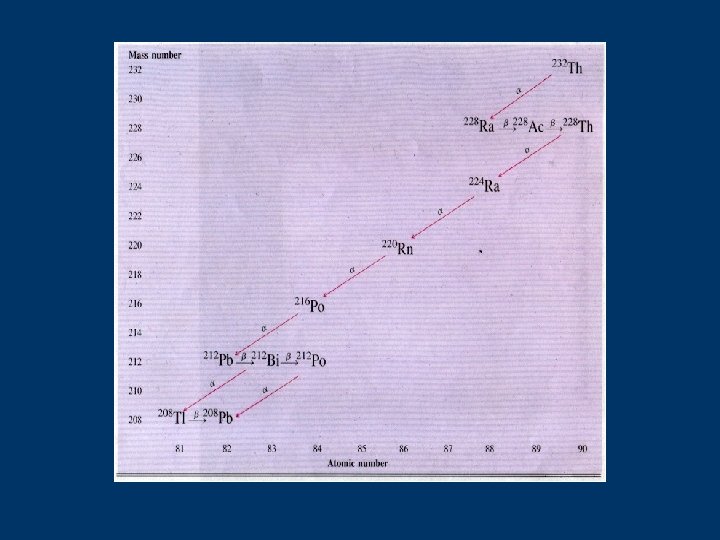
Radioactive Decay Series sometimes 1 transmutation isn’t enough to achieve stability some radioisotopes go through several changes before achieve stability (no longer radioactive)


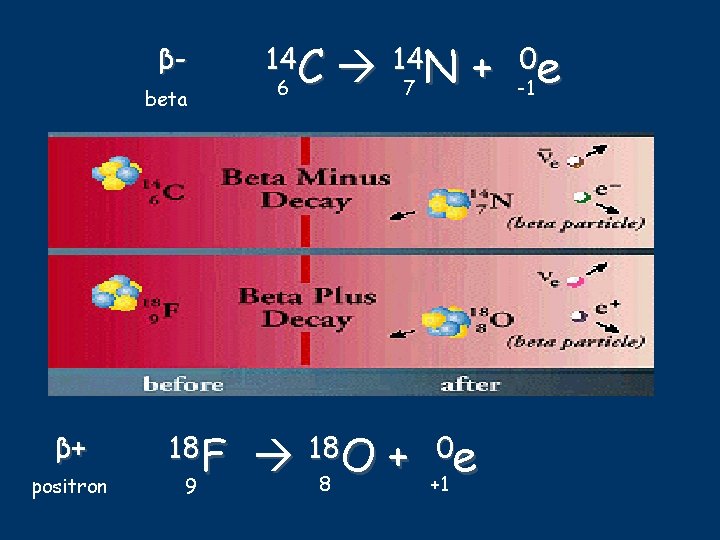
βbeta β+ positron 18 F 9 14 C 6 147 N + -10 e 188 O + +10 e

How does the mass number or atomic number change in or decay? go to Table N; find isotope that decays by α, or ; write equation; see how mass number (or atomic number) changes 226 Ra 4 + X so X has to be 222 X 88 2 X is Ra-222 • mass number decreases by 4 • atomic number decreases by 2 86


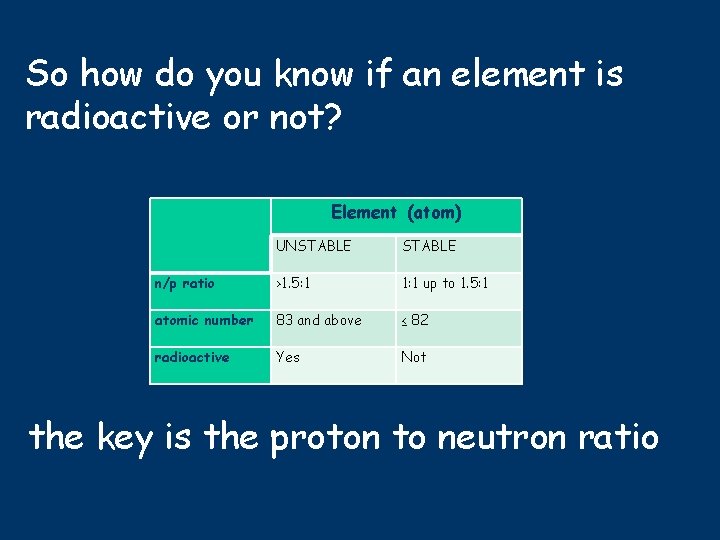
So how do you know if an element is radioactive or not? Element (atom) UNSTABLE n/p ratio >1. 5: 1 1: 1 up to 1. 5: 1 atomic number 83 and above ≤ 82 radioactive Yes Not the key is the proton to neutron ratio