Nuclear Radiation Henri Becquerel Marie Curie Radioactivity Nuclear
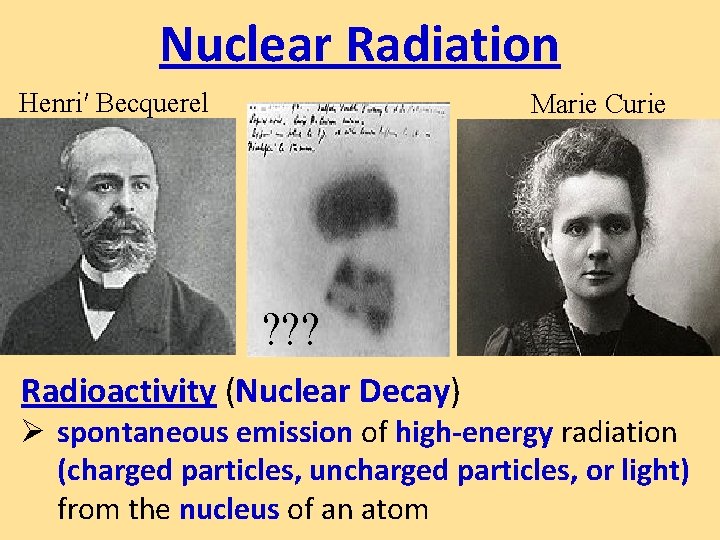
Nuclear Radiation Henri′ Becquerel Marie Curie ? ? ? Radioactivity (Nuclear Decay) Ø spontaneous emission of high-energy radiation (charged particles, uncharged particles, or light) from the nucleus of an atom

• The becquerel (symbol: Bq) is the SI derived unit of radioactivity. • 1896 Becquerel accidentally develops photo plate with U salts w/o sunlight. • Curies conducted experiments and named the “invisible rays” radioactivity. Both won Nobel Prize in Physics, she won for Chem later. Marie Curie died in 1934 of Aplastic anemia.
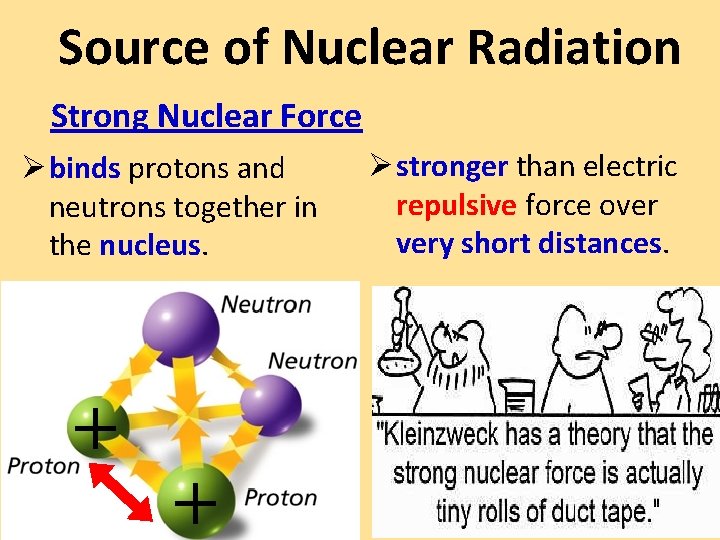
Source of Nuclear Radiation Strong Nuclear Force Ø binds protons and neutrons together in the nucleus. + + Ø stronger than electric repulsive force over very short distances.
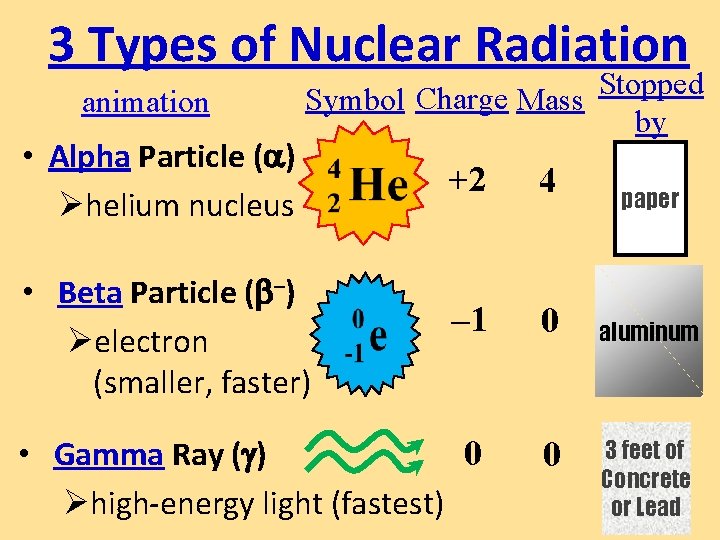
3 Types of Nuclear Radiation animation • Alpha Particle ( ) Øhelium nucleus Stopped Symbol Charge Mass by +2 4 – 1 0 • Gamma Ray ( ) 0 Øhigh-energy light (fastest) 0 • Beta Particle ( –) Øelectron (smaller, faster) paper aluminum 3 feet of Concrete or Lead
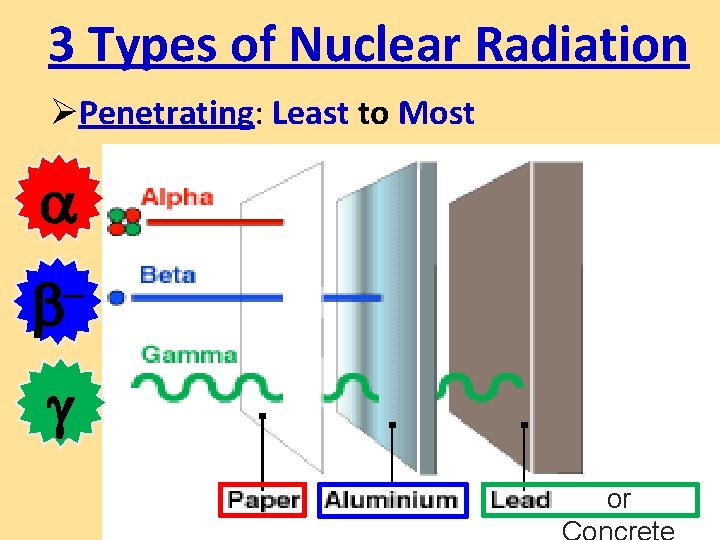
3 Types of Nuclear Radiation ØPenetrating: Least to Most – or
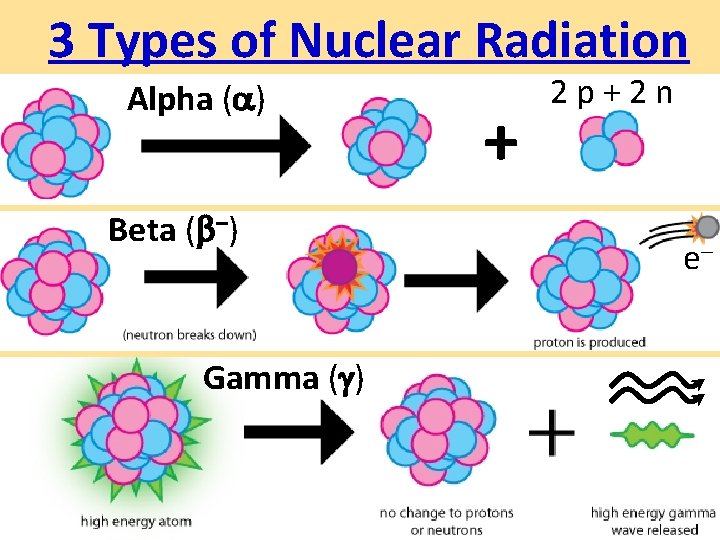
3 Types of Nuclear Radiation Alpha ( ) Beta ( –) Gamma ( ) + 2 p+2 n e–

Quick Quiz! 1. Certain elements are radioactive because their atoms have A. B. C. D. more neutrons than electrons. an unstable nucleus. a small nucleus. more neutrons than protons.

Quick Quiz. 2. Which property does NOT describe an alpha particle? A. B. C. D. +2 charge a relatively large mass a negative charge low penetrating power

Quick Quiz. 3. When a radioactive nucleus releases a high-speed electron, the process can be described as A. B. C. D. oxidation. alpha emission. beta emission. gamma radiation.
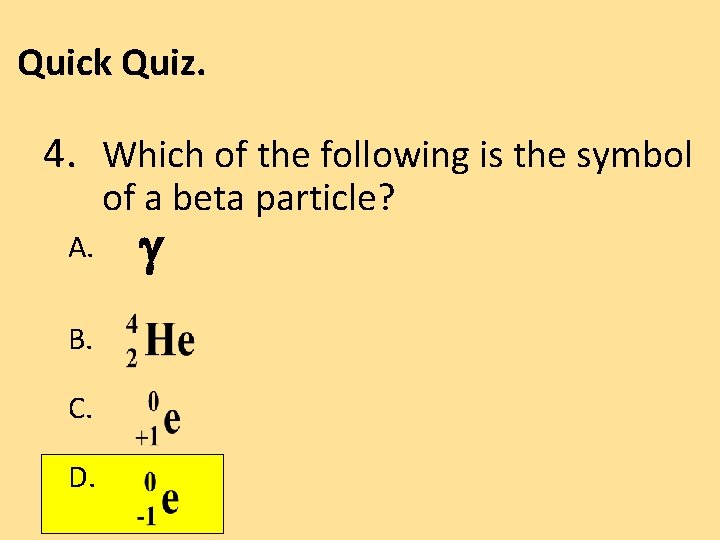
Quick Quiz. 4. Which of the following is the symbol of a beta particle? A. B. C. D.
- Slides: 10