Nuclear Physics l The Three Mile Island nuclear
















- Slides: 23

Nuclear Physics

l The Three Mile Island nuclear plant on Thursday, March 11, 2004 in Pennsylvania's Londonderry Twp. It was 25 years ago on March 28, 1979 that an accident in the Unit Two nuclear reactor at the plant caused a near meltdown of the unit's core.

Nuclear Physics l PRISCILLA was a 37 kiloton balloon shot fired June 24, 1957 at the Nevada Test Site.

The Nucleus l Rutherford’s experiment showed that all of the atoms positive charge and most of its mass is contained in the nucleus l The nucleus is the core of an atom made up of one or more protons and one or more neutrons l The protons and neutrons that make up the nucleus are called nucleons

Nuclear Force l In the nucleus, protons are only 10 -15 meters apart. l Consequently, a large repulsive Coulomb force exists between them. l Why doesn’t the nucleus blow apart?

Nuclear Force l Gravitational force holding them together? – No way! We already learned that the electrostatic forces are much stronger than the forces of gravity.

Nuclear Force l There must exist a very strong attractive very force to keep the protons concentrated in the nucleus of the atom! l We call this force the Nuclear Force.

Nuclear Force l The nuclear force (also called the “strong force”) is an attractive force between protons and neutrons that is responsible for the stability of the nucleus. l The nuclear force of attraction between two protons in a nucleus is about 100 times stronger than the electrostatic force of repulsion.

Nuclear Force l Why don’t we perceive the nuclear force in our daily lives? – At distances greater than a few nucleon diameters , the nuclear force diminishes rapidly and becomes much less than the gravitational or electrostatic forces. l Although nuclear forces are the strongest forces known to exist, they are effective only over very short distances.

Universal Mass Unit l The universal mass unit (or atomic mass unit) is defined as 1/12 th the mass of an atom of carbon 12. – In universal mass units, the mass of the proton is 1. 0073 u – The mass of a neutron is 1. 0087 u – The mass of an electron is 0. 0005 u. l In SI units, 1 universal mass unit is equal to 1. 66 x 10 -27 kg.

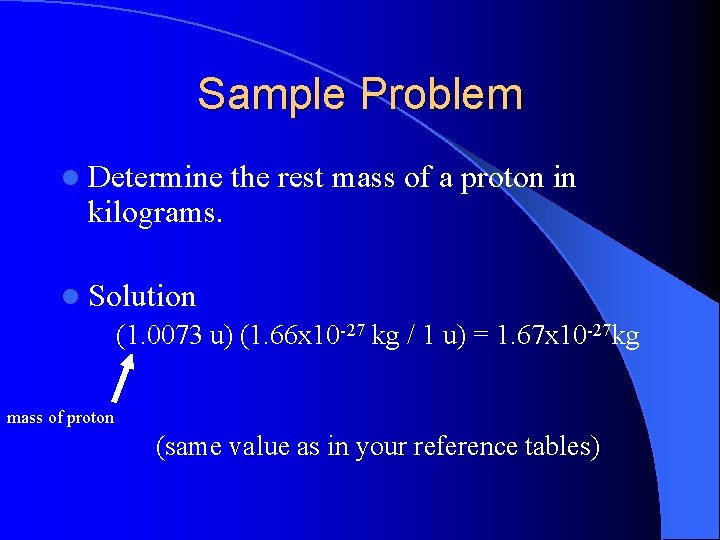
Sample Problem l Determine the rest mass of a proton in kilograms. l Solution (1. 0073 u) (1. 66 x 10 -27 kg / 1 u) = 1. 67 x 10 -27 kg mass of proton (same value as in your reference tables)

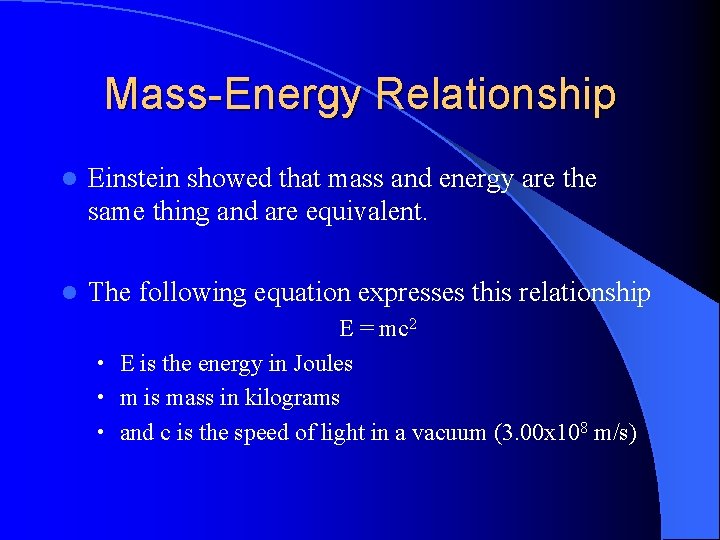
Mass-Energy Relationship l Einstein showed that mass and energy are the same thing and are equivalent. l The following equation expresses this relationship E = mc 2 • E is the energy in Joules • m is mass in kilograms • and c is the speed of light in a vacuum (3. 00 x 108 m/s)

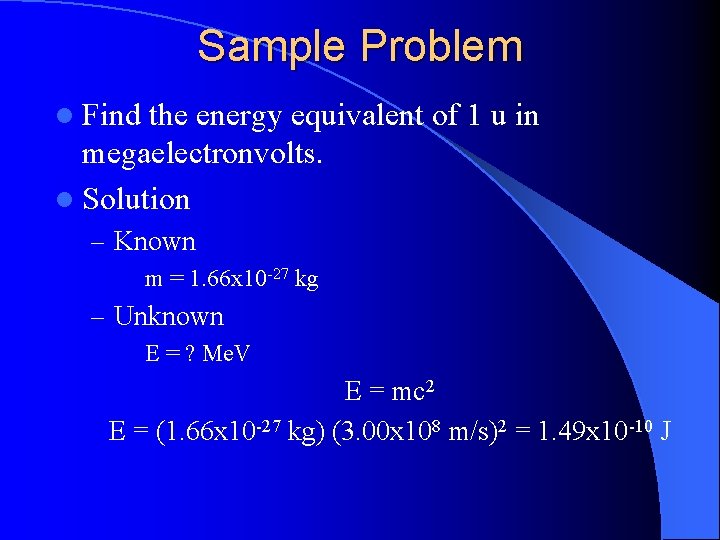
Sample Problem l Find the energy equivalent of 1 u in megaelectronvolts. l Solution – Known m = 1. 66 x 10 -27 kg – Unknown E = ? Me. V E = mc 2 E = (1. 66 x 10 -27 kg) (3. 00 x 108 m/s)2 = 1. 49 x 10 -10 J

Solution Continued l Need to convert to e. V’s (1. 49 x 10 -10 J) (1 e. V / 1. 60 x 10 -19 J) = 9. 31 x 108 e. V • Need to convert to Me. V (9. 31 x 108 e. V) (1 Me. V / 106 e. V) = 931 Me. V (same value as given in your reference tables)

Making the Connection Between the Nuclear Force and the Mass. Energy Relationship l It is the nuclear force (the “strong force”) that we are tapping into when we convert mass to energy (i. e. nuclear reactors).

Nuclear Mass and Energy l Mass-Energy is conserved at all levels, from cosmic to subatomic. l Lets look at an ordinary chemical reaction… C (1 kg) + O 2 (2. 66 kg) CO 2 + Energy (3. 3 x 107 J) According to Einstein, mass & energy must be conserved. Where did that energy come from (it wasn’t there in the beginning)? If it came from a conversion of mass to energy, why don’t I see it?

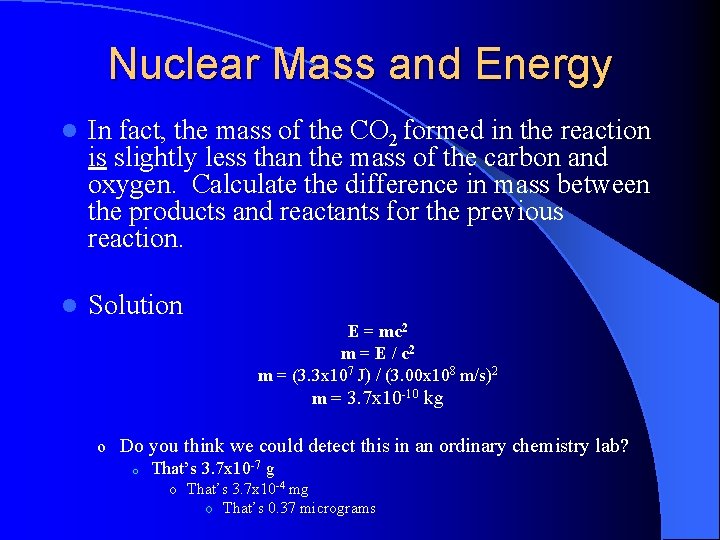
Nuclear Mass and Energy l In fact, the mass of the CO 2 formed in the reaction is slightly less than the mass of the carbon and oxygen. Calculate the difference in mass between the products and reactants for the previous reaction. l Solution E = mc 2 m = E / c 2 m = (3. 3 x 107 J) / (3. 00 x 108 m/s)2 m = 3. 7 x 10 -10 kg o Do you think we could detect this in an ordinary chemistry lab? o That’s 3. 7 x 10 -7 g o That’s 3. 7 x 10 -4 mg o That’s 0. 37 micrograms

Nuclear Mass and Energy l However, in reactions involving the nuclei of atoms, the changes in energy relative to the masses involved are much larger, and the corresponding changes in mass can be measured. l The mass of helium-4 nucleus (two protons, two neutrons) is 4. 0016 u. l But… the mass of a proton is 1. 0073 u and the mass of a neutron is 1. 0087 u. If we add these up, we get a different number…. (2 protons)(1. 0073 u) + (2 neutrons)(1. 0087 u) = 4. 0320 u o Thus, the mass of the nucleus is less than the sum of the mass of the individual parts.

Nuclear Mass and Energy l What happened to the missing mass? – When nucleons come together to form a nucleus, energy is released an equivalent amount of matter is lost. This process is referred to as fusion.

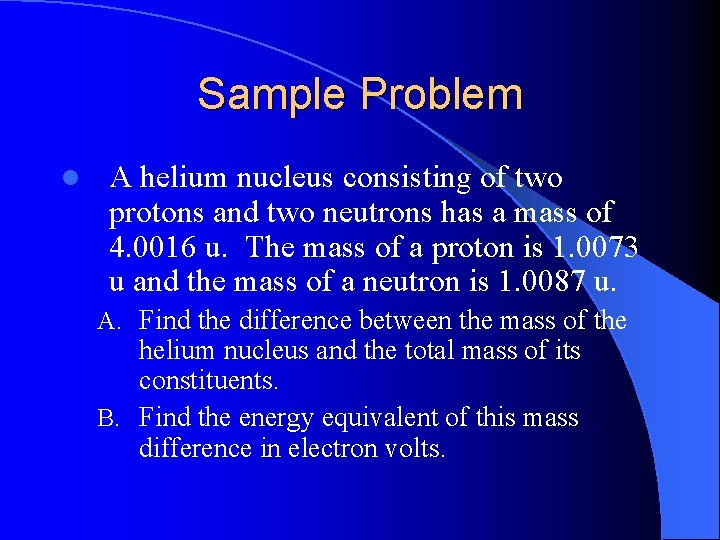
Sample Problem l A helium nucleus consisting of two protons and two neutrons has a mass of 4. 0016 u. The mass of a proton is 1. 0073 u and the mass of a neutron is 1. 0087 u. A. Find the difference between the mass of the helium nucleus and the total mass of its constituents. B. Find the energy equivalent of this mass difference in electron volts.

Solution Ø Determine the mass of the two protons and two neutrons… (2 protons)(1. 0073 u) + (2 neutrons)(1. 0087 u) = 4. 0320 u Ø Find the difference between the mass of the nucleons and the mass of the helium nucleus mass difference = 4. 0320 u - 4. 0016 u = 0. 0304 u

Solution l Find the energy equivalent of this mass difference in electronvolts – Use the relationship we derived yesterday in class (its also in your reference tables) 1 u = 931 Me. V E = (0. 0304 u) (931 Me. V / 1 u) = 28. 3 Me. V (28. 3 Me. V) (106 e. V / Me. V) = 2. 83 x 107 e. V

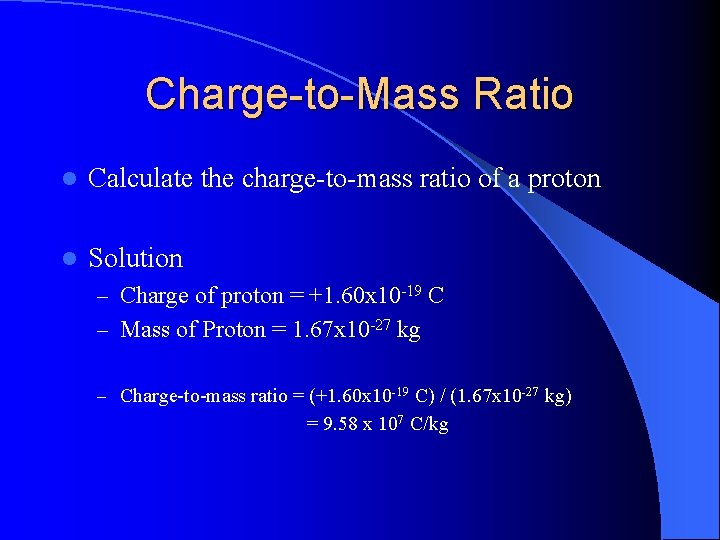
Charge-to-Mass Ratio l Calculate the charge-to-mass ratio of a proton l Solution – Charge of proton = +1. 60 x 10 -19 C – Mass of Proton = 1. 67 x 10 -27 kg – Charge-to-mass ratio = (+1. 60 x 10 -19 C) / (1. 67 x 10 -27 kg) = 9. 58 x 107 C/kg