Nuclear Reactions Do Now 1 What is the




- Slides: 15

Nuclear Reactions

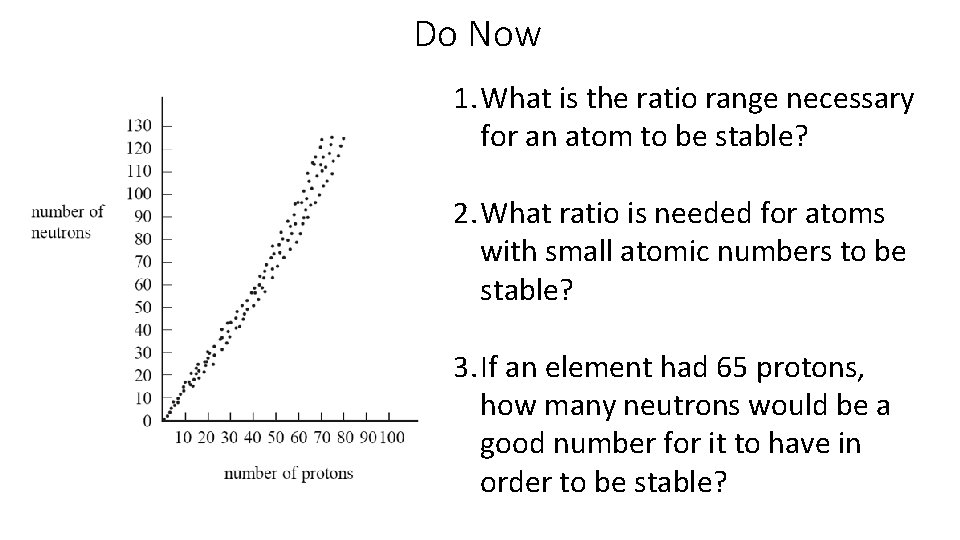
Do Now 1. What is the ratio range necessary for an atom to be stable? 2. What ratio is needed for atoms with small atomic numbers to be stable? 3. If an element had 65 protons, how many neutrons would be a good number for it to have in order to be stable?

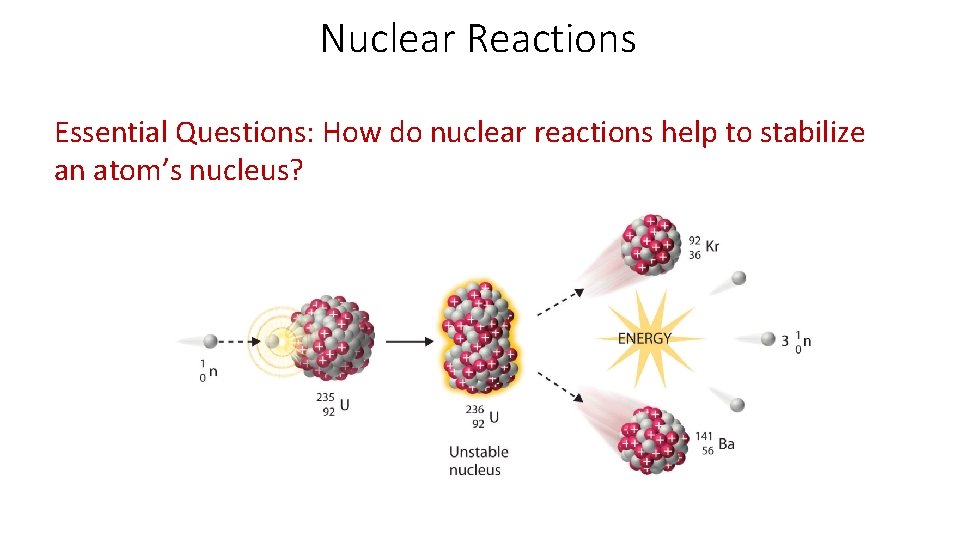
Nuclear Reactions Essential Questions: How do nuclear reactions help to stabilize an atom’s nucleus?

Nuclear Reactions What is a nuclear reaction? • A nuclear reaction is a reaction that changes the amount of particles in the nucleus of an atom.

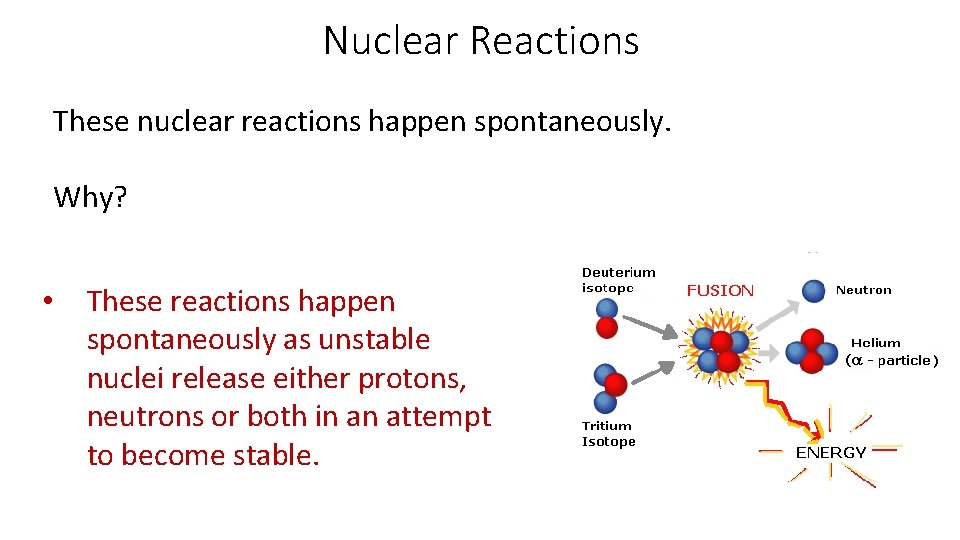
Nuclear Reactions These nuclear reactions happen spontaneously. Why? • These reactions happen spontaneously as unstable nuclei release either protons, neutrons or both in an attempt to become stable.

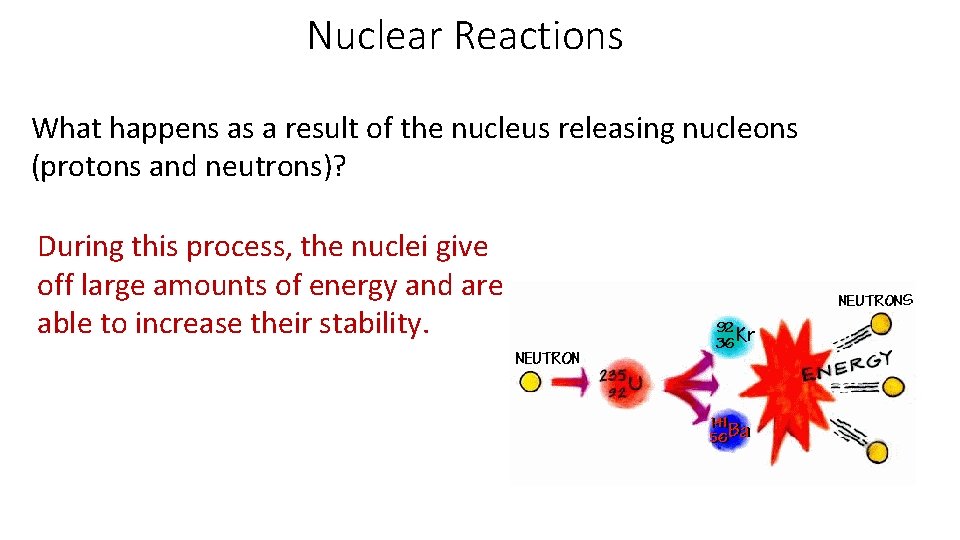
Nuclear Reactions What happens as a result of the nucleus releasing nucleons (protons and neutrons)? During this process, the nuclei give off large amounts of energy and are able to increase their stability.

Stop and Think 1)Why would a nuclei be unstable? 2)Explain why a nuclear reaction would happen spontaneously? 3)What are the end results of a spontaneous nuclear reaction?

Nuclear Reactions What is the difference between a nuclear reaction and a chemical reaction? • Chemical reactions involve an atom’s electrons • Nuclear reactions involve the atom’s nucleus. Nuclear reaction Chemical reaction

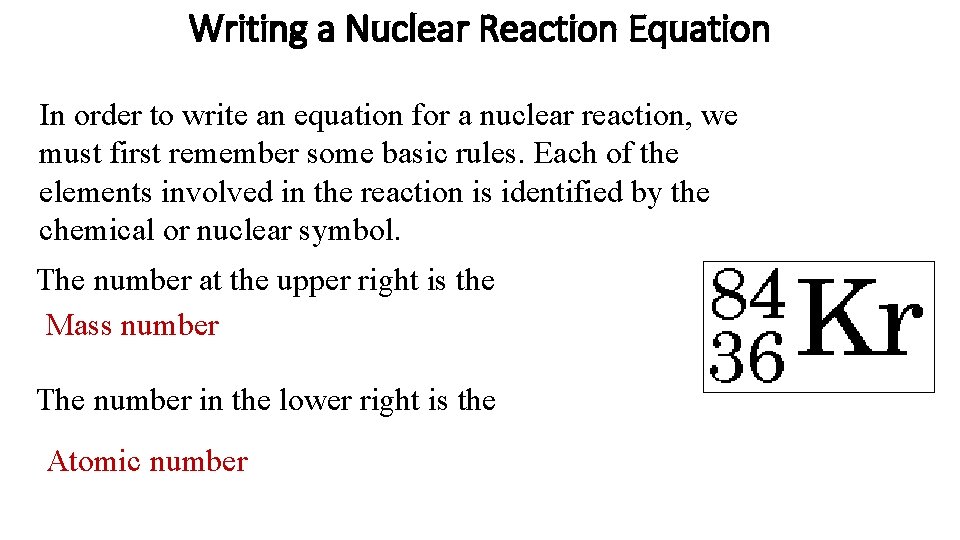
Writing a Nuclear Reaction Equation In order to write an equation for a nuclear reaction, we must first remember some basic rules. Each of the elements involved in the reaction is identified by the chemical or nuclear symbol. The number at the upper right is the Mass number The number in the lower right is the Atomic number

Stop and Think 1. Give two reasons why the mass number is so important. 2. What information can be found from the atomic number? 3. How do both numbers help to determine the stability of an atom?
Symbols Used in Nuclear Reactions

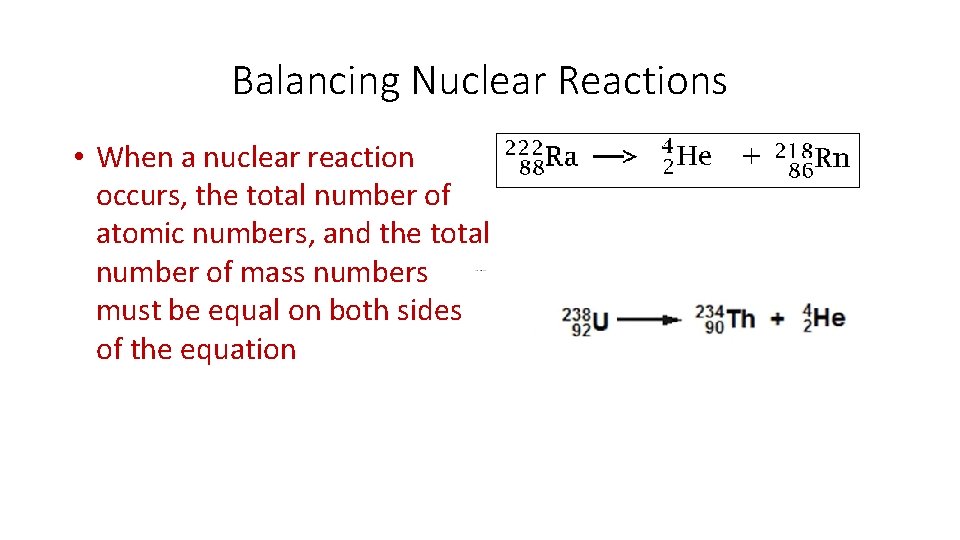
Balancing Nuclear Reactions • When a nuclear reaction occurs, the total number of atomic numbers, and the total number of mass numbers must be equal on both sides of the equation

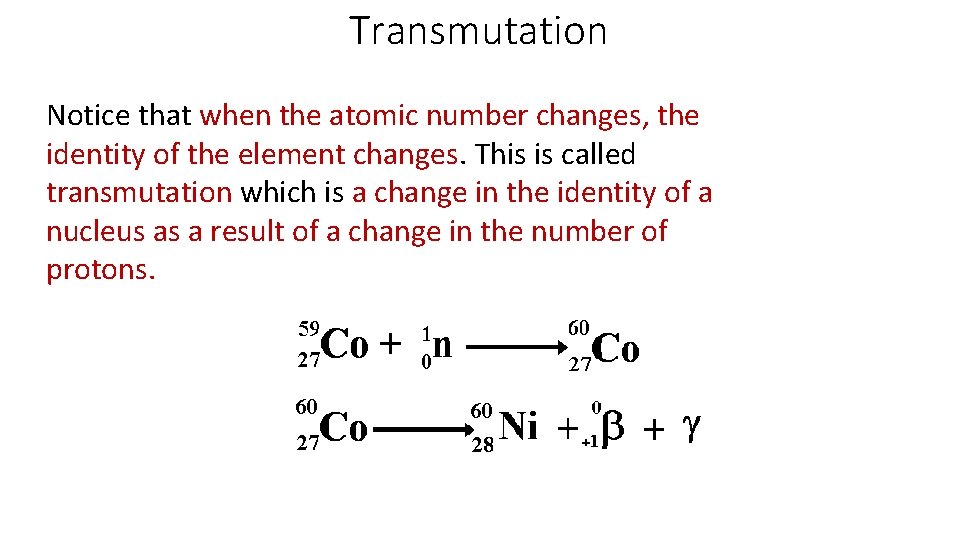
Transmutation Notice that when the atomic number changes, the identity of the element changes. This is called transmutation which is a change in the identity of a nucleus as a result of a change in the number of protons.

Stop and Think 1. Why must the total number of mass numbers and atomic numbers be the same on both sides of a nuclear equation? 2. How can you identify the new product created as a result of a nuclear reaction?

Balancing Nuclear reaction Practice