Nuclear Reactions Alpha Beta and Gamma Decay Learning

Nuclear Reactions Alpha, Beta, and Gamma Decay Learning Goals: I will understand what a radioisotope is, and the differences between alpha, beta and gamma radioactive decay
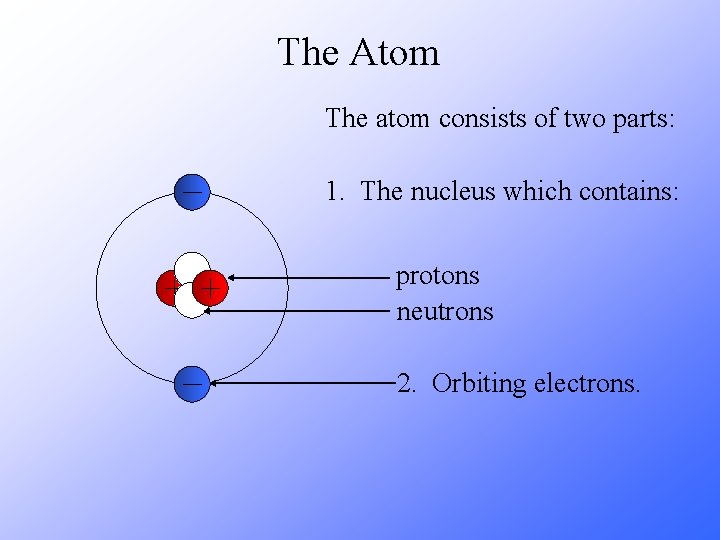
The Atom The atom consists of two parts: 1. The nucleus which contains: protons neutrons 2. Orbiting electrons.
The Atom All matter is made up of elements (e. g. carbon, hydrogen, etc. ). The smallest part of an element is called an atom. Atom of different elements contain different numbers of protons. The mass of an atom is almost entirely due to the number of protons and neutrons.
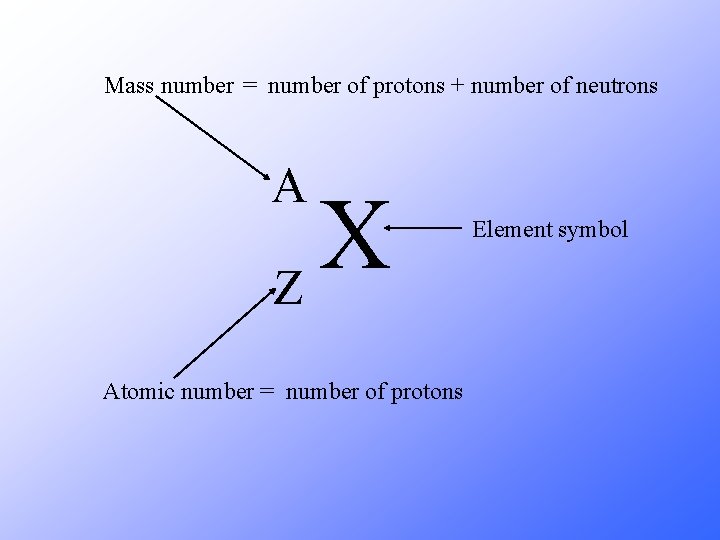
Mass number = number of protons + number of neutrons A X Z Atomic number = number of protons Element symbol

A X Z A = number of protons + number of neutrons Z = number of protons A – Z = number of neutrons Number of neutrons = Mass Number – Atomic Number
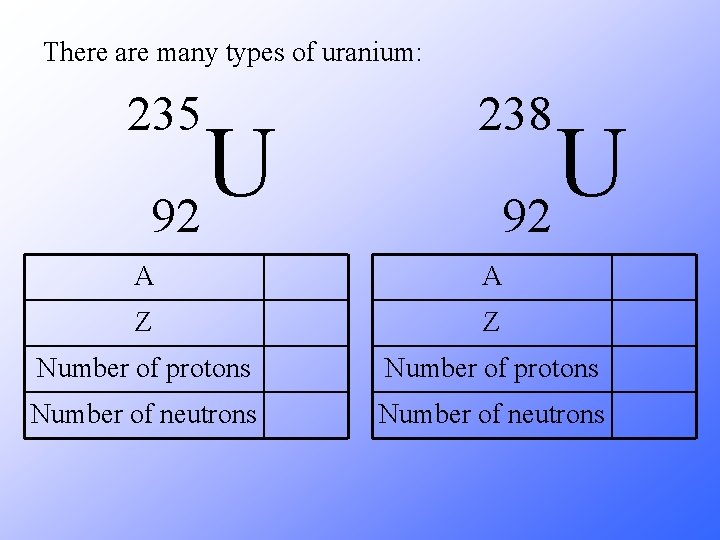
There are many types of uranium: 235 238 A A Z Z Number of protons Number of neutrons U 92
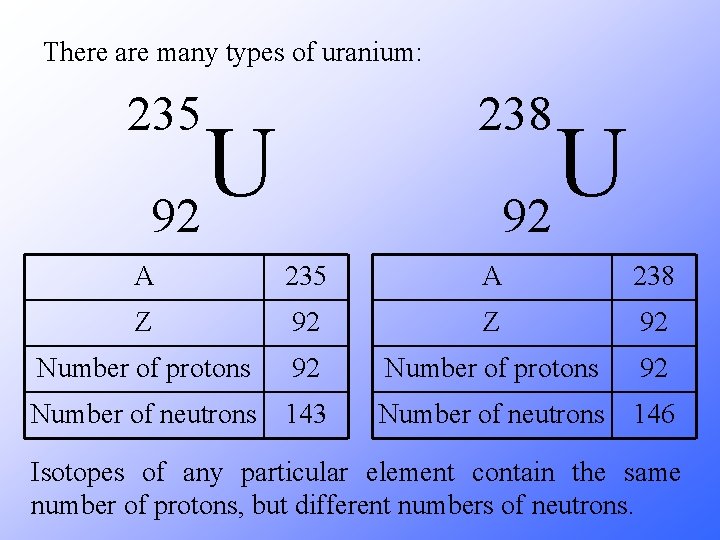
There are many types of uranium: 238 235 U 92 A 235 A 238 Z 92 Number of protons 92 Number of neutrons 143 Number of neutrons 146 Isotopes of any particular element contain the same number of protons, but different numbers of neutrons.

Most of the isotopes which occur naturally are stable. A few naturally occurring isotopes and all of the manmade isotopes are unstable because the # of protons is far from the # of neutrons. Unstable isotopes can become stable by releasing different types of particles. This process is called radioactive decay and the elements which undergo this process are called radioisotopes/radionuclides.

Radioactive Decay Radioactive decay results in the emission of either: • an alpha particle (a), • a beta particle (b), • or a gamma ray(g).

Alpha Decay An alpha particle is identical to that of a helium nucleus. It contains two protons and two neutrons.
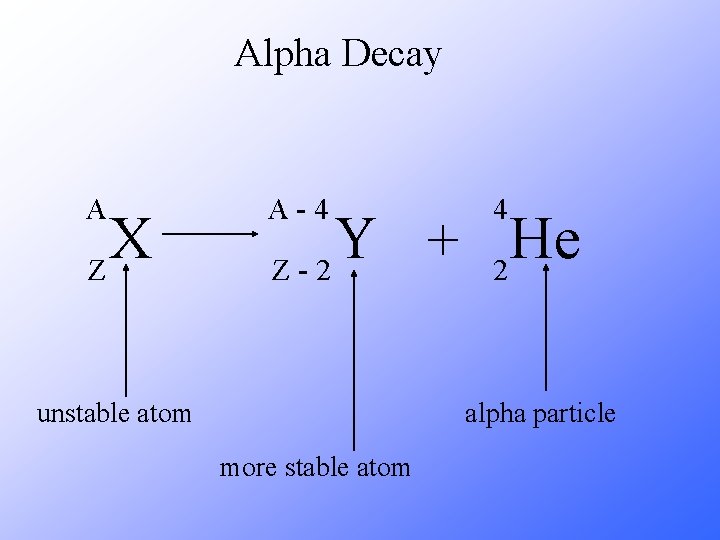
Alpha Decay A X Z A-4 4 Y He + Z-2 2 unstable atom alpha particle more stable atom
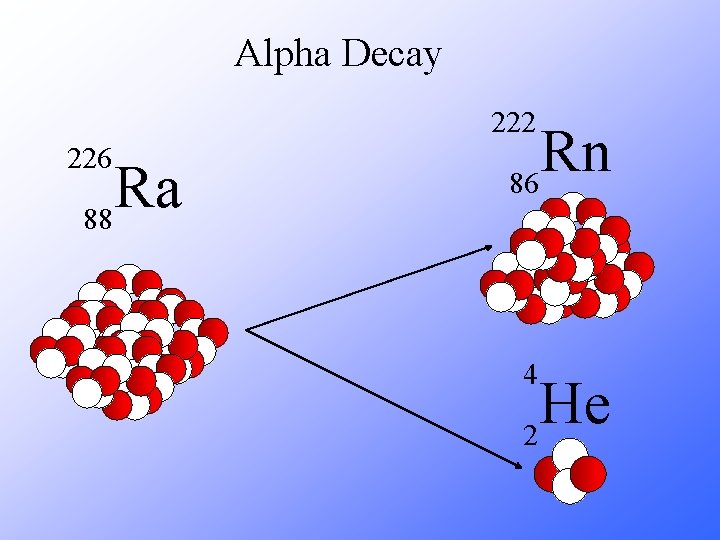
Alpha Decay 222 226 Ra 88 Rn 86 4 He 2
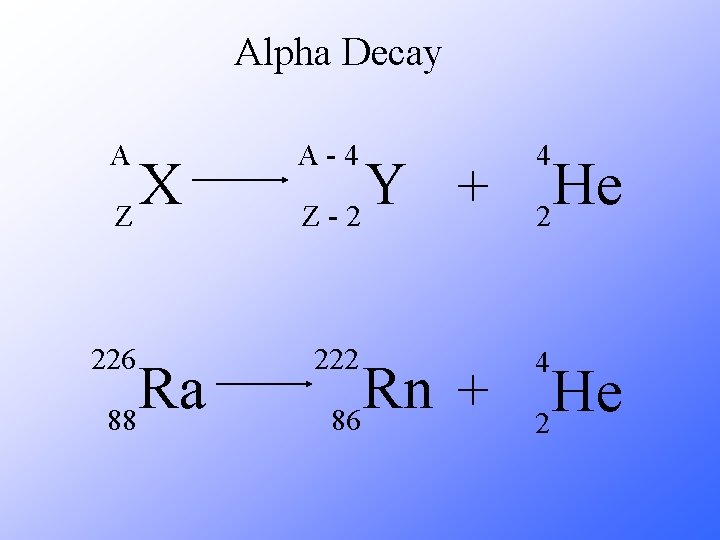
Alpha Decay A A-4 4 226 222 4 X Z Ra 88 Y + Z-2 Rn + 86 He 2
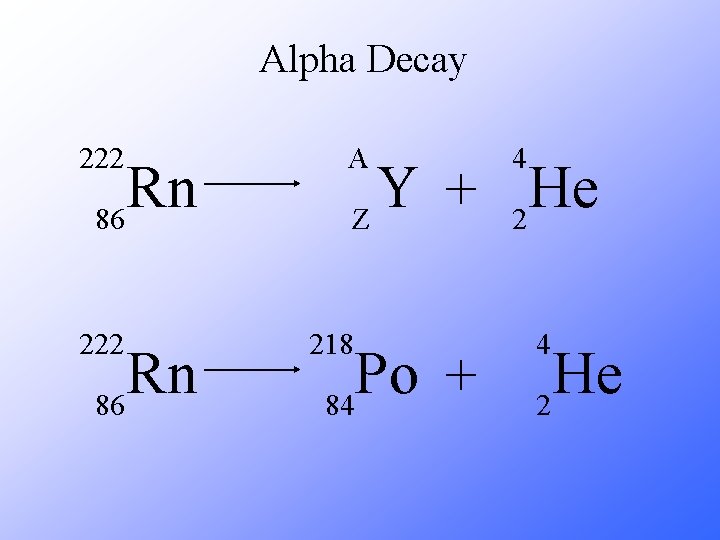
Alpha Decay 222 Rn 86 A 4 Y He + Z 2 218 Po + 84 4 He 2
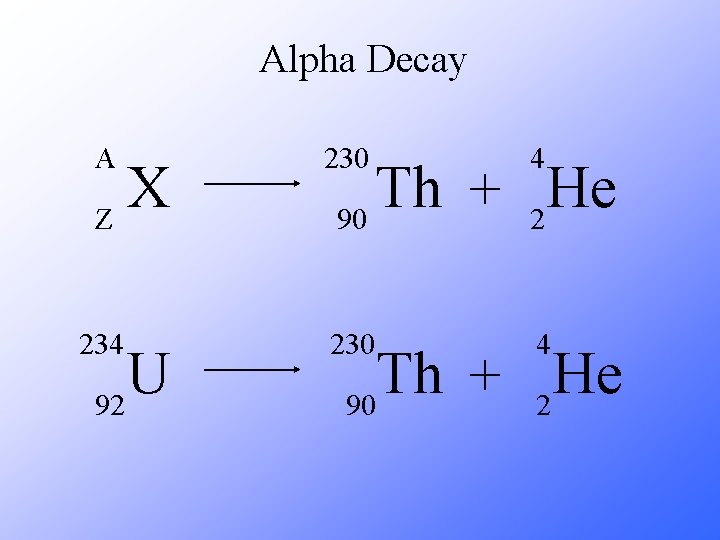
Alpha Decay A 230 4 X Z U 92 Th He + 90 2
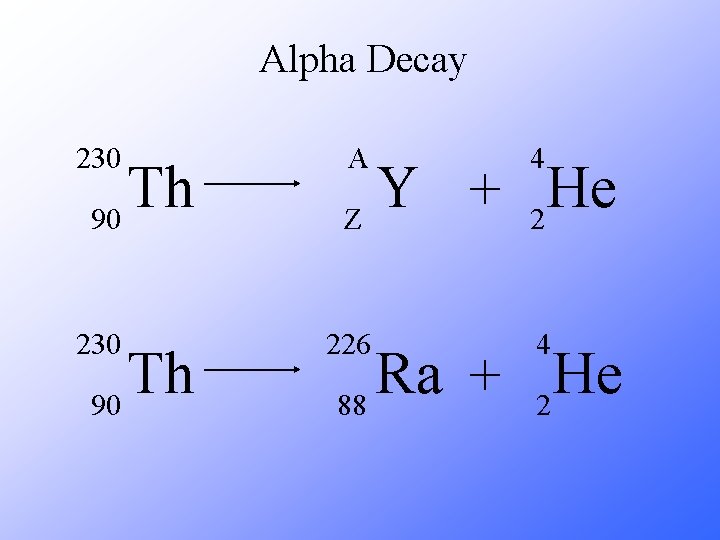
Alpha Decay 230 Th 90 A 4 226 4 Y He + Z 2 Ra He + 88 2
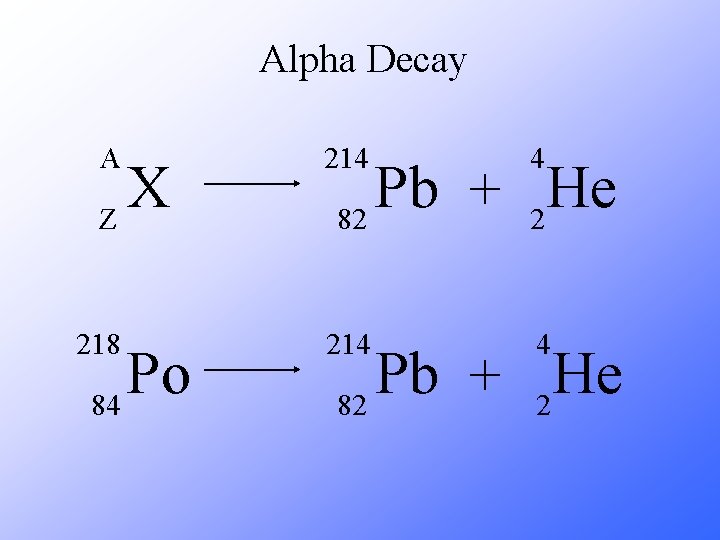
Alpha Decay A 214 4 218 214 4 X Z Po 84 Pb He + 82 2
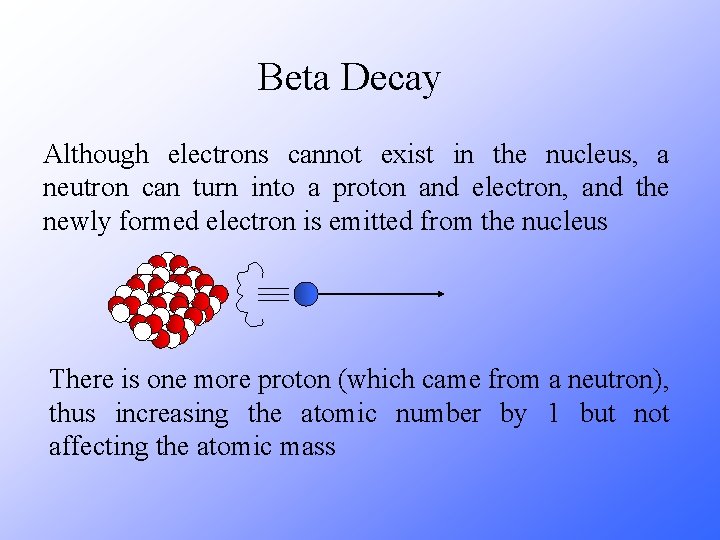
Beta Decay Although electrons cannot exist in the nucleus, a neutron can turn into a proton and electron, and the newly formed electron is emitted from the nucleus There is one more proton (which came from a neutron), thus increasing the atomic number by 1 but not affecting the atomic mass
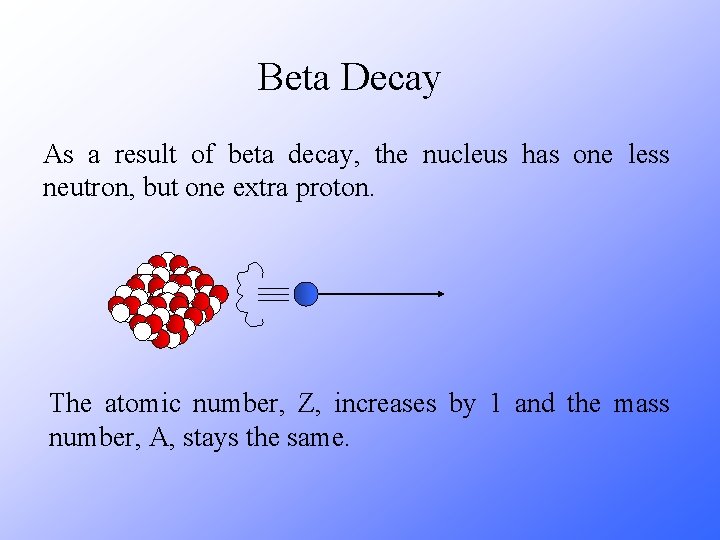
Beta Decay As a result of beta decay, the nucleus has one less neutron, but one extra proton. The atomic number, Z, increases by 1 and the mass number, A, stays the same.
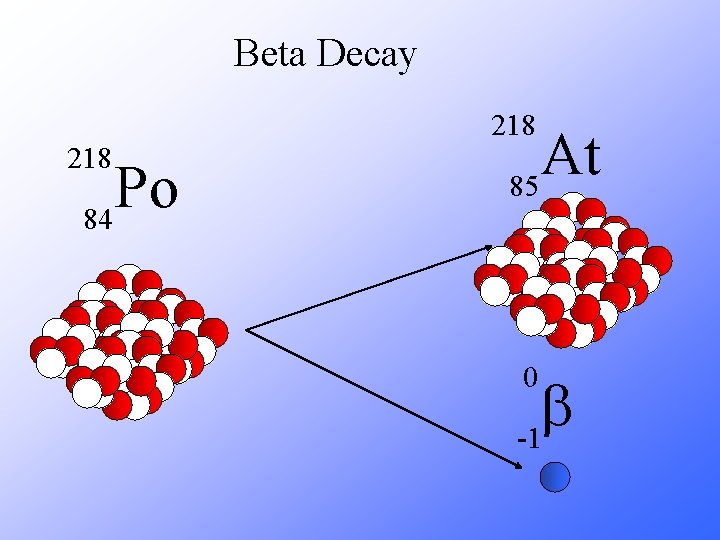
Beta Decay 218 Po 84 At 85 0 b -1
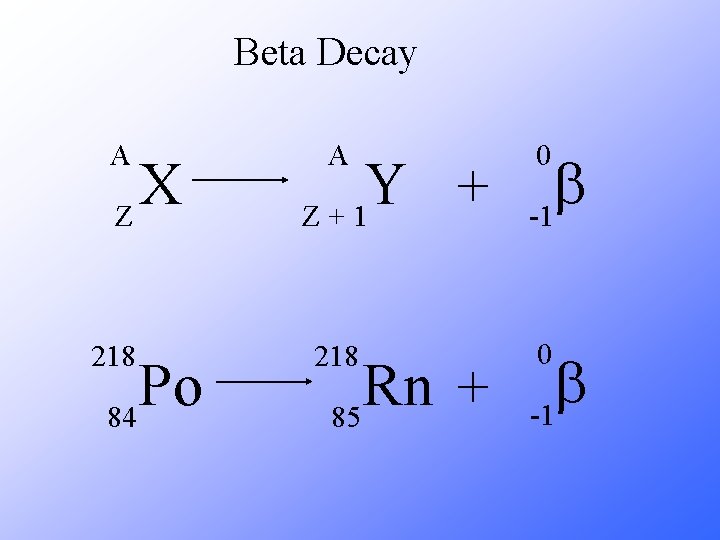
Beta Decay A X Z 218 Po 84 A Y + Z+1 218 Rn + 85 0 b -1
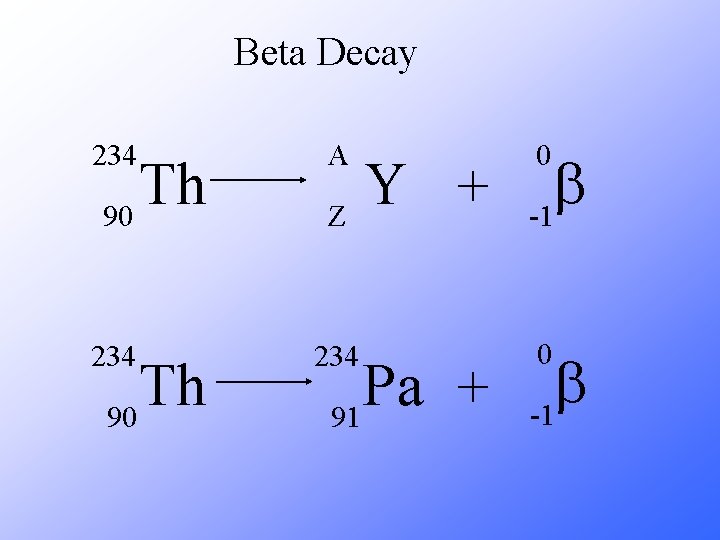
Beta Decay 234 A 234 Th 90 Y + Z Pa + 91 0 b -1
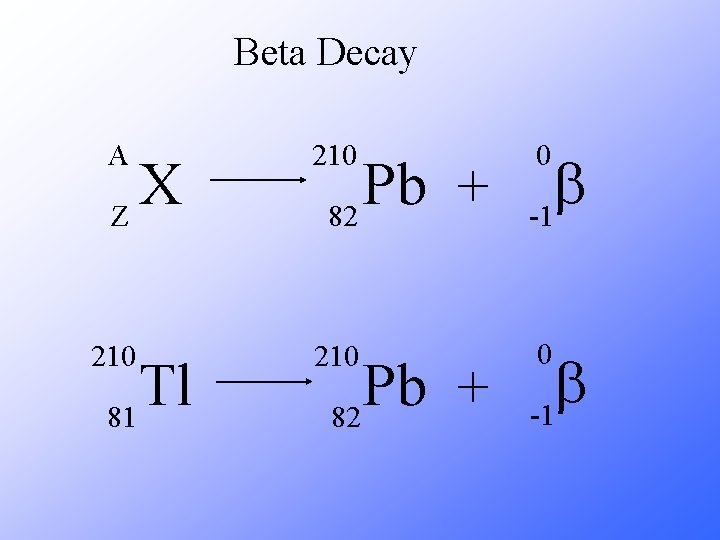
Beta Decay A 210 b -1 210 0 X Z Tl 81 Pb + 82 0 b -1
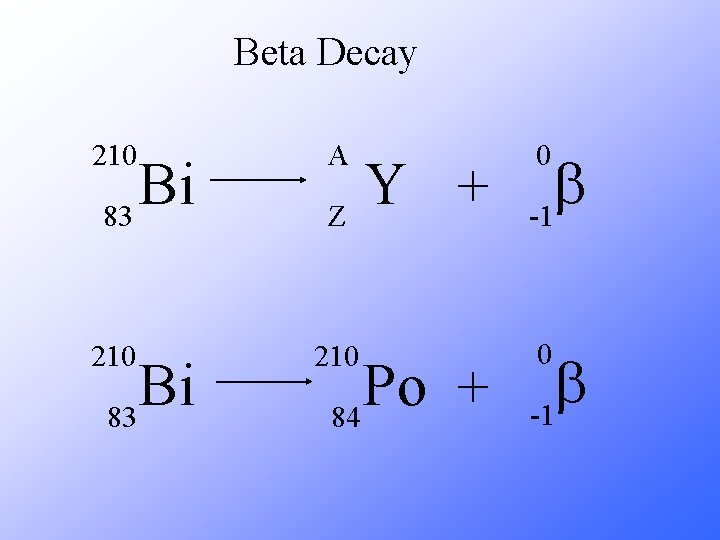
Beta Decay 210 A 210 Bi 83 Y + Z Po + 84 0 b -1
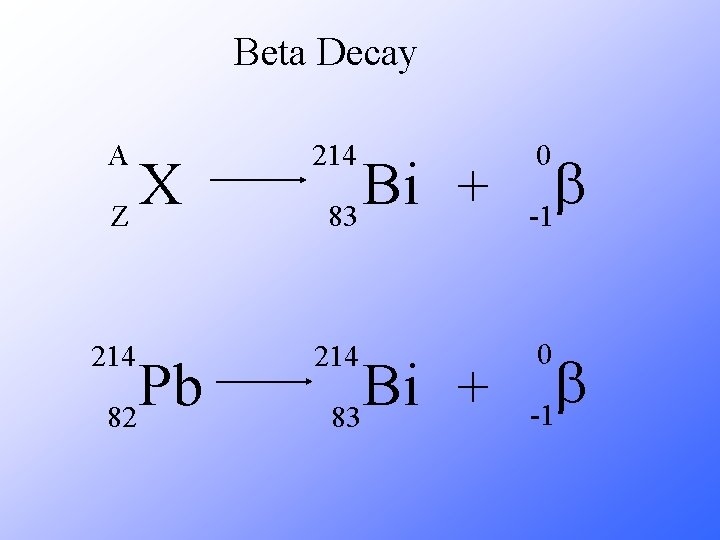
Beta Decay A 214 b -1 214 0 X Z Pb 82 Bi + 83 0 b -1

Gamma Decay Gamma rays are not charged particles like a and b particles. Gamma rays are electromagnetic radiation with high frequency.

How Did We Do? Learning Goals: I will understand what a radioisotope is, and the differences between alpha, beta and gamma radioactive decay
- Slides: 27