Nuclear PBL Unit Day 2 Radioactive Particles Chart

Nuclear PBL Unit Day 2 Radioactive Particles Chart Alpha, Beta Decay Practice

Objectives: ◦ Today I will be able to: Differentiate between the composition and materials required to shield alpha, beta, gamma, positron and electron capture emissions Correctly solve and balance radioactive equations Informal assessment – monitor student interactions as they complete the radioactive particles chart Formal assessment – analyzing responses to the radioactive particles chart and the alpha and beta decay practice Common Core Connection ◦ Build Strong Content Knowledge ◦ Value Evidence ◦ Construct viable arguments and critique the reasoning of others

Lesson Sequence Evaluate: Warm – Up Explore/Explain: Radioactive Particles Chart Explain: Alpha and Beta Decay Notes Elaborate: Alpha and Beta Decay Practice Evaluate: Exit Ticket

Warm – Up Where is the nuclear reactor in Maryland? How many nuclear reactors are in the United States? Which region of the United States has the highest concentration of nuclear reactors
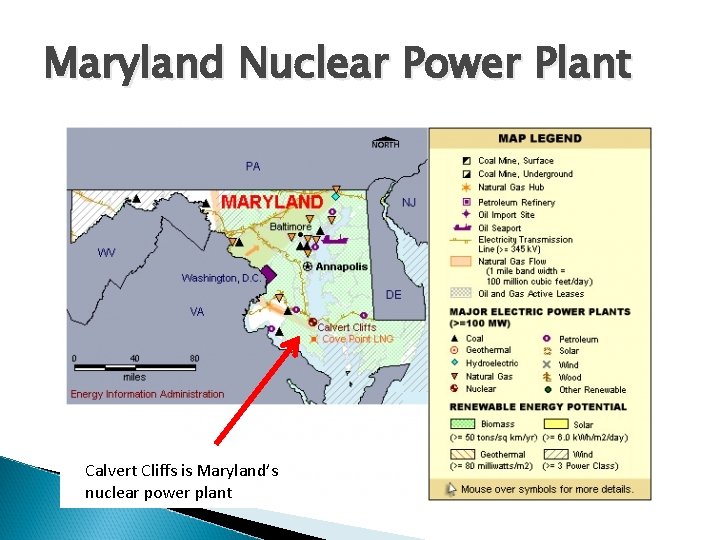
Maryland Nuclear Power Plant Calvert Cliffs is Maryland’s nuclear power plant
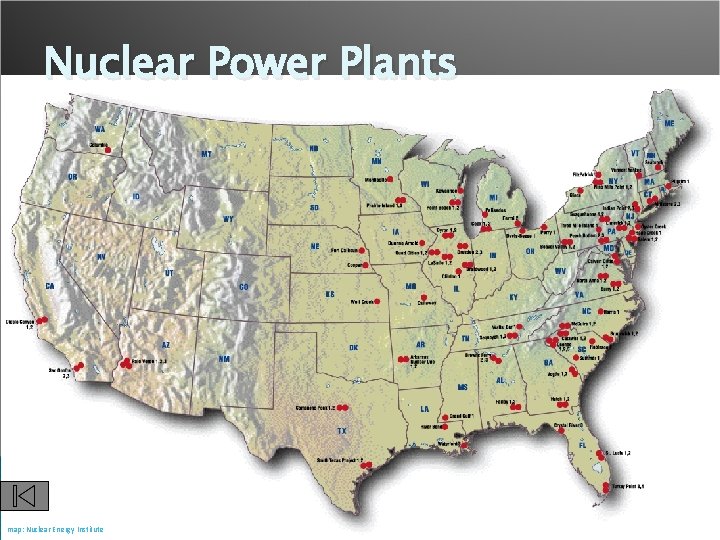
Nuclear Power Plants map: Nuclear Energy Institute

Objective Today I will be able to: ◦ Differentiate between the composition and materials required to shield alpha, beta, gamma, positron and electron capture emissions ◦ Correctly solve and balance radioactive equations

Homework Finish Alpha and Beta Decay Practice Answer these questions about your radioactive incident ◦ • What is the isotope for your radioactive incident? ◦ • Which type of nuclear decay is your isotope undergoing? ◦ • Write the decay equation for your isotope. Study for Atomic Structure, Mole and History of Atom Quiz on Thursday, October 9 STEM Fair Data Collection and Analysis Due Friday, October 10

Agenda Warm – Up Radioactive Particles Chart Alpha and Beta Decay Notes Alpha and Beta Decay Practice Exit Ticket

Radioactive Particles Chart
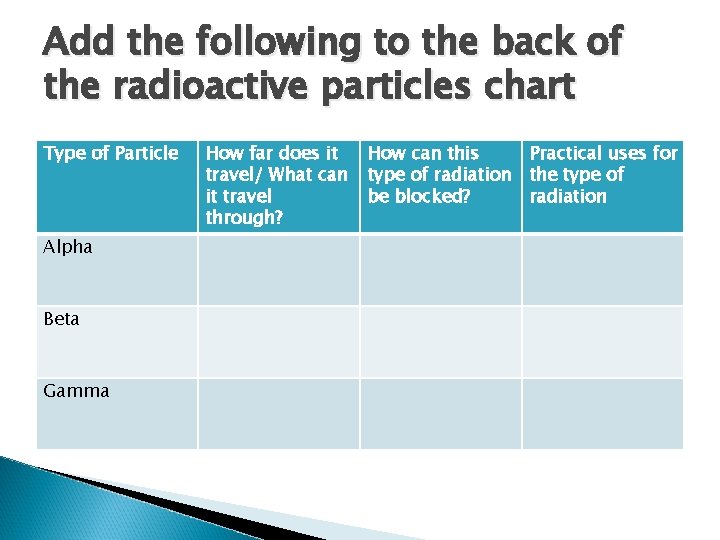
Add the following to the back of the radioactive particles chart Type of Particle Alpha Beta Gamma How far does it travel/ What can it travel through? How can this Practical uses for type of radiation the type of be blocked? radiation

Radioactivity Review Notes
Radioactive Decay Chains A nucleus goes through a series of decays and states before it reaches a stable configuration Each step in the chain will have its own unique characteristics of half-life and own type of radiation emitted Understanding helps make nuclear reactors (power) and weapons!
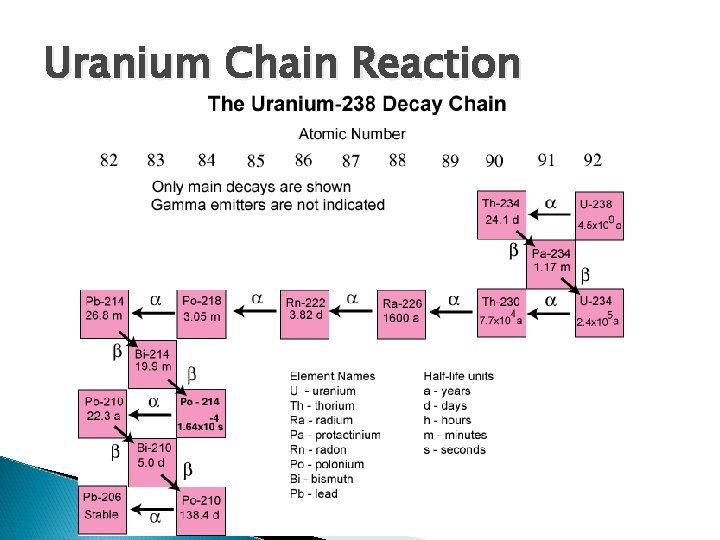
Uranium Chain Reaction
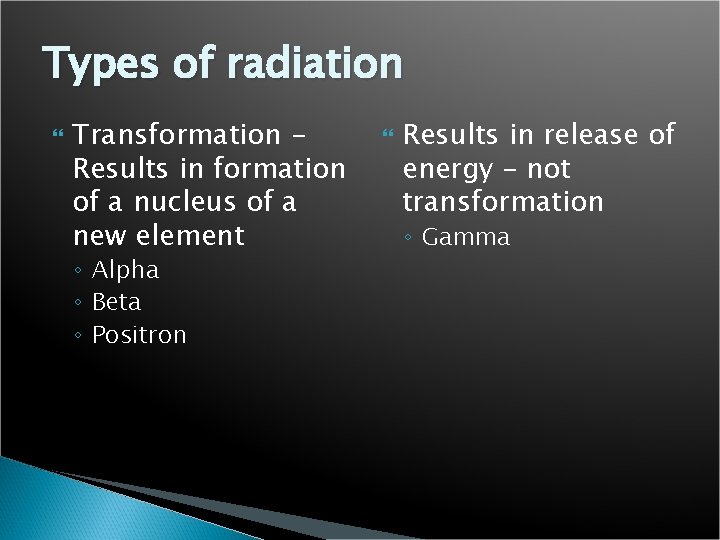
Types of radiation Transformation Results in formation of a nucleus of a new element ◦ Alpha ◦ Beta ◦ Positron Results in release of energy – not transformation ◦ Gamma

Radioactive Decay – Alpha Decay Stream of high energy alpha particles Consists of 2 protons and 2 neutrons (identical to a positively charged particle of a Helium nuclei) An alpha particle is composed of two protons and two neutrons, so it can be represented by a Helium-4 atom

Radioactive Decay – Alpha Decay When an alpha particle breaks away from the nucleus of a radioactive atom, it has no electrons, so it carries a +2 charge An alpha particle is typically shown with no charge, because it very rapidly picks up two electrons and becomes a neutral helium atom, instead of an ion α 4 2 He 4 2
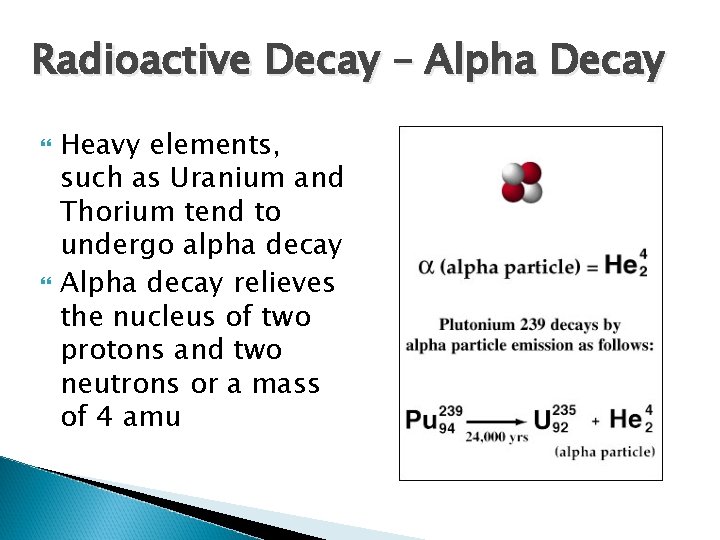
Radioactive Decay – Alpha Decay Heavy elements, such as Uranium and Thorium tend to undergo alpha decay Alpha decay relieves the nucleus of two protons and two neutrons or a mass of 4 amu

Radioactive Decay – Alpha Decay Easily stopped by clothes or paper Only travel several cm through air Usually does not pose a health risk unless the source of radiation enters the body

Radioactive Decay – Beta Decay Consists of a stream of high speed electrons A neutron breaks down into one p+ and one e. The p+ stays in nucleus and e- leaves at high speed Is able to pass through clothing and damage skin
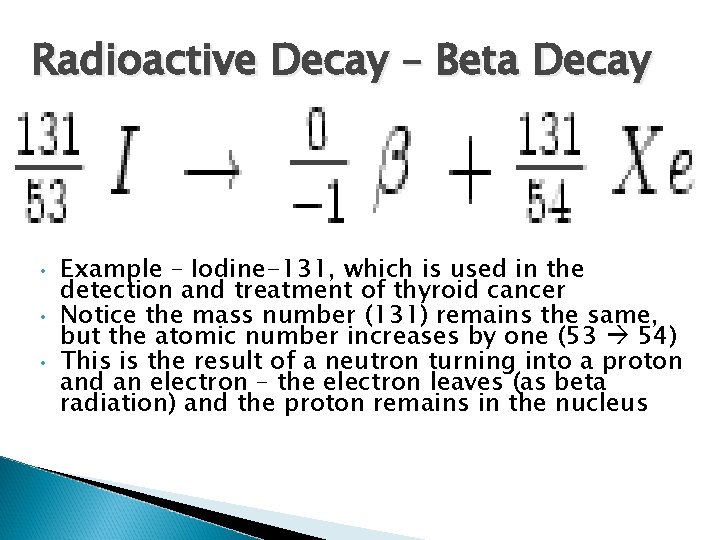
Radioactive Decay – Beta Decay • • • Example – Iodine-131, which is used in the detection and treatment of thyroid cancer Notice the mass number (131) remains the same, but the atomic number increases by one (53 54) This is the result of a neutron turning into a proton and an electron – the electron leaves (as beta radiation) and the proton remains in the nucleus
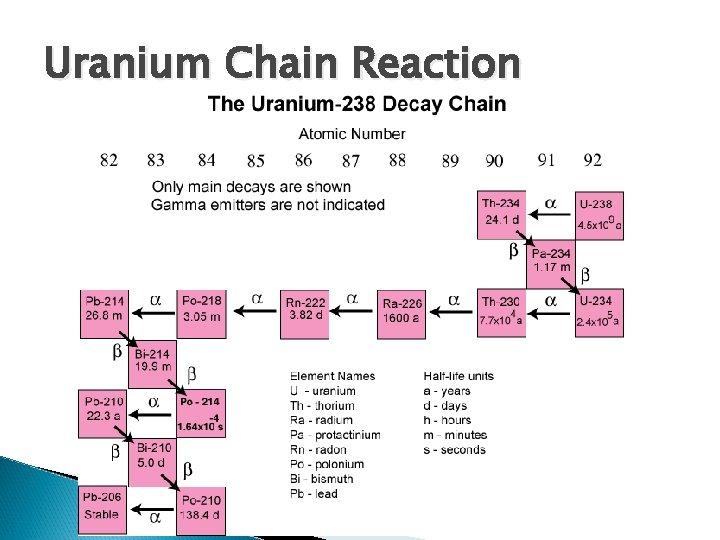
Uranium Chain Reaction

Radioactive Decay – Gamma Radiation Is a form of light (energy) that our eyes don’t see Similar to X-rays – high energy, short wavelength radiation No mass change associated with gamma radiation

Radioactive Decay – Gamma Radiation Does not consist of particles Usually accompanies alpha and beta radiation Isotopes, such as Cobalt-60 release gamma radiation Very penetrating – only stopped by lead or concrete
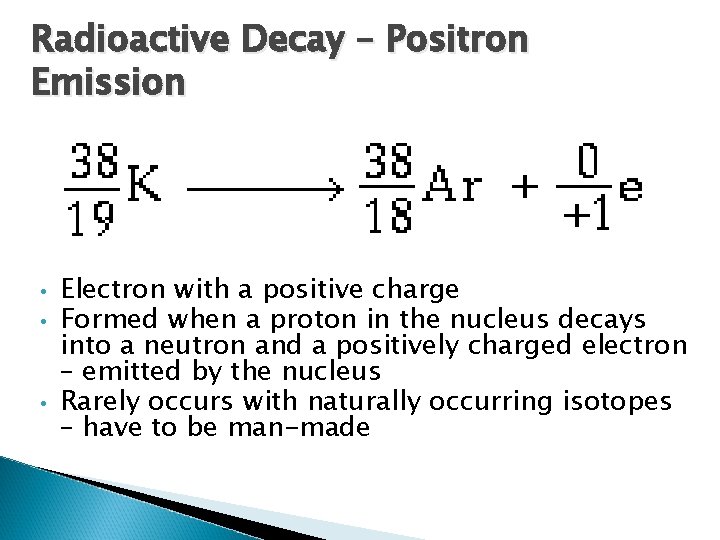
Radioactive Decay – Positron Emission • • • Electron with a positive charge Formed when a proton in the nucleus decays into a neutron and a positively charged electron – emitted by the nucleus Rarely occurs with naturally occurring isotopes – have to be man-made
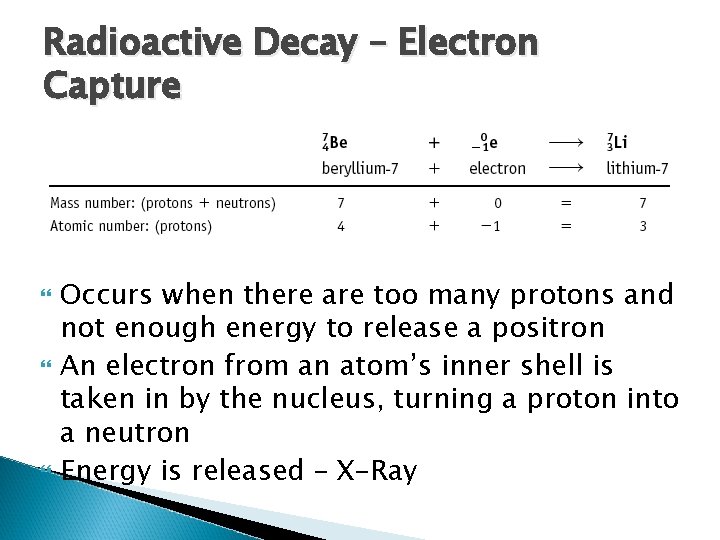
Radioactive Decay – Electron Capture Occurs when there are too many protons and not enough energy to release a positron An electron from an atom’s inner shell is taken in by the nucleus, turning a proton into a neutron Energy is released – X-Ray

Radioactive Decay – Practical Uses PET scans Smoke detectors Nuclear weapons (WWII) Source of electricity Food preservation Cancer treatment

Radioactive Decay – Practical Uses • Radioactive Dating - Uses the isotope, Carbon-14 - Produced in the atmosphere by cosmic radiation - A very small amount of CO 2 contains C-14, which is taken in by plants during photosynthesis - Animals eat plants, therefore C-14 is part of all living things

Radioactive Decay – Practical Uses Radioactive Dating (continued) - Once an organism dies, the amount of C-14 begins to decrease and the half-life (5, 730 years) can be used to determine the age - For non-living substance, other isotopes, such as potassium-40 are used
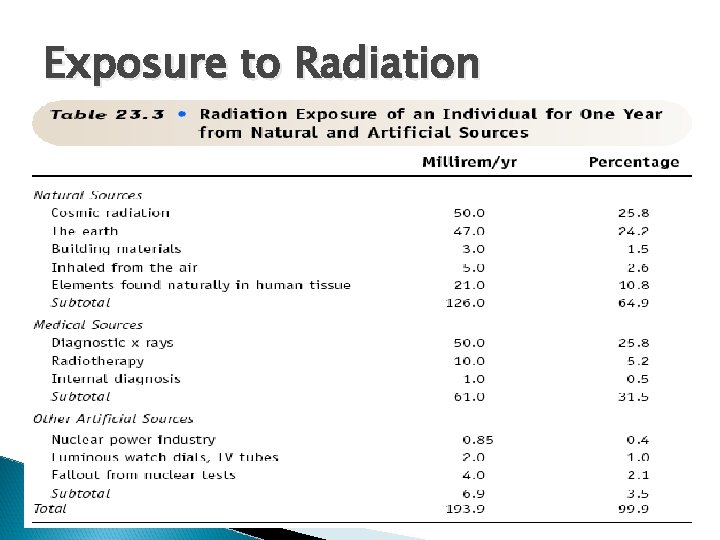
Exposure to Radiation
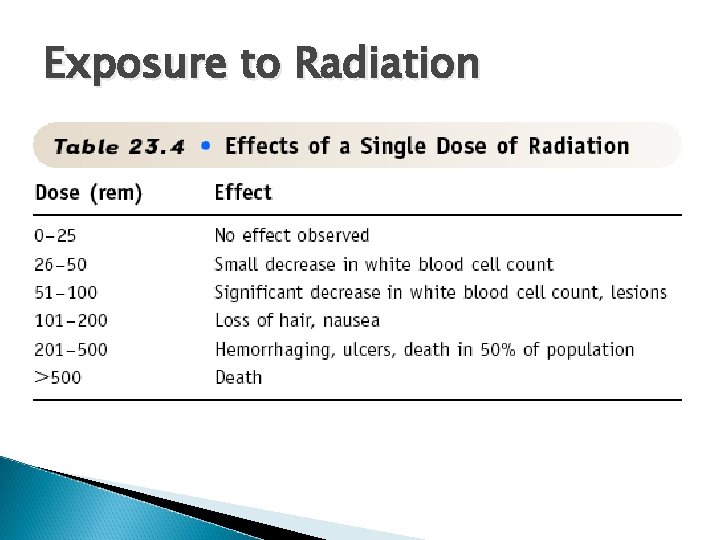
Exposure to Radiation

Alpha and Beta Decay Practice

Exit Ticket Write the radioactive decay equation for: ◦ The alpha decay of Uranium-238 ◦ The beta decay of Lead- 213
- Slides: 33