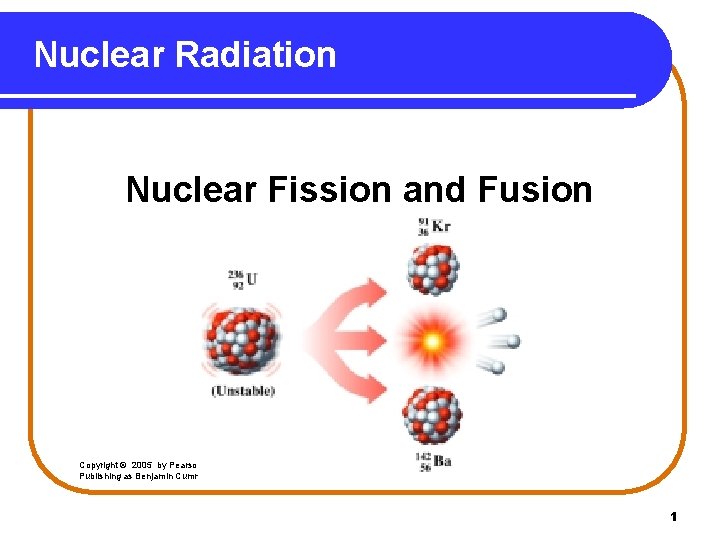
Nuclear Radiation Nuclear Fission and Fusion Copyright 2005









- Slides: 23

Nuclear Radiation Nuclear Fission and Fusion Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 1

Energy can be obtained two ways l l l Fission Splitting atoms Get energy if the nucleus is big. The smaller ones are more stable. What we do in nuclear reactors. l l l Fusion Joining atoms Get energy if the nuclei are small. The larger one is more stable. This is how the sun works. 2

Nuclear Fission l l l Fission is the splitting of atoms These are usually very large, so that they are not as stable Fission chain has three general steps: 1. Initiation. Reaction of a single atom starts the chain (e. g. , 235 U + neutron) 2. Propagation. 236 U fission releases neutrons that initiate other fissions 3. Termination. 3

Nuclear Fission A very heavy nucleus splits into more stable nuclei of intermediate mass. l The mass of the products is less than the mass of the reactants. l Missing mass is converted to energy l 4

Nuclear Fission In nuclear fission, • a large nucleus is bombarded with a small particle. • the nucleus splits into smaller nuclei and several neutrons. • large amounts of energy are released. 5

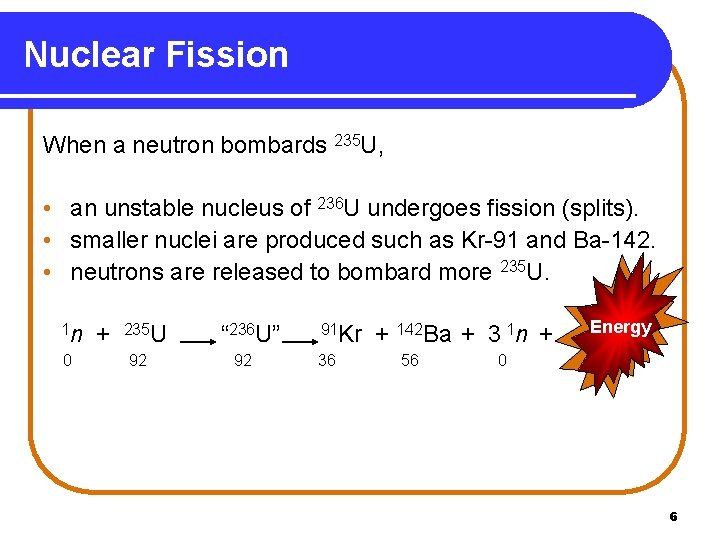
Nuclear Fission When a neutron bombards 235 U, • an unstable nucleus of 236 U undergoes fission (splits). • smaller nuclei are produced such as Kr-91 and Ba-142. • neutrons are released to bombard more 235 U. 1 n 0 + 235 U 92 “ 236 U” 92 91 Kr 36 + 142 Ba + 3 1 n + 56 Energy 0 6

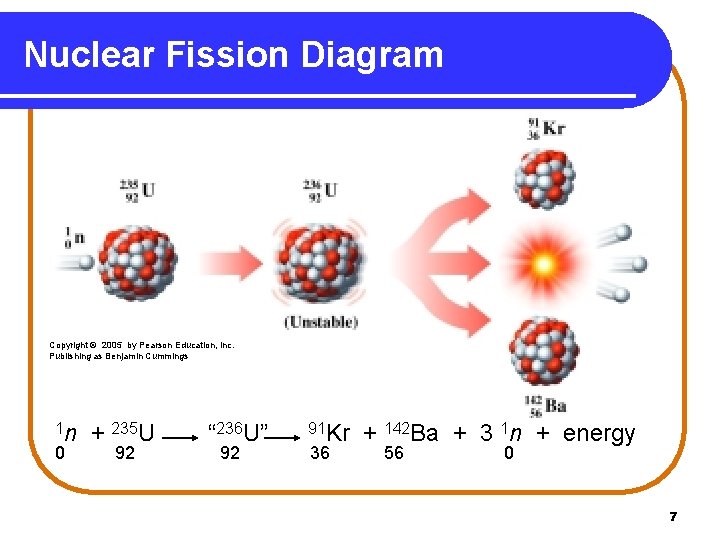
Nuclear Fission Diagram Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 1 n 0 + 235 U 92 “ 236 U” 92 91 Kr 36 + 142 Ba + 3 1 n + energy 56 0 7

l. Where does all this energy come from? 8

l E = mc 2 E = Energy (joules) m = mass (kg) c = speed of light = 3 x 10^8 m/s 9

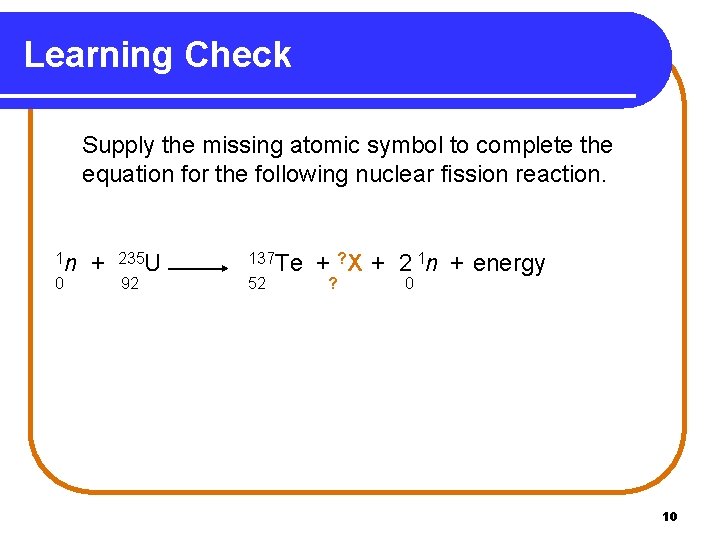
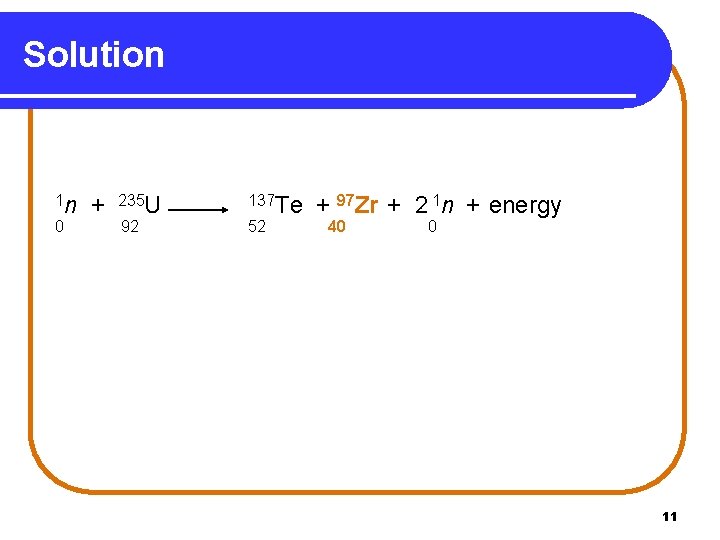
Learning Check Supply the missing atomic symbol to complete the equation for the following nuclear fission reaction. 1 n 0 + 235 U 92 137 Te 52 + ? X + 2 1 n + energy ? 0 10

Solution 1 n 0 + 235 U 92 137 Te 52 + 97 Zr + 2 1 n + energy 40 0 11

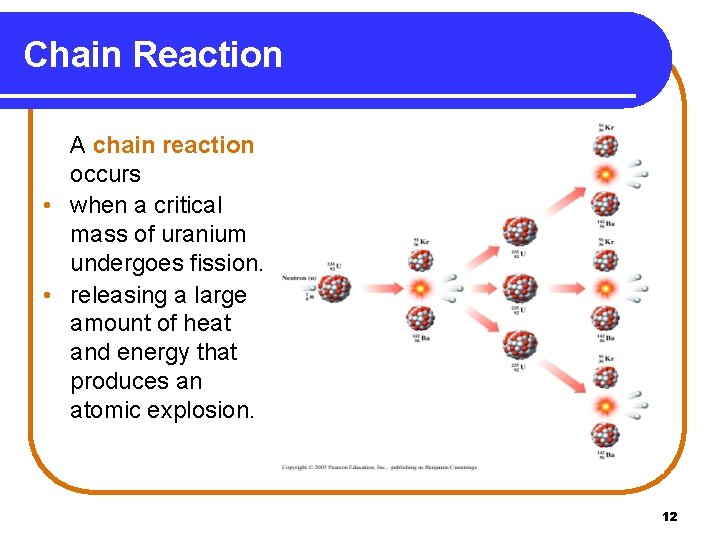
Chain Reaction A chain reaction occurs • when a critical mass of uranium undergoes fission. • releasing a large amount of heat and energy that produces an atomic explosion. 12

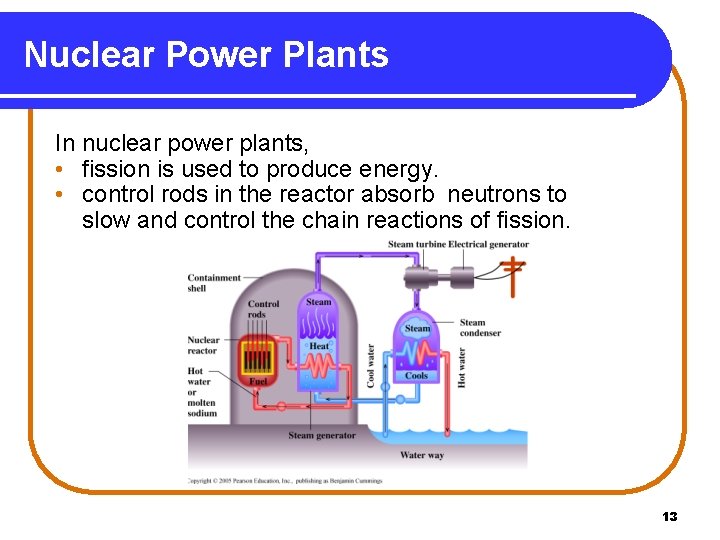
Nuclear Power Plants In nuclear power plants, • fission is used to produce energy. • control rods in the reactor absorb neutrons to slow and control the chain reactions of fission. 13

Fusion l Light-mass nuclei combine to form a heavier, more stable nucleus. l More energetic than fission reactions l Source of energy for the H-bomb l Origin of the elements 14

The most destructive force on the planet H-bombs 1000 s of times more powerful than A-bombs 15

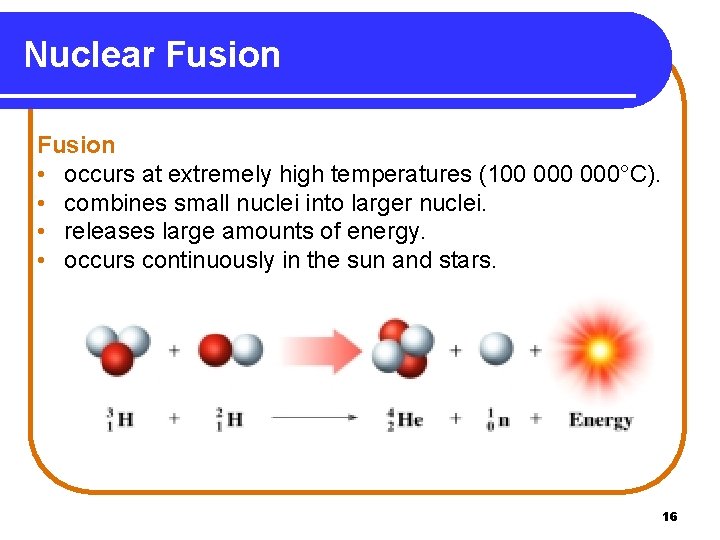
Nuclear Fusion • occurs at extremely high temperatures (100 000°C). • combines small nuclei into larger nuclei. • releases large amounts of energy. • occurs continuously in the sun and stars. 16

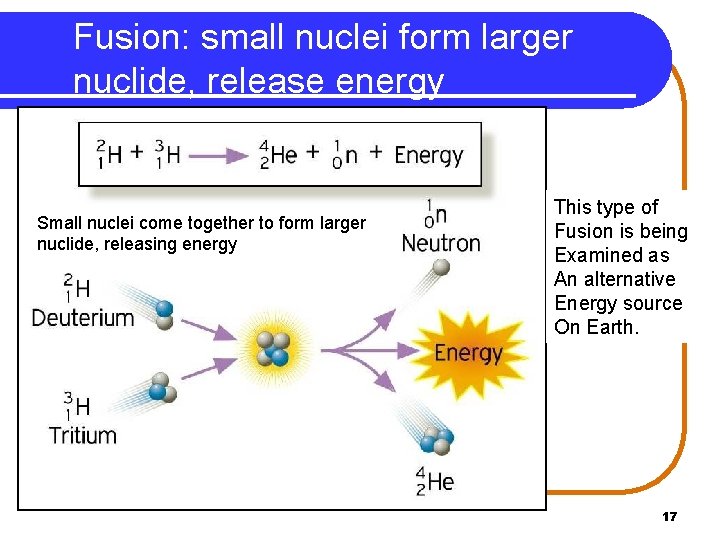
Fusion: small nuclei form larger nuclide, release energy Small nuclei come together to form larger nuclide, releasing energy This type of Fusion is being Examined as An alternative Energy source On Earth. 17

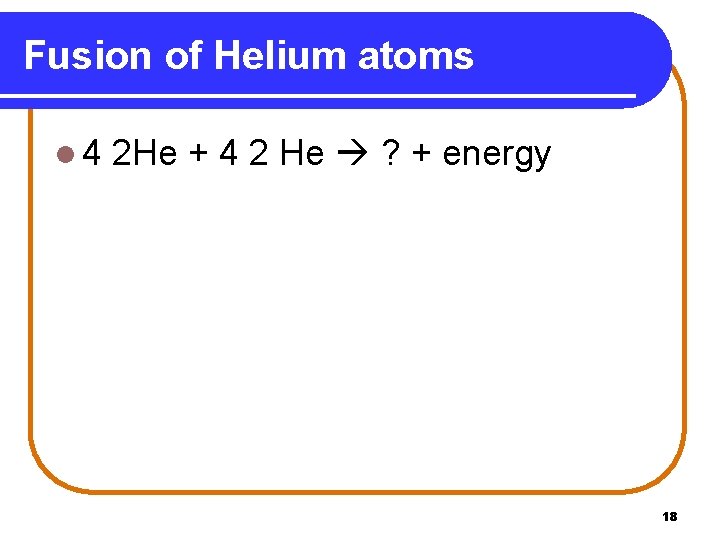
Fusion of Helium atoms l 4 2 He + 4 2 He ? + energy 18

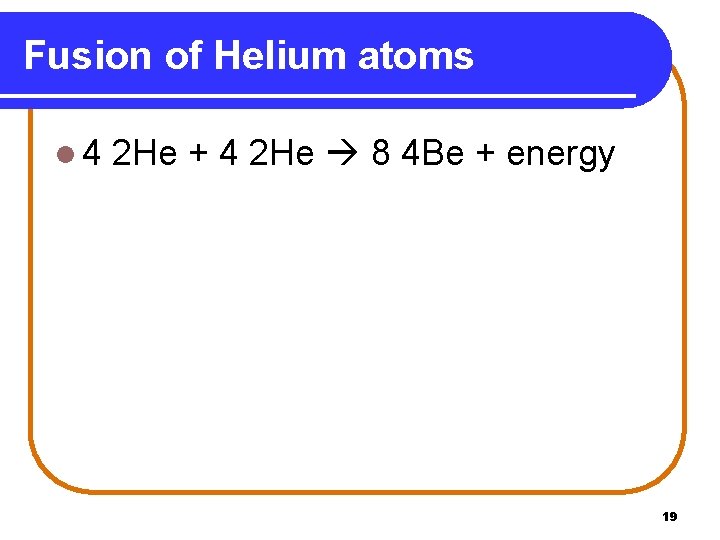
Fusion of Helium atoms l 4 2 He + 4 2 He 8 4 Be + energy 19

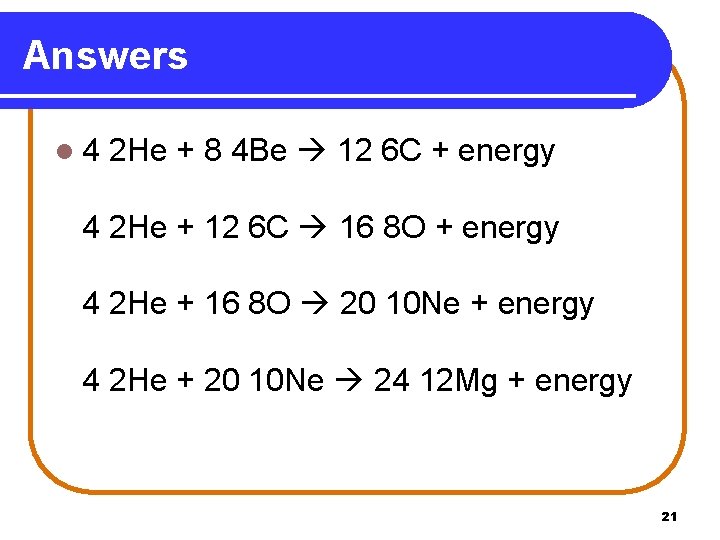
Practice l 4 2 He + 8 4 Be ? + energy 4 2 He + ? 16 8 O + energy 4 2 He + 16 8 O ? + energy 4 2 He + 20 10 Ne ? + energy 20

Answers l 4 2 He + 8 4 Be 12 6 C + energy 4 2 He + 12 6 C 16 8 O + energy 4 2 He + 16 8 O 20 10 Ne + energy 4 2 He + 20 10 Ne 24 12 Mg + energy 21

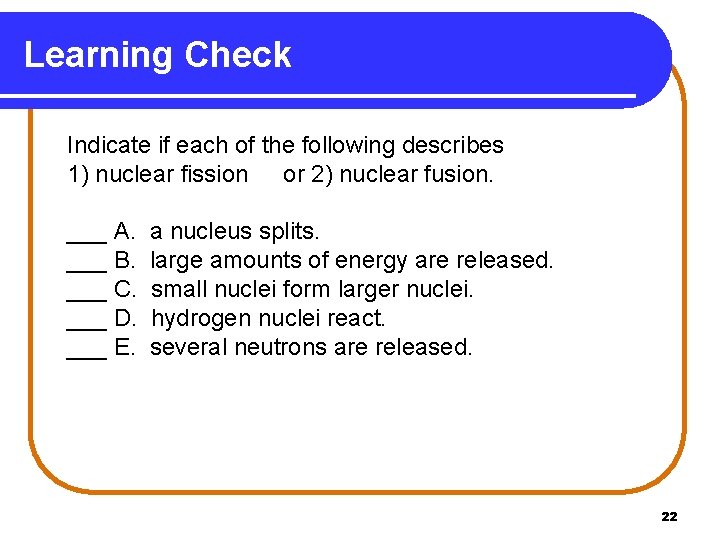
Learning Check Indicate if each of the following describes 1) nuclear fission or 2) nuclear fusion. ___ A. ___ B. ___ C. ___ D. ___ E. a nucleus splits. large amounts of energy are released. small nuclei form larger nuclei. hydrogen nuclei react. several neutrons are released. 22

Solution Indicate if each of the following is 1) nuclear fission or 2) nuclear fusion. 1 1, 2 2 2 1 A. B. C. D. E. a nucleus splits. large amounts of energy are released. small nuclei form larger nuclei. hydrogen nuclei react. several neutrons are released. 23