Nuclear Physics DISCUSS THE FOLLOWING QUESTIONS WITH YOUR






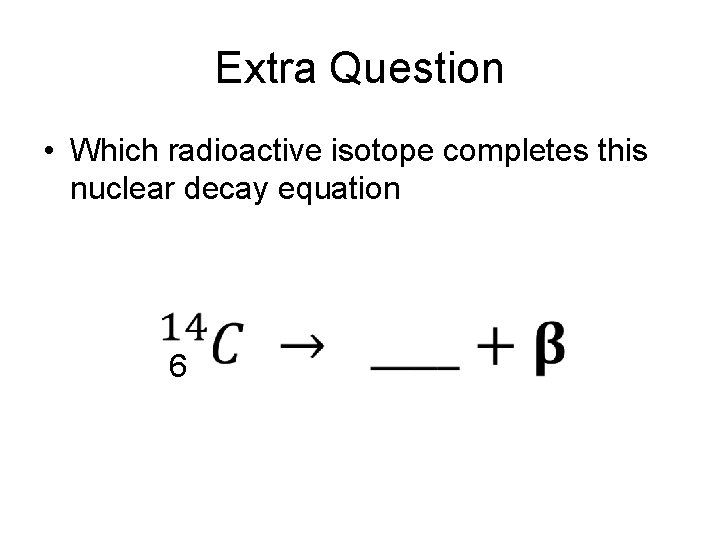










- Slides: 38

Nuclear Physics

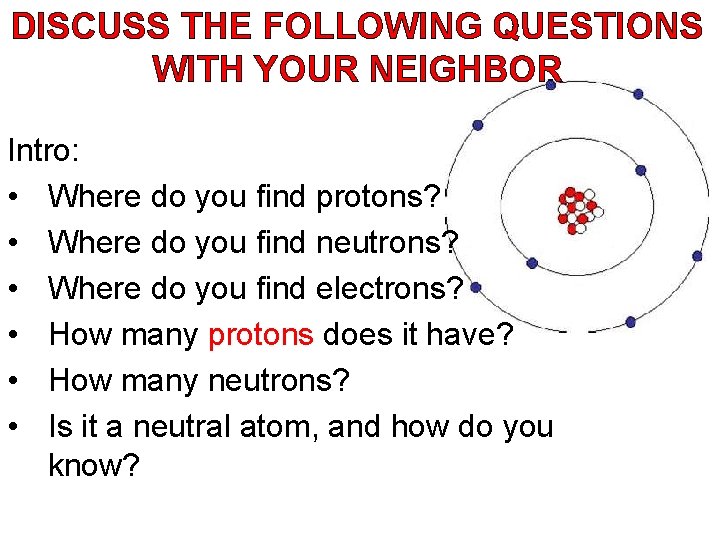
DISCUSS THE FOLLOWING QUESTIONS WITH YOUR NEIGHBOR Intro: • Where do you find protons? • Where do you find neutrons? • Where do you find electrons? • How many protons does it have? • How many neutrons? • Is it a neutral atom, and how do you know?

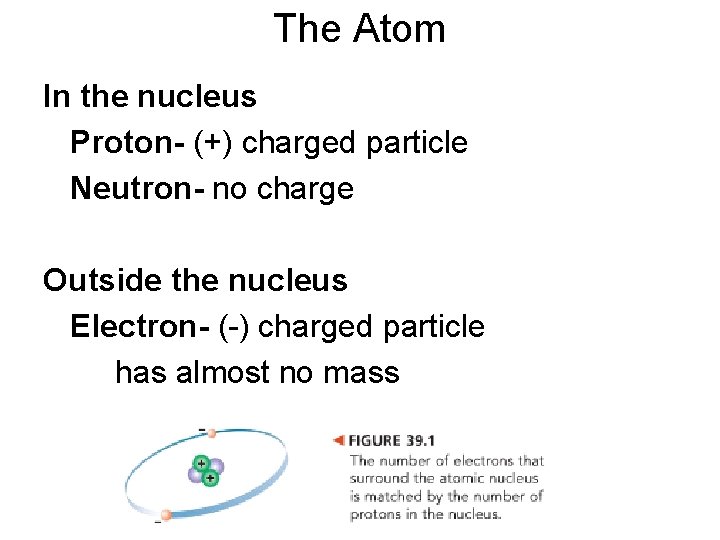
The Atom In the nucleus Proton- (+) charged particle Neutron- no charge Outside the nucleus Electron- (-) charged particle has almost no mass

Nucleons • Are subatomic particles inside the nucleus • Consist of + charged protons and neutral neutrons • Have almost 2000 times the mass of electrons • Because of the two types of nucleons, the nucleus is positively charged

• Where can you find the number of protons? • It’s the atomic number (found on the periodic table)

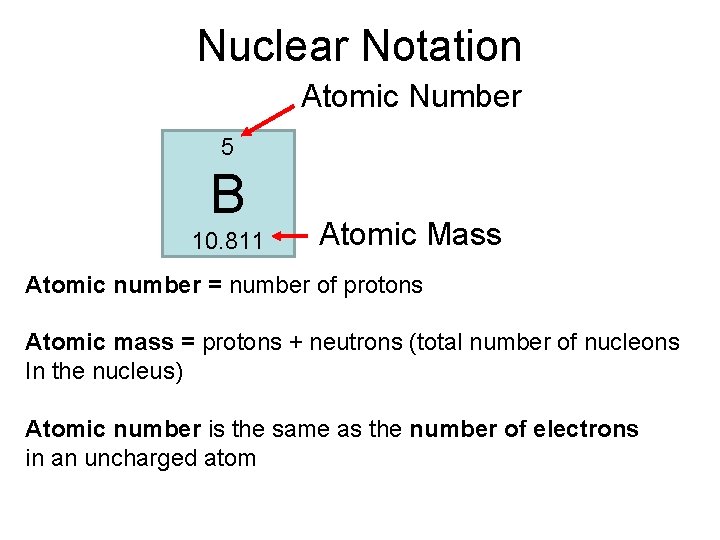
Nuclear Notation Atomic Number 5 B 10. 811 Atomic Mass Atomic number = number of protons Atomic mass = protons + neutrons (total number of nucleons In the nucleus) Atomic number is the same as the number of electrons in an uncharged atom

Question 1 You may see atomic number written many ways. The smaller number is the atomic number and the larger is the atomic mass 1 a. How many protons? 1 b. How many neutrons? 1 c. How many nucleons?

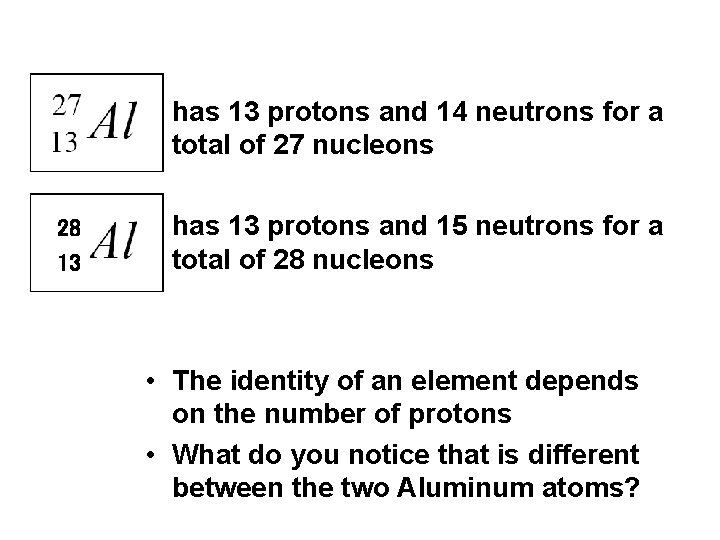
• has 13 protons and 14 neutrons for a total of 27 nucleons 28 13 • has 13 protons and 15 neutrons for a total of 28 nucleons • The identity of an element depends on the number of protons • What do you notice that is different between the two Aluminum atoms?

Isotopes: • Atoms of the same element with different numbers of neutrons (giving them different masses) Most common stable isotope of carbon Unstable radioactive isotope of carbon

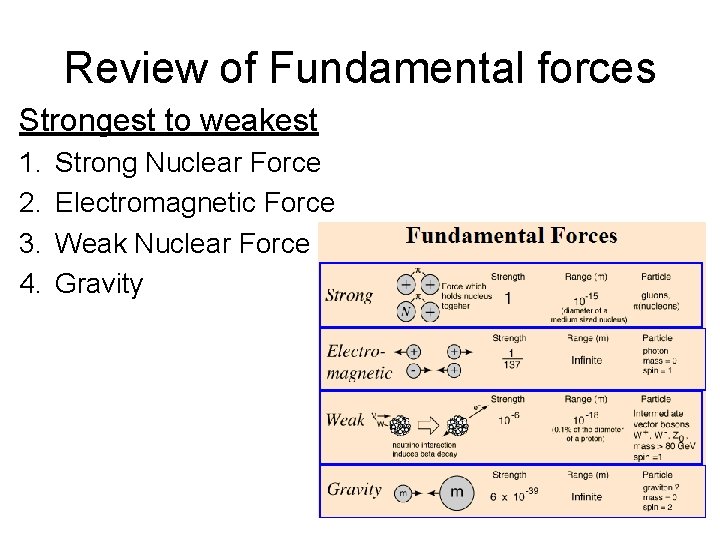
Review of Fundamental forces Strongest to weakest 1. 2. 3. 4. Strong Nuclear Force Electromagnetic Force Weak Nuclear Force Gravity

2 Forces Acting on Nucleons: Forces of attraction between nucleons Strong forces – Holds the nucleons in the nucleus – Are independent of the charge of the nucleon – Are short range (exist only between closest neighbors) Electrical force (electrostatic) – Force of repulsions between positively charged protons – Are long range

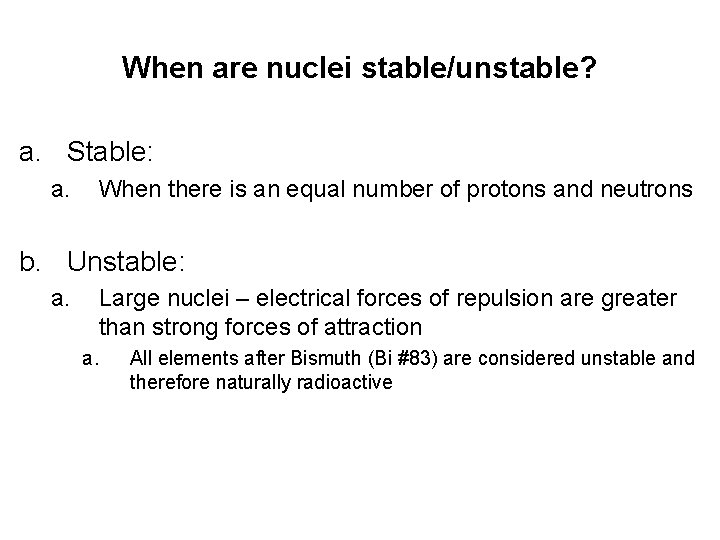
When are nuclei stable/unstable? a. Stable: a. When there is an equal number of protons and neutrons b. Unstable: a. Large nuclei – electrical forces of repulsion are greater than strong forces of attraction a. All elements after Bismuth (Bi #83) are considered unstable and therefore naturally radioactive

A radioactive isotope: • Has an unstable nucleus • Spontaneously emits a particle and decays into another element (to become more stable)

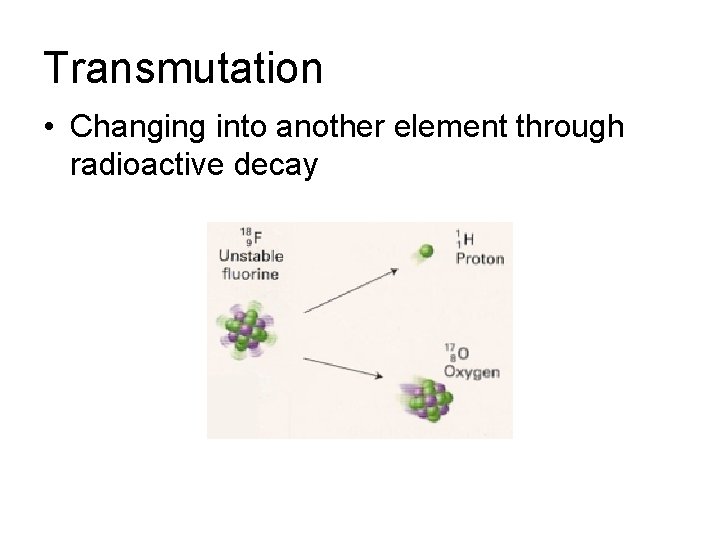
Transmutation • Changing into another element through radioactive decay

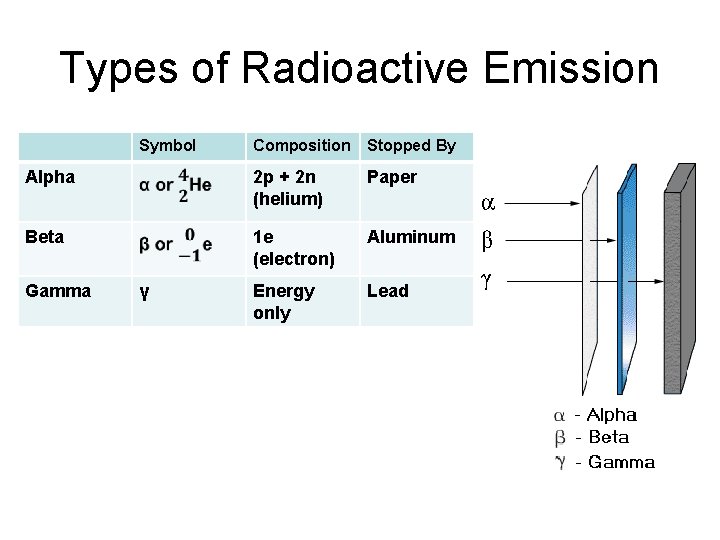
Types of Radioactive Emission Symbol Composition Stopped By Alpha 2 p + 2 n (helium) Paper Beta 1 e (electron) Aluminum Energy only Lead Gamma γ

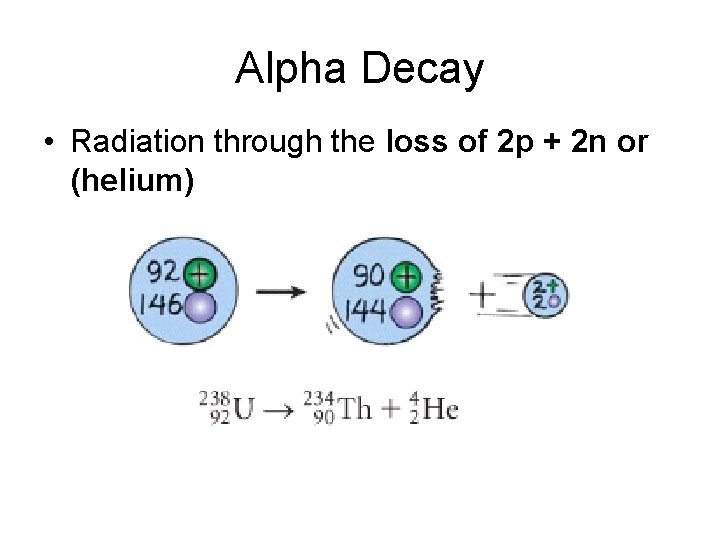
Alpha Decay • Radiation through the loss of 2 p + 2 n or (helium)

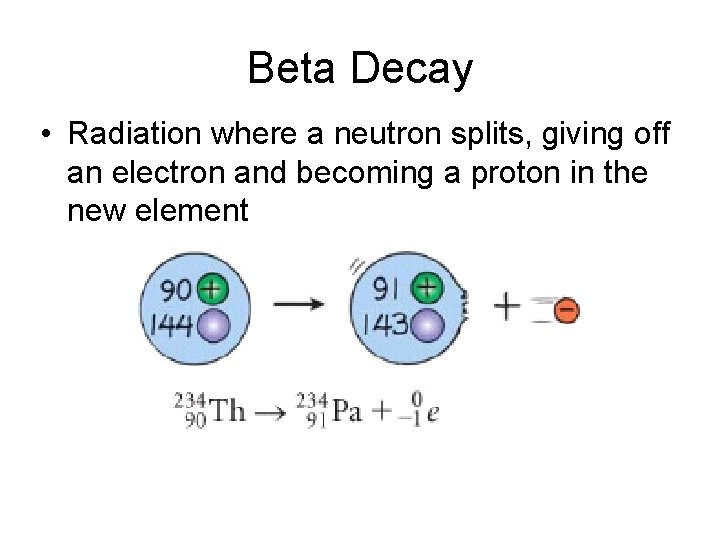
Beta Decay • Radiation where a neutron splits, giving off an electron and becoming a proton in the new element

Beta Decay

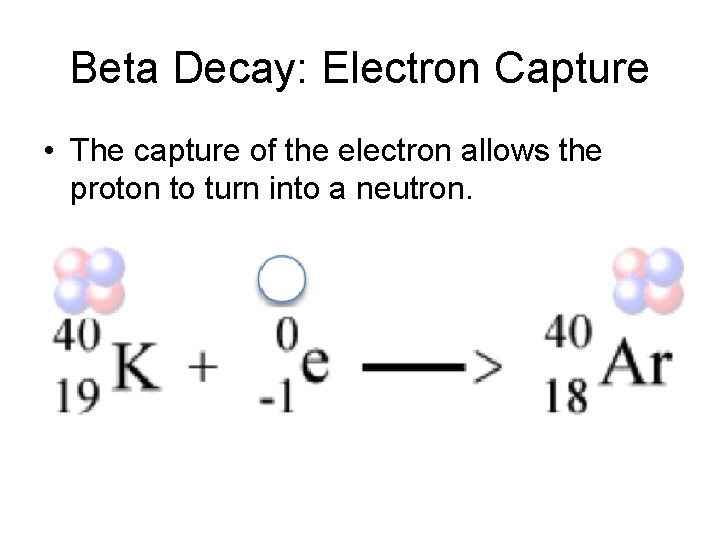
Beta Decay: Electron Capture • The capture of the electron allows the proton to turn into a neutron.

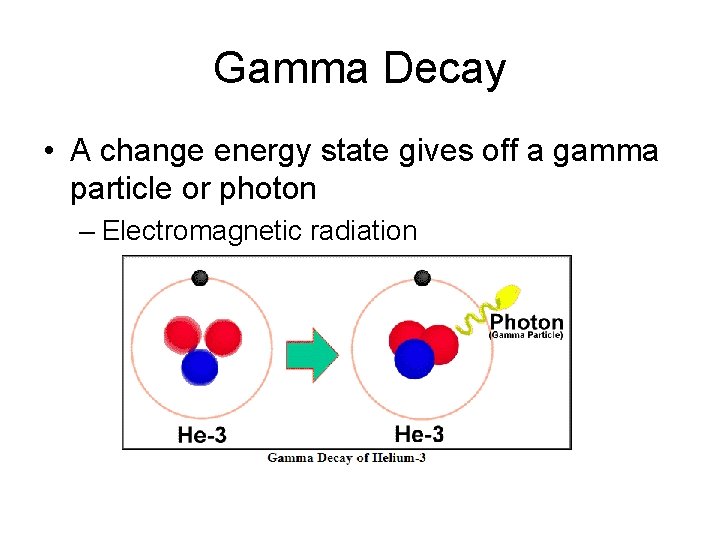
Gamma Decay • A change energy state gives off a gamma particle or photon – Electromagnetic radiation

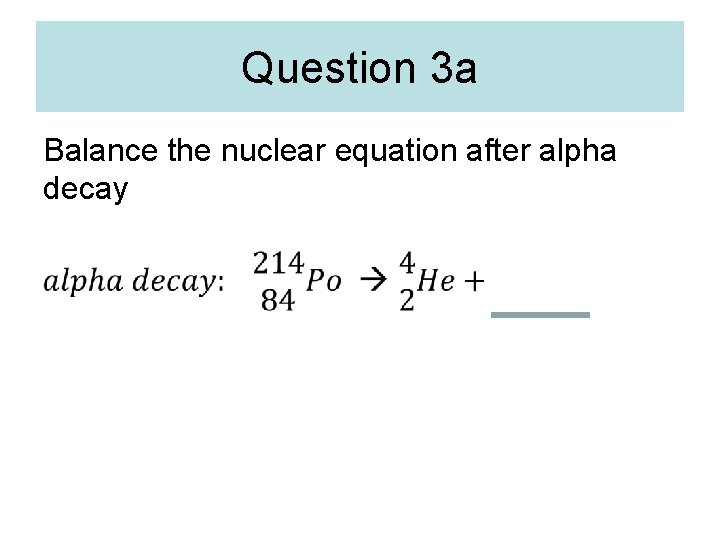
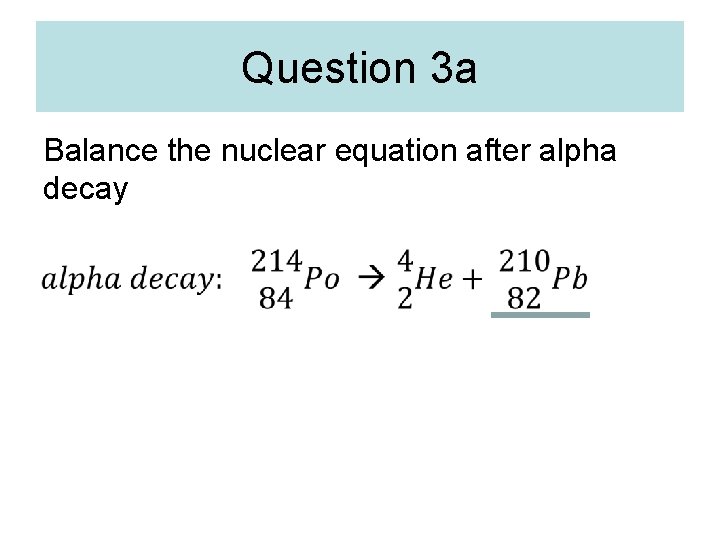
Question 3 a Balance the nuclear equation after alpha decay

Question 3 a Balance the nuclear equation after alpha decay

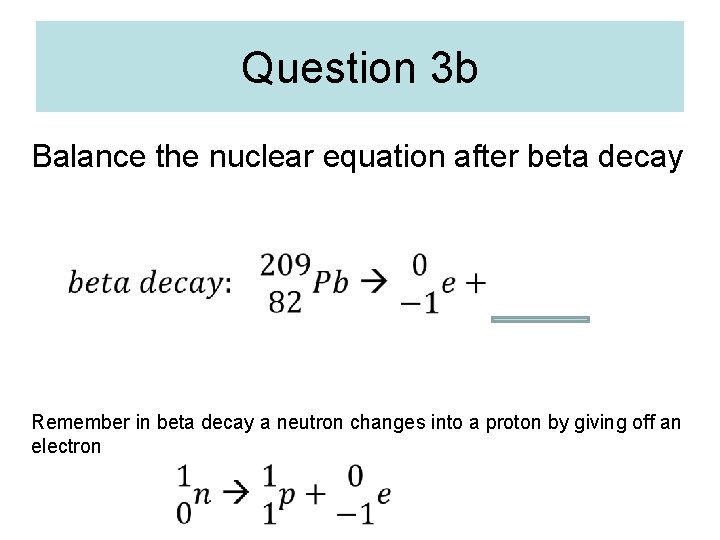
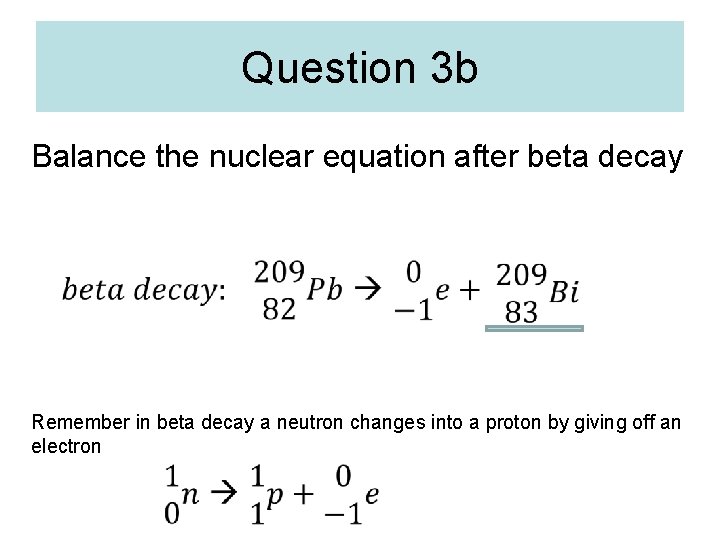
Question 3 b Balance the nuclear equation after beta decay Remember in beta decay a neutron changes into a proton by giving off an electron

Question 3 b Balance the nuclear equation after beta decay Remember in beta decay a neutron changes into a proton by giving off an electron
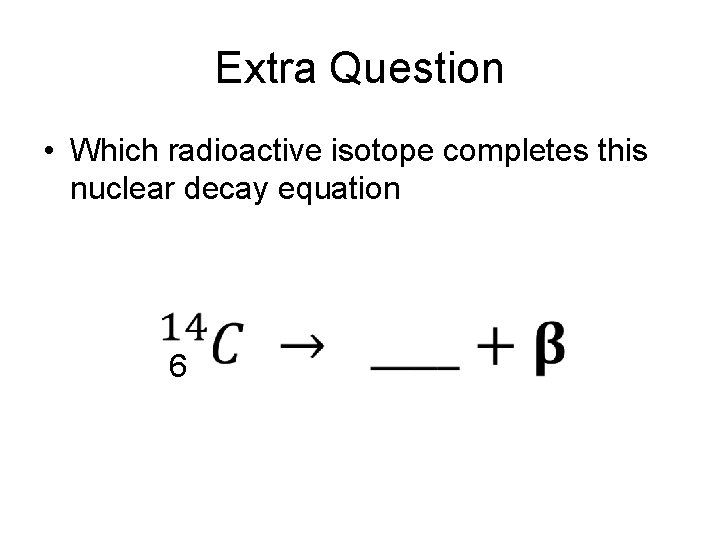
Extra Question • Which radioactive isotope completes this nuclear decay equation 6

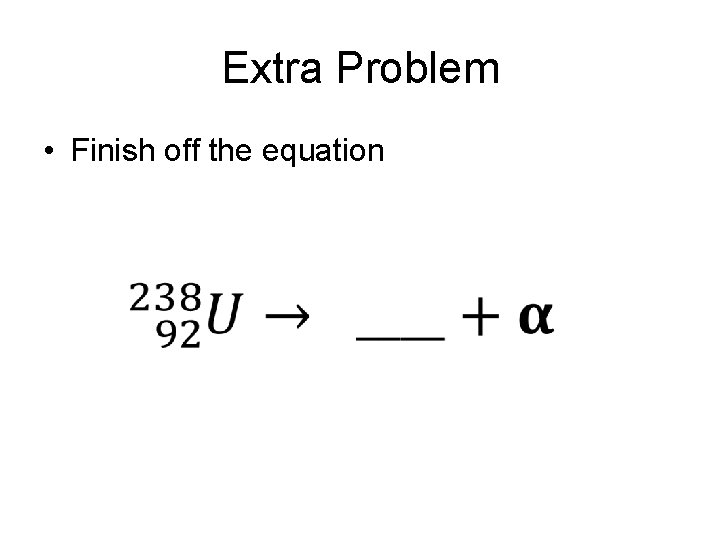
Extra Problem • Finish off the equation

Show what you know





Warm-up Questions 1. An element decays multiple times, emitting 2 alpha particles and 1 beta particle. What happens to its atomic number? 2. The nucleus of an atom consists of… 3. The force that holds the nucleus together is the ______ force. 4. If 1/8 of a sample is left and it has a half life of 1 billions years, what is the samples age? 5. An alpha particle consists of… 6. If a radioactive material has a half life of 3 year, what percentage would be left after 3 years?

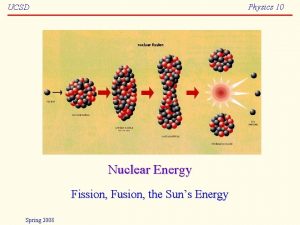
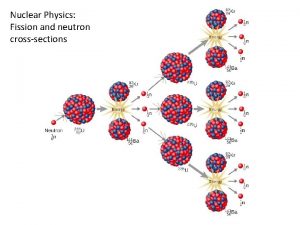
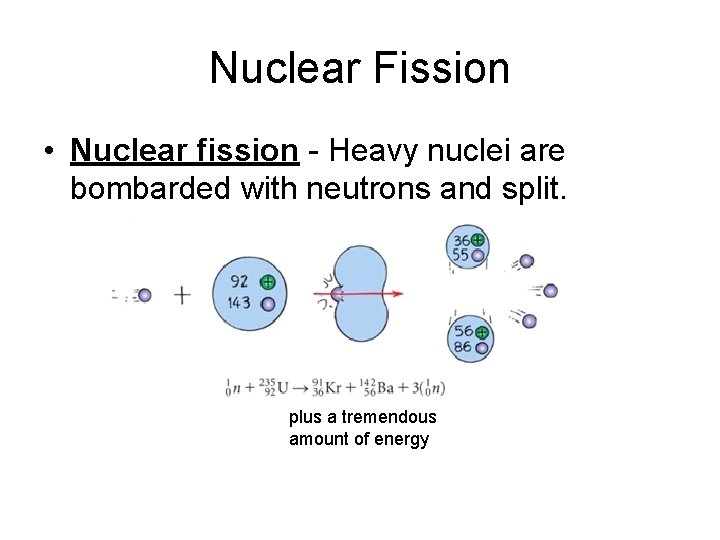
Nuclear Fission • Nuclear fission - Heavy nuclei are bombarded with neutrons and split. plus a tremendous amount of energy

Nuclear fission • Mass of particles produced is slightly less than the mass of the reactants. This mass is converted into energy. (E=mc 2)

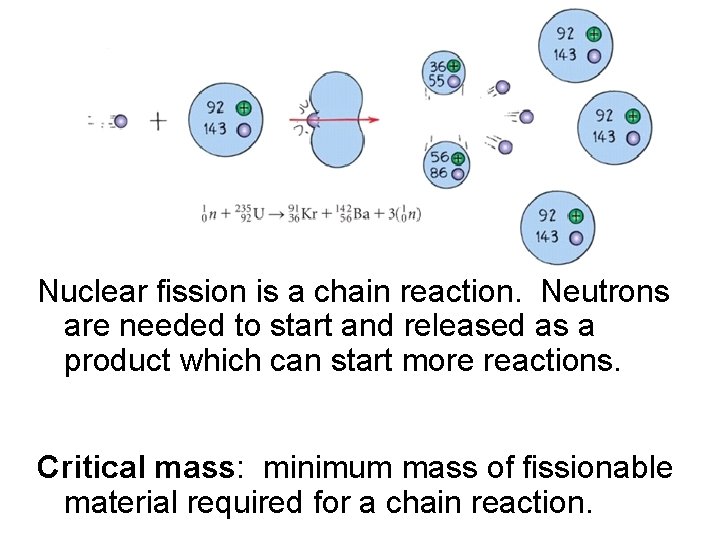
Nuclear fission is a chain reaction. Neutrons are needed to start and released as a product which can start more reactions. Critical mass: minimum mass of fissionable material required for a chain reaction.

Problems with Fission • Nuclear fission produces radioactive waste that has a large half life. U-235 Uranium 235 – Half life of U-235 is 713 million years • We cannot get rid of this dangerous product so we store it away from anything it can harm. – We deeply bury • Meltdown if cooling system fails the reactor can overheat and melt releasing radioactive materials

• Nuclear fusion – combination of small nuclei into larger with release of energy. • Mass of particles produced is much less than the mass of the reactants. • This mass is converted into energy. (E=mc 2) • Can release up to 10 times that of fission • Occurs naturally in our sun and other stars • Does not give off radioactive waste

Problems with Fusion • Fusion requires high temperatures like those in the stars. • We cannot sustain these temperatures without vaporizing the container of the fusion reaction. • Today many are looking into ways of making fusion work under sustainable conditions
 Discuss the following questions
Discuss the following questions Discuss the questions with your partner
Discuss the questions with your partner Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear In pairs discuss the following questions
In pairs discuss the following questions Work in pairs and discuss the questions
Work in pairs and discuss the questions Discuss the following questions
Discuss the following questions Discuss the following questions
Discuss the following questions Discuss the following questions in groups
Discuss the following questions in groups Work in pairs. answer the questions.
Work in pairs. answer the questions. Discuss the following questions
Discuss the following questions Amateurs talk tactics professionals talk logistics
Amateurs talk tactics professionals talk logistics Discuss these questions with your partner
Discuss these questions with your partner Physics topic 12
Physics topic 12 Shell model of nucleus
Shell model of nucleus Skobeltsyn institute of nuclear physics
Skobeltsyn institute of nuclear physics Nuclear energy
Nuclear energy Scattering cross section in nuclear physics
Scattering cross section in nuclear physics Budker institute of nuclear physics
Budker institute of nuclear physics Petersburg nuclear physics institute
Petersburg nuclear physics institute Physics topic 12
Physics topic 12 Nuclear physics
Nuclear physics Nuclear physics topics for presentation
Nuclear physics topics for presentation Nuclear physics
Nuclear physics Nuclear physics
Nuclear physics Nuclear physics
Nuclear physics Nuclear physics b
Nuclear physics b Magic number
Magic number Nuclear physics
Nuclear physics Budker
Budker Nuclear physics day
Nuclear physics day Your notebook define the following terms in your own words
Your notebook define the following terms in your own words Write down your answer
Write down your answer Answer the following question
Answer the following question Do the following in your notebook
Do the following in your notebook In your journal notebook answer the following question
In your journal notebook answer the following question Trimester fill in worksheet answers
Trimester fill in worksheet answers Answer the following question on your notebook
Answer the following question on your notebook Answer the following question on your notebook
Answer the following question on your notebook