Nuclear Reactions AN INTRODUCTION TO FISSION FUSION Introduction


















- Slides: 22

Nuclear Reactions: AN INTRODUCTION TO FISSION & FUSION

Introduction Ø Nuclear reactions deal with interactions between the nuclei of atoms Ø The focus of this presentation are the processes of nuclear fission and nuclear fusion Ø Both fission and fusion processes deal with matter and energy

Matter and Energy Ø In chemical reactions “matter and energy cannot be created nor destroyed” Ø Nuclear chemistry is differerent Ø We now need to understand that Matter and Energy are two forms of the same thing

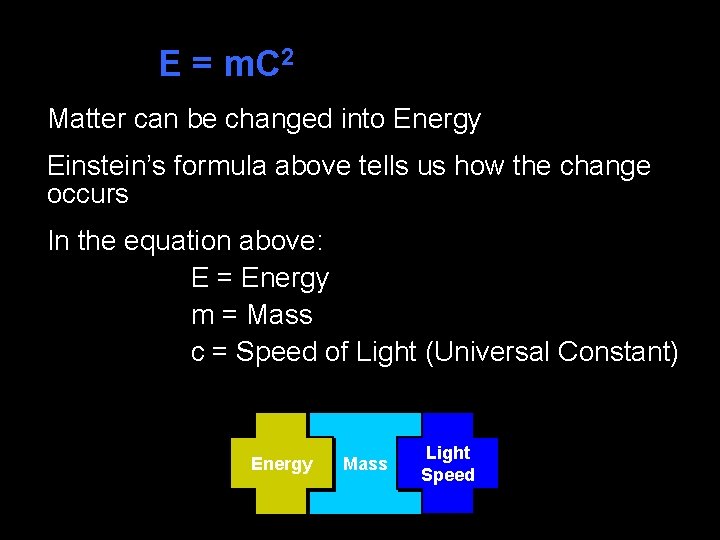
E = m. C 2 Matter can be changed into Energy Einstein’s formula above tells us how the change occurs In the equation above: E = Energy m = Mass c = Speed of Light (Universal Constant) Energy Mass Light Speed

E = m. C 2 Ø The equation may be read as follows: Energy (E) is equal to Mass (m) multiplied by the Speed of Light (c) squared Ø This tells us that a small amount of mass can be converted into a very large amount of energy because the speed of light (c) is an extremely large number Ø C= celeretas Latin for swiftness Ø C = 186, 000 miles/second)

Fission o Fission may be defined as the process of splitting an atomic nucleus into fission fragments o (Fission splits Big Atoms into little atoms + no) o The fission fragments are generally in the form of smaller atomic nuclei and neutrons o Large amounts of energy are produced by the fission process

Fission q Fissile nuclei (nuclei that can split) are generally heavy atoms with large numbers of nucleons (p+ and no) q The nuclei of such heavy atoms are struck by neutrons initiating the fission process q This sticking collision causes spontaneous instability. q Fission occurs due to electrostatic repulsion created by large numbers of protons within the nuclei of heavy atoms

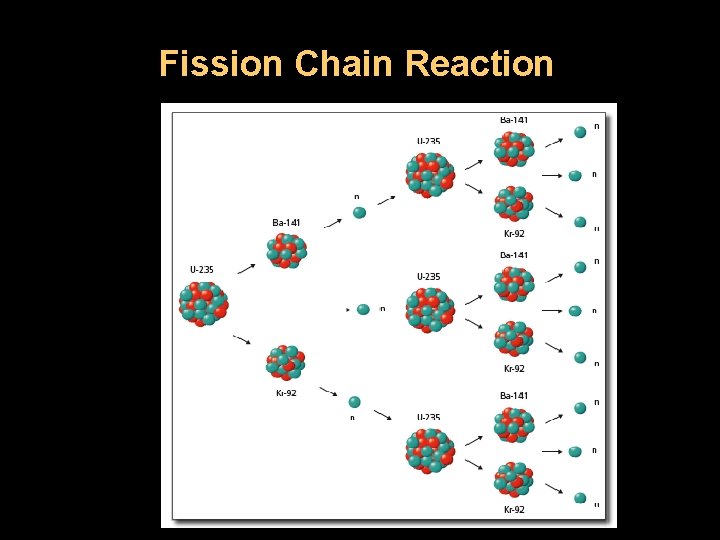
Fission Chain Reaction

U-235 Fission U-235 + 1 no U-236 3 no s + Kr-92 + Ba-141 + Energy (235 +1) = (236) = (3 + 92 + 141 ) 1. In this example, a stray neutron strikes an atom of U-235. 2. It absorbs the neutron and becomes an unstable atom of U -236. 3. It then undergoes fission. Notice that more neutrons are released in the reaction. 4. These neutrons can strike other U-235 atoms to initiate their fission and cause a chain reaction if there is enough U -235.

Fission ã The fission process is an a natural one as a French researcher found a natural uranium reactor in Gabon, West Africa; it has been estimated to be over 2 billion years old ã Fission produces large amounts of heat energy and it is this heat that is captured by nuclear power plants to produce electricity

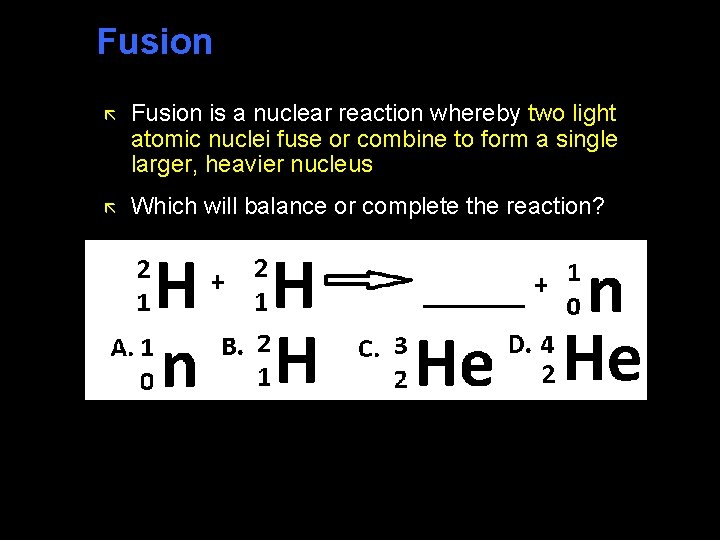
Fusion ã Fusion is a nuclear reaction whereby two light atomic nuclei fuse or combine to form a single larger, heavier nucleus ã Which will balance or complete the reaction?

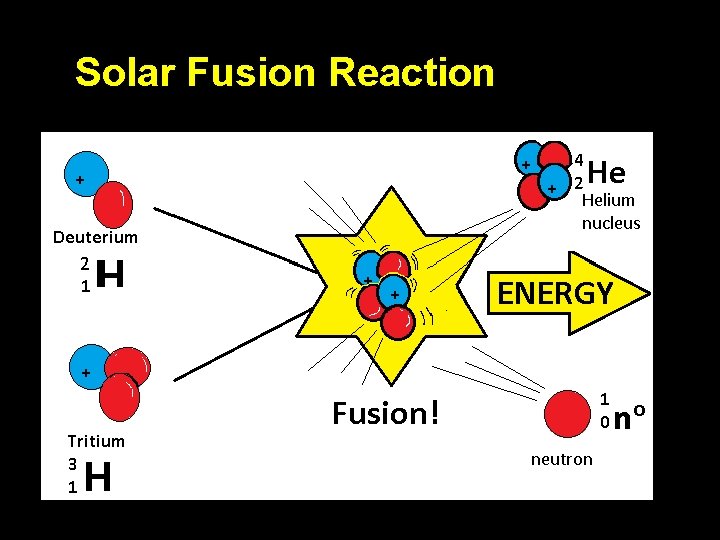
Solar Fusion Reaction

Fusion Ø The fusion process generates tremendous amounts of energy; refer back to Einstein’s equation Ø For fusion to occur, a large amount of energy is needed to overcome the electrical charges of the nuclei and fuse them together

Fusion Ø Fusion reactions do not occur naturally on our planet but are the principal type of reaction found in stars Ø The large masses, densities, and high temperatures of stars provide the initial energies needed to fuel fusion reactions Ø The sun fuses hydrogen atoms to produce helium, subatomic particles, and vast amounts of energy

Review Ø Mass and Energy are two forms of the same thing; neither can be created nor destroyed but mass can be converted into energy (E = mc 2) Ø Fission is a nuclear reaction in which a heavy atomic nucleus is split into lighter atomic nuclei Ø Fusion is a nuclear reaction in which 2 light atomic nuclei are combined into a single, heavier atomic nucleus

Quiz 1. Which nuclear process produces large amounts of energy? A. Fission B. Fusion C. Both fission & fusion D. Neither fission nor fusion

Quiz 2. Fission is the process that _____ atomic nuclei. A. Combines B. Burns up C. Stores D. Splits

Quiz 3. Mass may be converted into energy. A. True B. False

Quiz ã The fission process requires heavy atomic nuclei. A. True B. False

Quiz 4. Name a nuclear reaction that occurs within the sun:

Quiz 5. Fission is a natural process that occurs on the planet Earth. A. True B. False

Quiz 6. Explain this equation to your neighbor: E = mc 2 A. What do the parts mean? B. What is this used for today? C. Who gave us this equation?