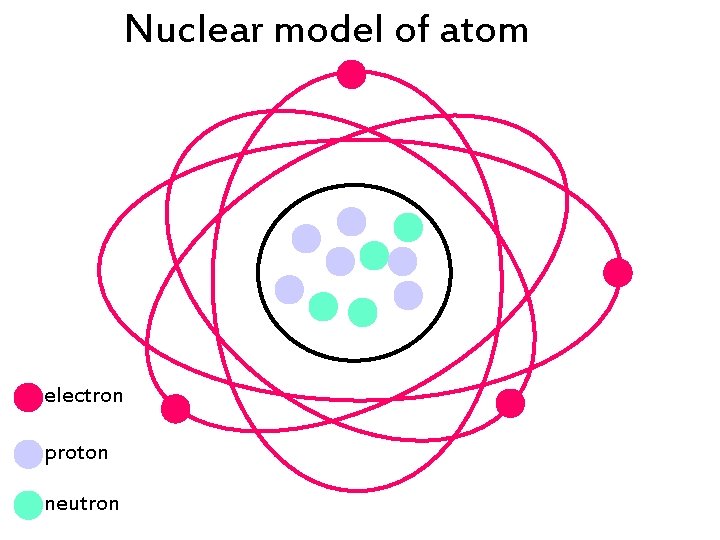
Nuclear model of atom electron proton neutron PARTICLE













- Slides: 56

Nuclear model of atom electron proton neutron -

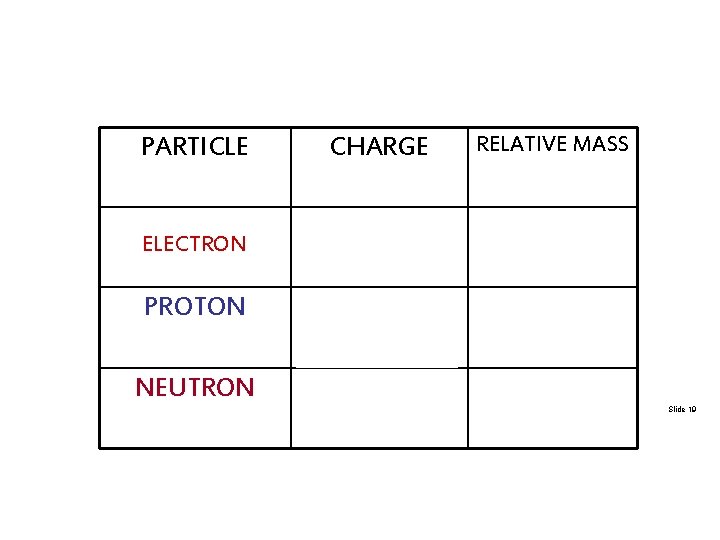
PARTICLE CHARGE RELATIVE MASS ELECTRON -1 (NEGATIVE) 1/2000 TH +1 (POSITIVE) NO CHARGE 1 UNIT PROTON NEUTRON (NEGLIGIBLE) 1 UNIT Slide 19

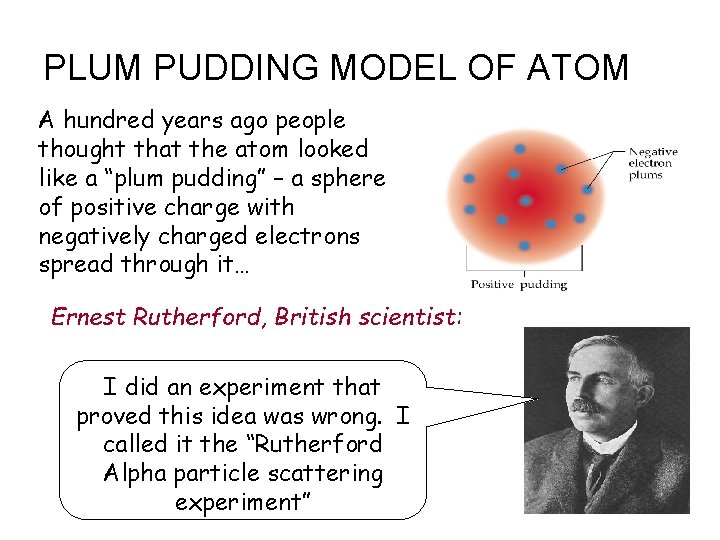
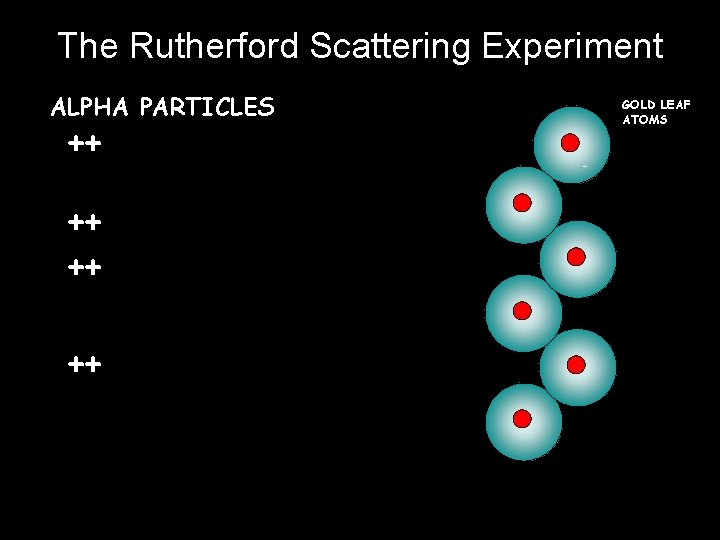
PLUM PUDDING MODEL OF ATOM A hundred years ago people thought that the atom looked like a “plum pudding” – a sphere of positive charge with negatively charged electrons spread through it… Ernest Rutherford, British scientist: I did an experiment that proved this idea was wrong. I called it the “Rutherford Alpha particle scattering experiment” _ -


The Rutherford Scattering Experiment ALPHA PARTICLES ++ ++ GOLD LEAF ATOMS -

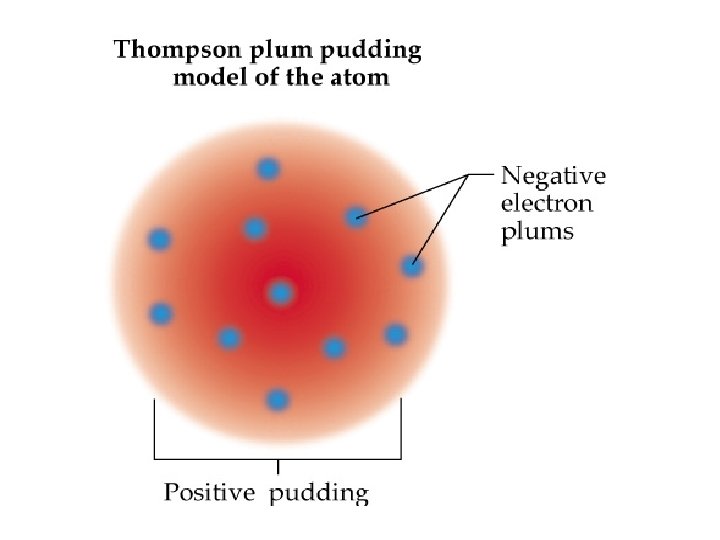
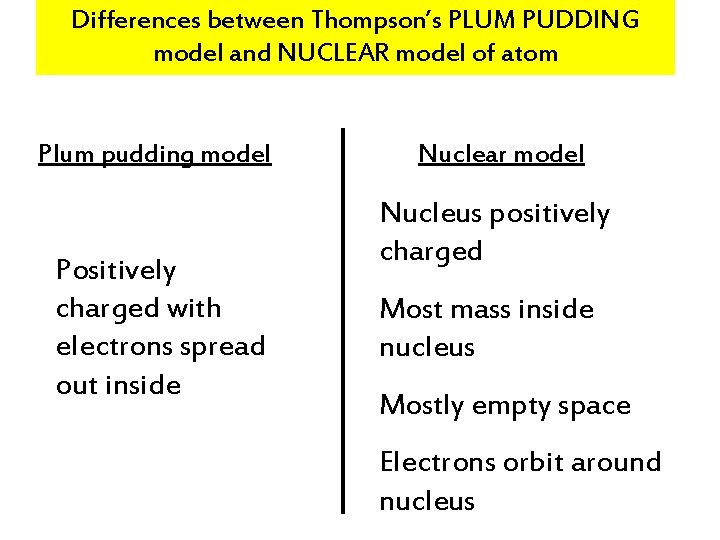
Differences between Thompson’s PLUM PUDDING model and NUCLEAR model of atom Plum pudding model Positively charged with electrons spread out inside Nuclear model Nucleus positively charged Most mass inside nucleus Mostly empty space Electrons orbit around nucleus

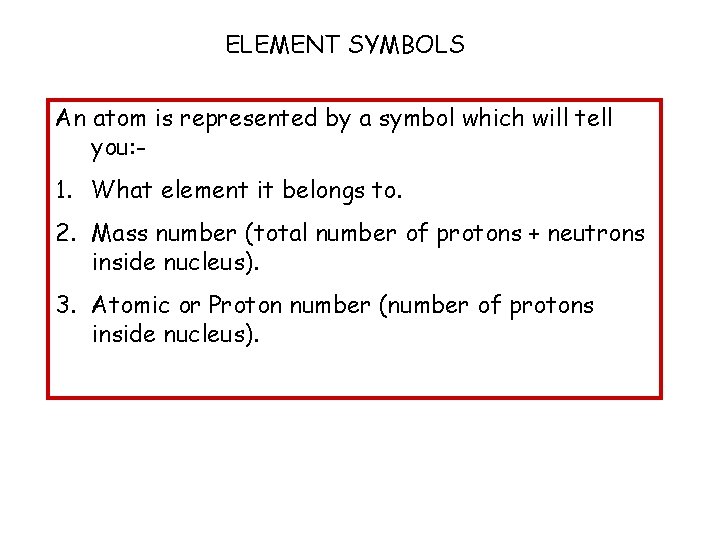
ELEMENT SYMBOLS An atom is represented by a symbol which will tell you: - 1. What element it belongs to. 2. Mass number (total number of protons + neutrons inside nucleus). 3. Atomic or Proton number (number of protons inside nucleus).

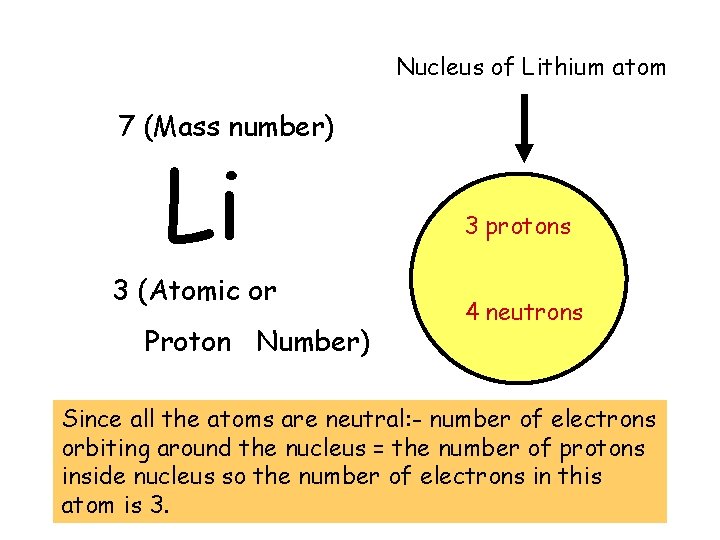
Nucleus of Lithium atom 7 (Mass number) Li 3 (Atomic or Proton Number) 3 protons 4 neutrons Since all the atoms are neutral: - number of electrons orbiting around the nucleus = the number of protons inside nucleus so the number of electrons in this atom is 3.

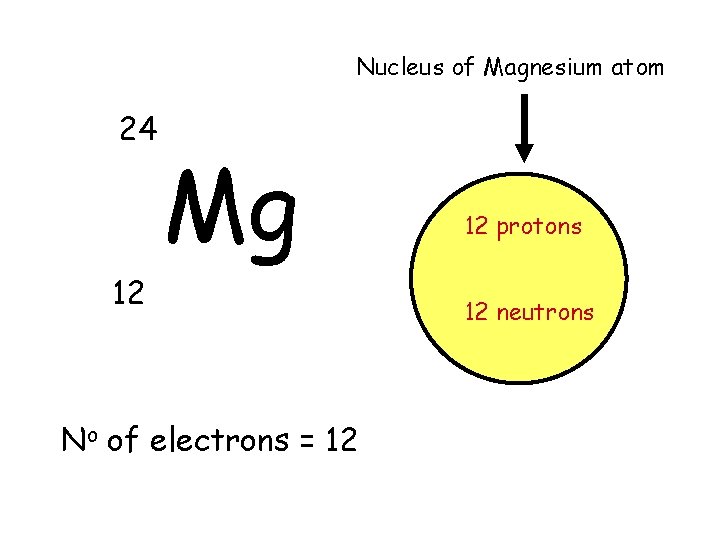
Nucleus of Magnesium atom 24 Mg 12 No of electrons = 12 12 protons 12 neutrons

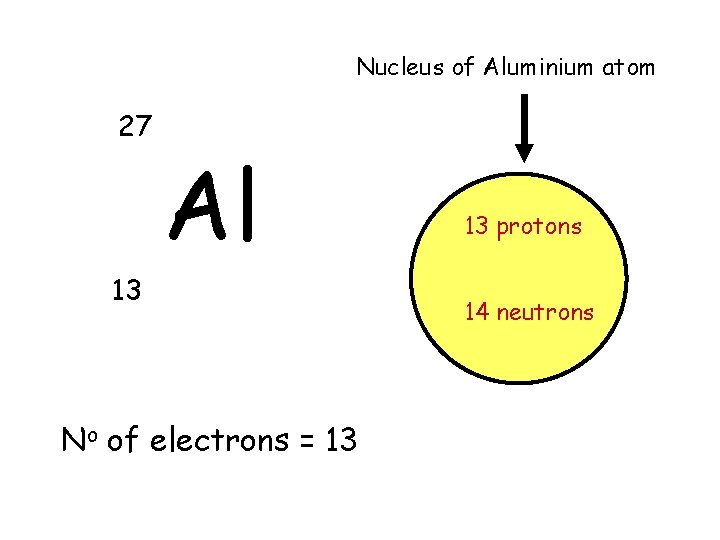
Nucleus of Aluminium atom 27 Al 13 No of electrons = 13 13 protons 14 neutrons

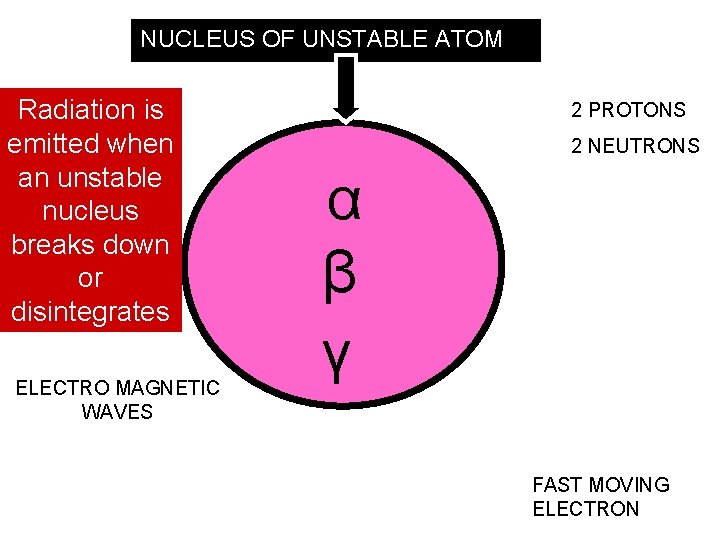
There are 3 types of radiation α (alpha particle) Helium nuclei 2 protons and 2 neutrons β (beta particle) fast moving electron γ (gamma rays) electro magnetic waves All 3 types come from the nucleus of the atom!!

Radioactive substances have atoms with unstable nuclei This means that there an unequal number of protons and neutrons inside the nucleus

NUCLEUS OF UNSTABLE ATOM Radiation is emitted when an unstable nucleus breaks down or disintegrates ELECTRO MAGNETIC WAVES 2 PROTONS 2 NEUTRONS α β γ FAST MOVING ELECTRON

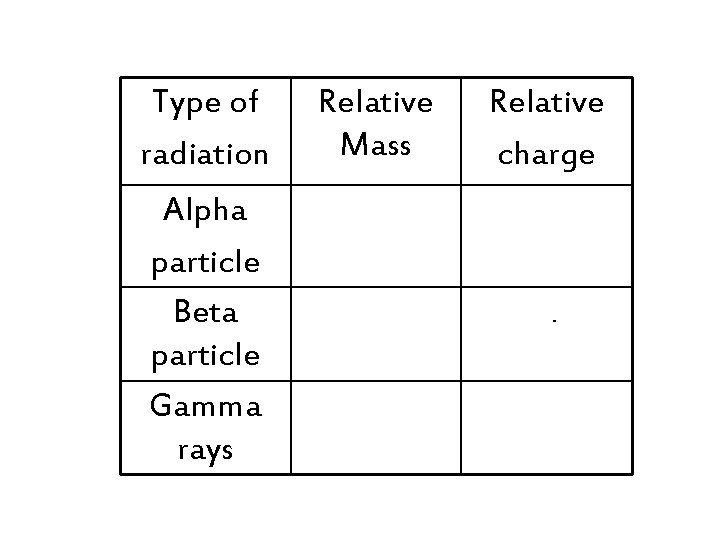
Type of radiation Relative Mass Relative charge Alpha particle Beta particle Gamma rays 4 +2 1/2000 th -1 no mass no charge

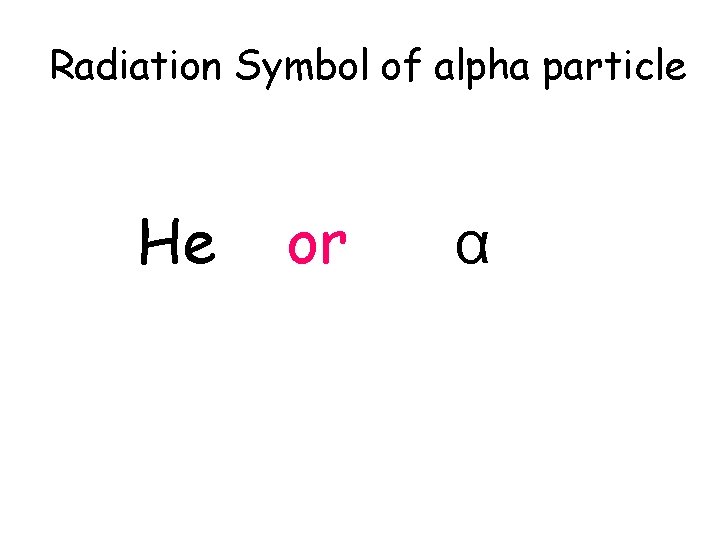
Radiation Symbol of alpha particle 4 2 He or 4 2 α

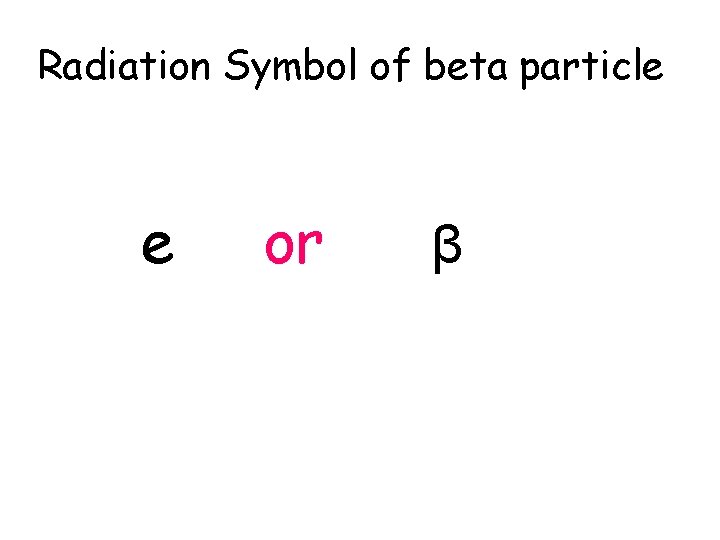
Radiation Symbol of beta particle 0 -1 e or 0 -1 β

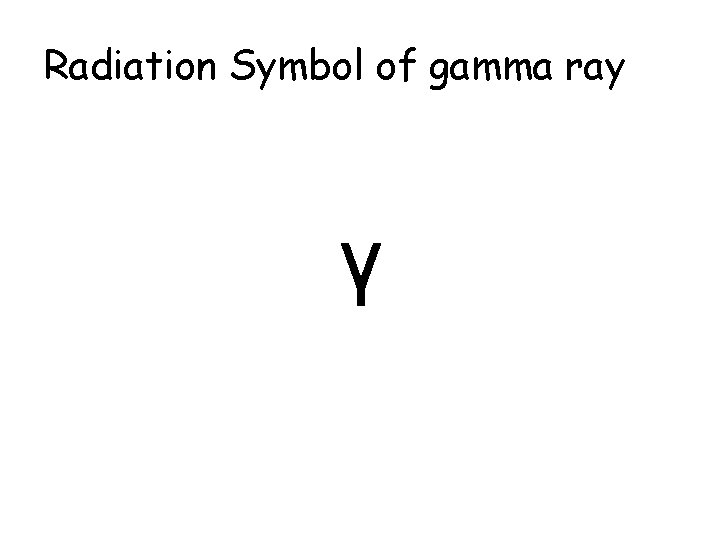
Radiation Symbol of gamma ray γ

NUCLEAR EQUATIONS Nuclear equations have to balance, just like chemical equations!!!!!!!

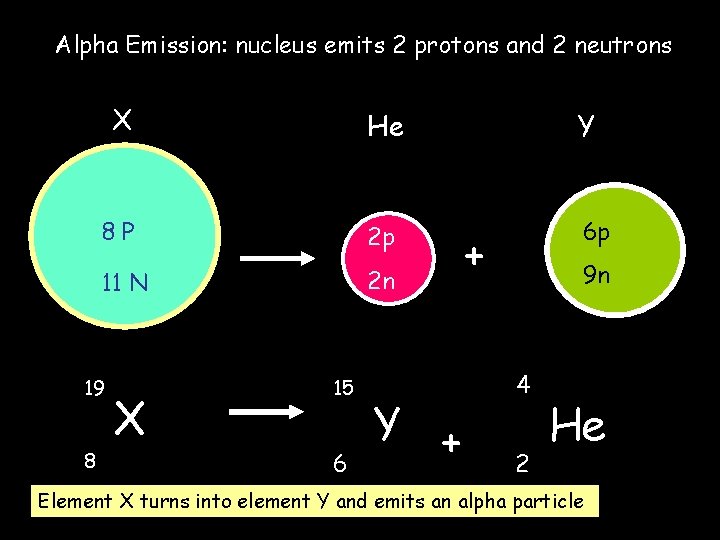
Alpha Emission: nucleus emits 2 protons and 2 neutrons X He Y 8 P 2 p 6 p 11 N 2 n 19 8 X 15 6 Y + + 9 n 4 2 He Element X turns into element Y and emits an alpha particle

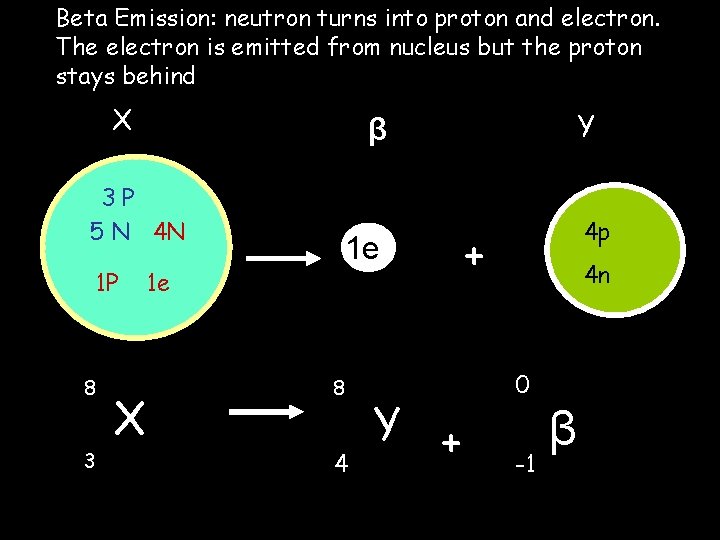
Beta Emission: neutron turns into proton and electron. The electron is emitted from nucleus but the proton stays behind X 3 P 5 N 4 N 1 P 8 3 X Y β 1 e 1 e 8 4 Y + 4 p + 4 n 0 -1 β

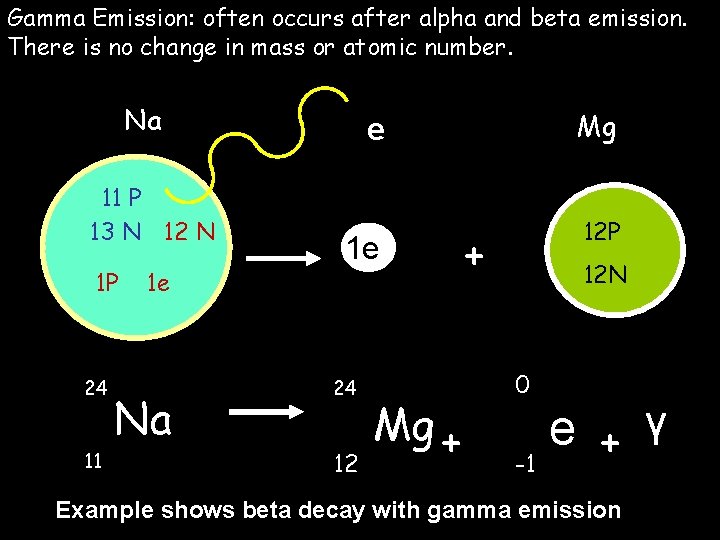
Gamma Emission: often occurs after alpha and beta emission. There is no change in mass or atomic number. Na 11 P 13 N 12 N 1 P 24 11 1 e Na Mg e 1 e 24 12 Mg + 12 P + 12 N 0 e + γ -1 Example shows beta decay with gamma emission


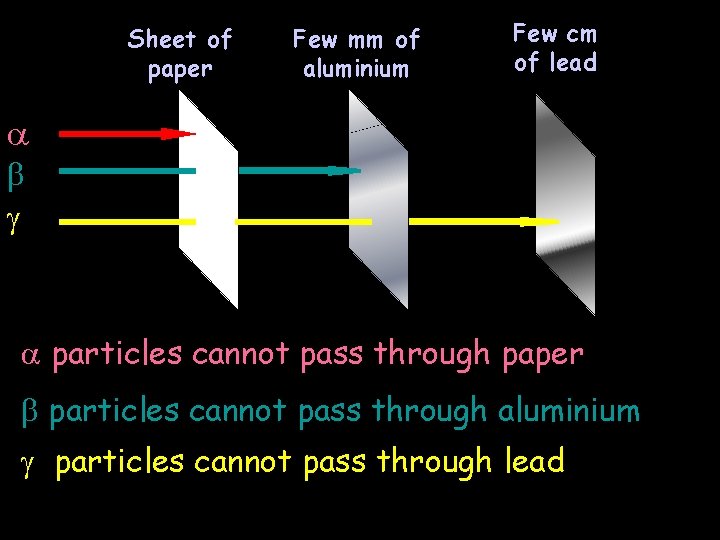
Few cm Powers of Radiation Sheet. Penetrating of Few mm of of lead paper aluminium particles cannot pass through paper particles cannot pass through aluminium particles cannot pass through lead

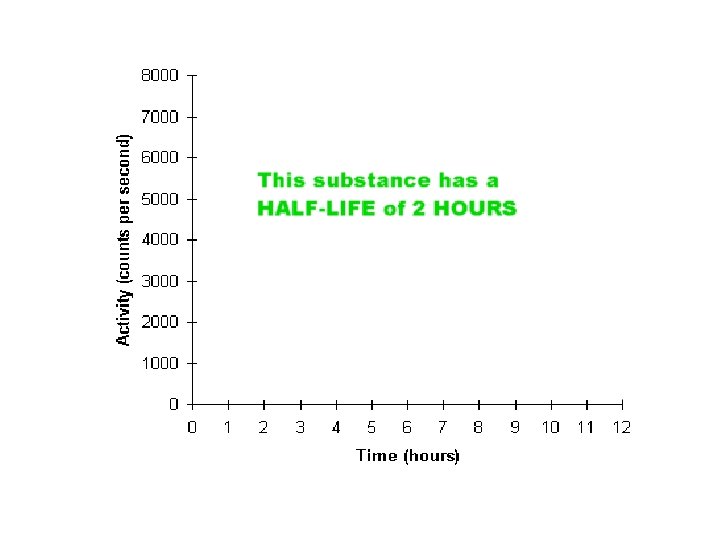
HALF LIFE TIME TAKEN FOR ½ THE RADIOACTIVE ATOMS TO DISINTEGRATE OR TIME IT TAKES FOR THE RADIATION TO FALL TO ½ ITS ORIGINAL LEVEL

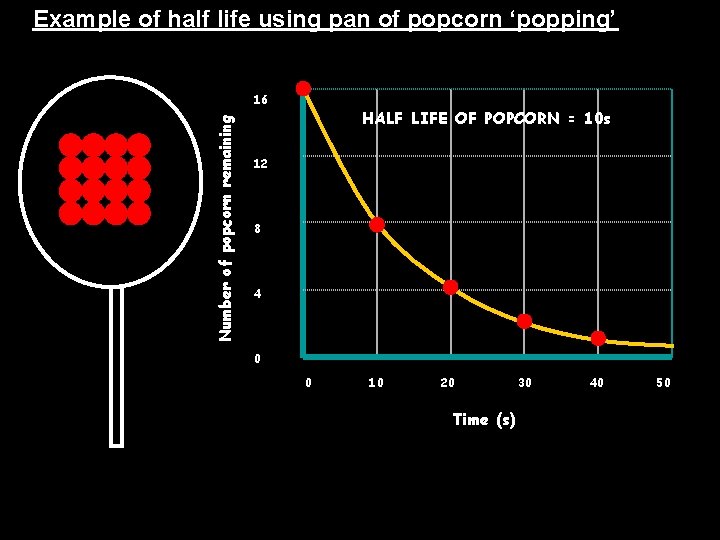
Example of half life using pan of popcorn ‘popping’ Number of popcorn remaining 16 HALF LIFE OF POPCORN = 10 s 12 8 4 0 0 10 20 Time (s) 30 40 50

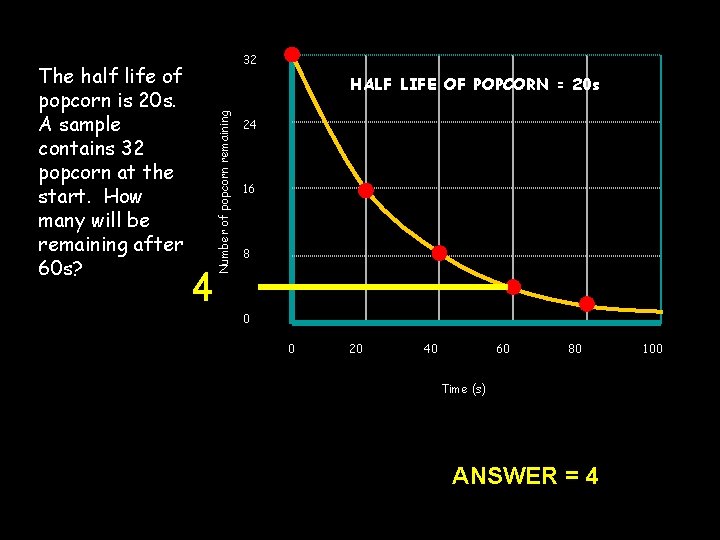
Question on half life of pop corn HALF LIFE OF POPCORN = 20 s 4 Number of popcorn remaining The half life of popcorn is 20 s. A sample contains 32 popcorn at the start. How many will be remaining after 60 s? 32 24 16 8 0 0 20 40 60 80 Time (s) 32 16 20 s 44 8 20 s ANSWER = 4 100


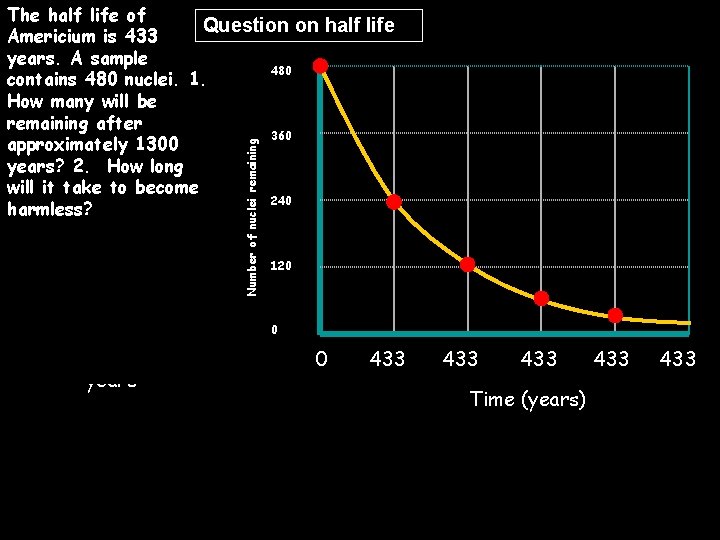
Number of nuclei remaining The half life of Question on half life Americium is 433 years. A sample 480 contains 480 nuclei. 1. How many will be remaining after 360 approximately 1300 years? 2. How long will it take to become 240 harmless? 1. 60 nuclei 2. 6 x 433 years 3. 120 0 = 2598 years 480 240 433 433 Time (years) 120 433 60 433 30 433 16 433 8 433 433


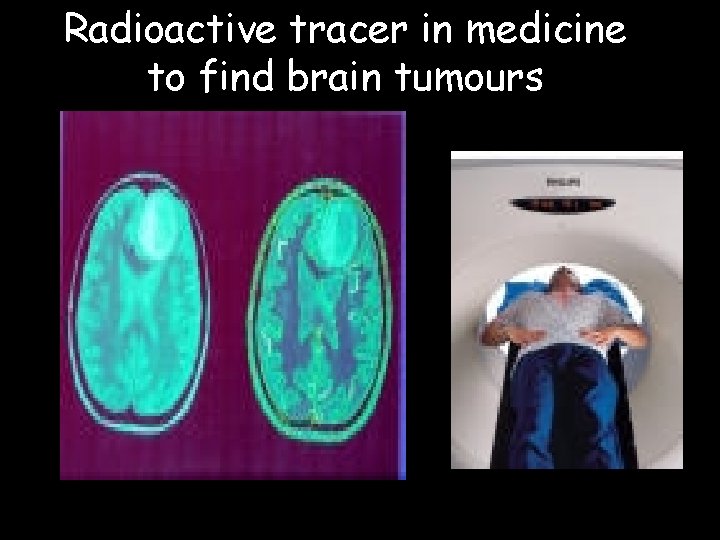
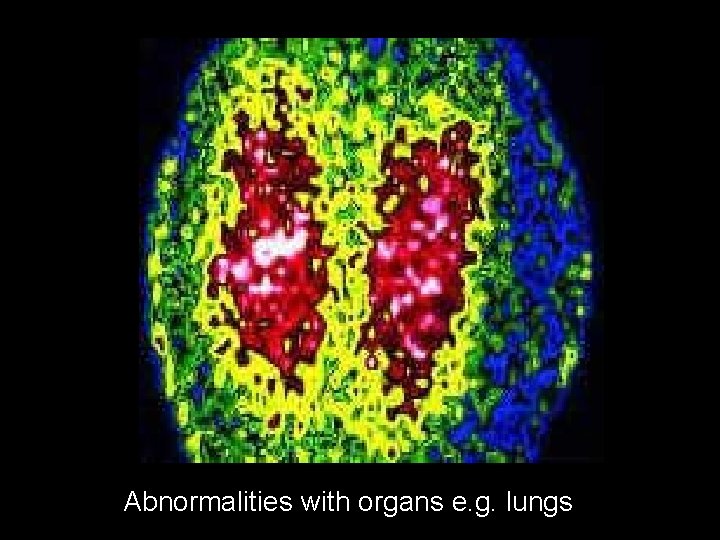
Radioactive tracer in medicine to find brain tumours

Abnormalities with organs e. g. lungs

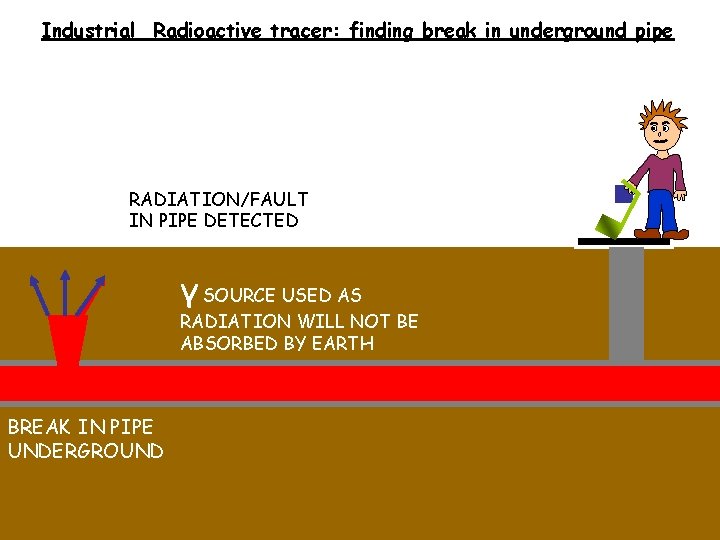
Industrial Radioactive tracer: finding break in underground pipe RADIATION/FAULT IN PIPE DETECTED γ SOURCE USED AS RADIATION WILL NOT BE ABSORBED BY EARTH BREAK IN PIPE UNDERGROUND

STERILIZATION OF SURGICAL EQUIPMENT AND FRESH FOOD

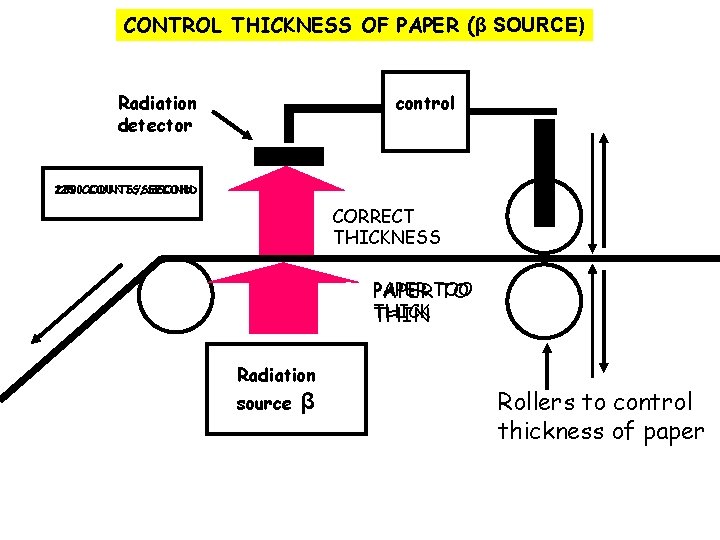
CONTROL THICKNESS OF PAPER (β SOURCE) Radiation detector control 200 150 250 COUNTS/SECOND CORRECT THICKNESS PAPER TO TOO PAPER TO THICK THIN Radiation source β Rollers to control thickness of paper

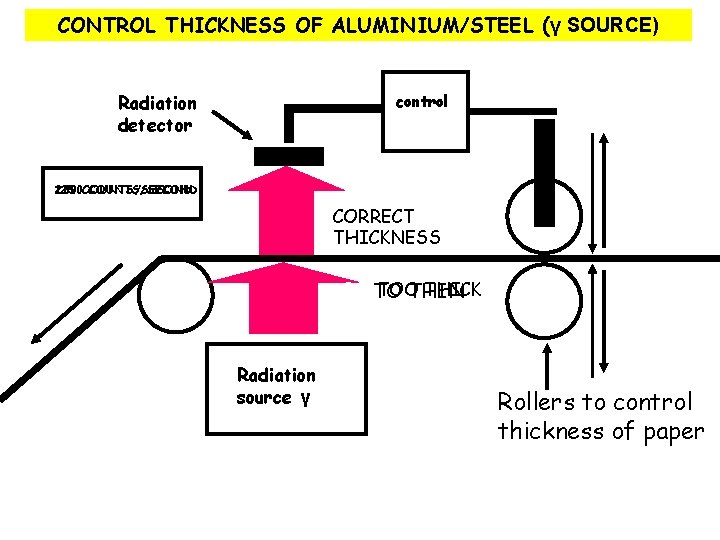
CONTROL THICKNESS OF ALUMINIUM/STEEL (γ SOURCE) Radiation detector control 200 150 250 COUNTS/SECOND CORRECT THICKNESS PAPER TOO TO THICK TO THIN THICK Radiation source γ Rollers to control thickness of paper

CARBON DATING OF ONCE LIVING THINGS ETC. FOSSILS

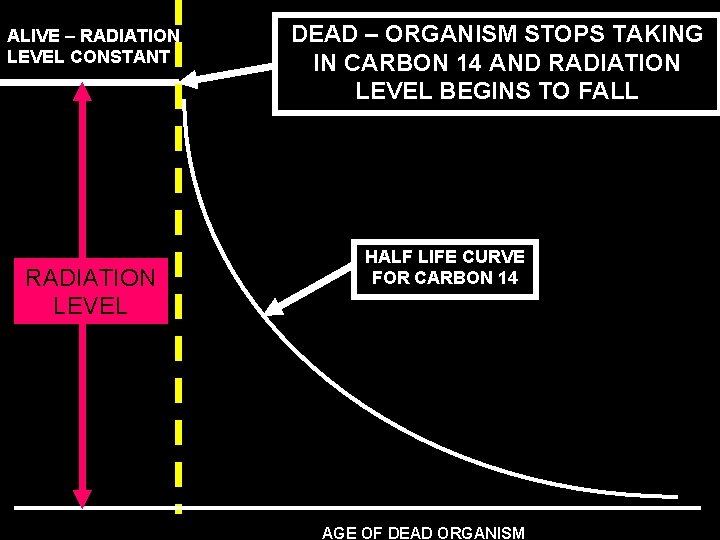
ALIVE – RADIATION LEVEL CONSTANT RADIATION LEVEL DEAD – ORGANISM STOPS TAKING IN CARBON 14 AND RADIATION LEVEL BEGINS TO FALL HALF LIFE CURVE FOR CARBON 14 AGE OF DEAD ORGANISM

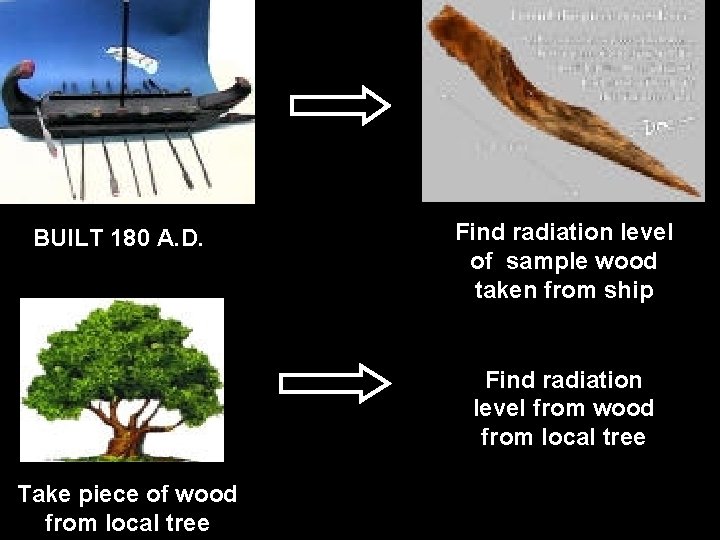
EXAMPLES OF CARBON DATING TO FIND THE AGE OF : - • ROMAN SHIP • TURIN SHROUD

BUILT 180 A. D. Find radiation level of sample wood taken from ship Find radiation level from wood from local tree Take piece of wood from local tree

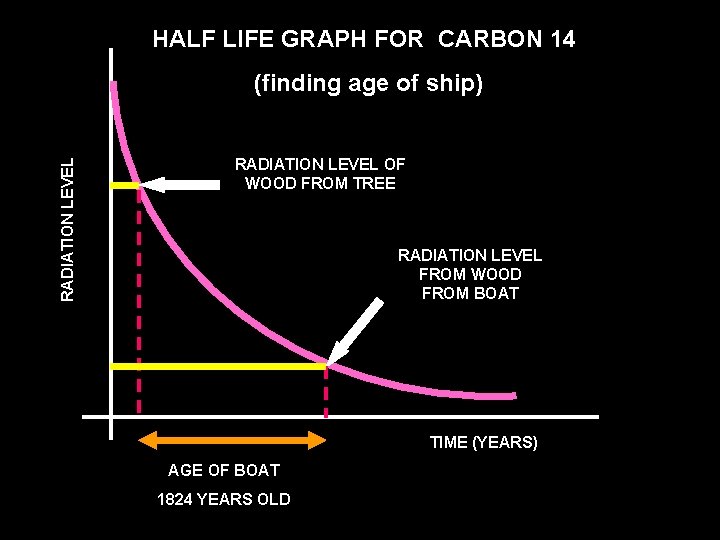
HALF LIFE GRAPH FOR CARBON 14 RADIATION LEVEL (finding age of ship) RADIATION LEVEL OF WOOD FROM TREE RADIATION LEVEL FROM WOOD FROM BOAT TIME (YEARS) AGE OF BOAT 1824 YEARS OLD

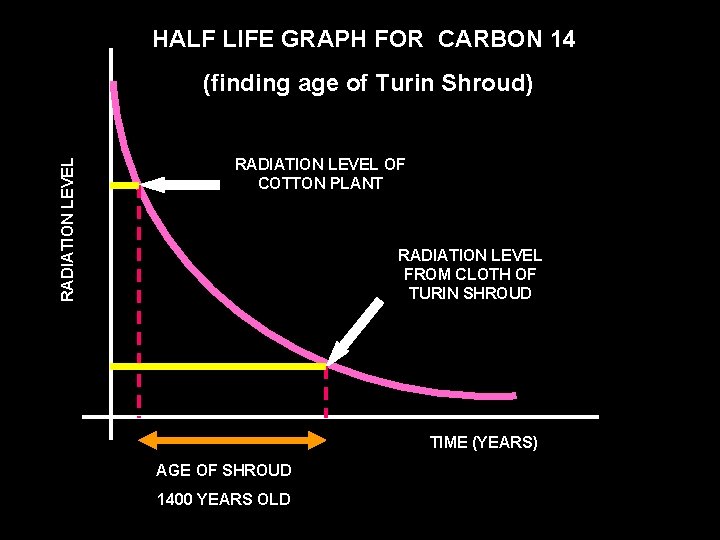
Turin shroud Cotton plant

HALF LIFE GRAPH FOR CARBON 14 RADIATION LEVEL (finding age of Turin Shroud) RADIATION LEVEL OF COTTON PLANT RADIATION LEVEL FROM CLOTH OF TURIN SHROUD TIME (YEARS) AGE OF SHROUD 1400 YEARS OLD

Finding the age of rocks Uranium Lead Approximately 4500 000 years

Rock Uranium Lead

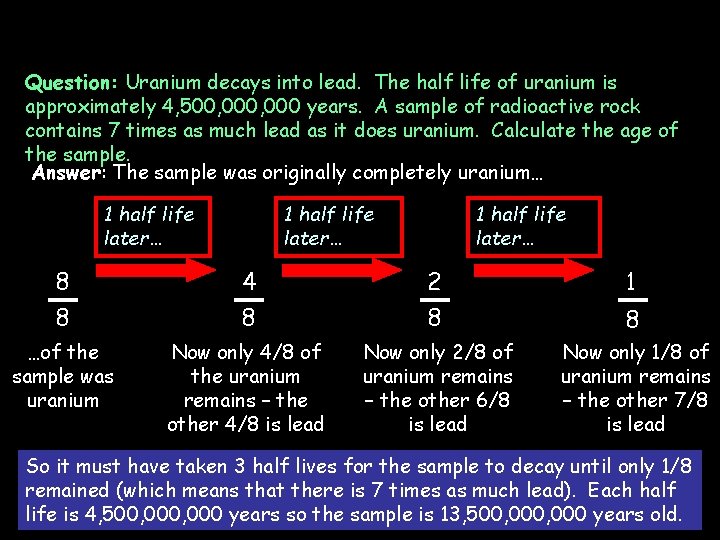
Question: Uranium decays into lead. The half life of uranium is approximately 4, 500, 000 years. A sample of radioactive rock contains 7 times as much lead as it does uranium. Calculate the age of the sample. Answer: The sample was originally completely uranium… 1 half life later… 8 8 4 8 2 8 1 …of the sample was uranium Now only 4/8 of the uranium remains – the other 4/8 is lead Now only 2/8 of uranium remains – the other 6/8 is lead Now only 1/8 of uranium remains – the other 7/8 is lead 8 So it must have taken 3 half lives for the sample to decay until only 1/8 remained (which means that there is 7 times as much lead). Each half life is 4, 500, 000 years so the sample is 13, 500, 000 years old.

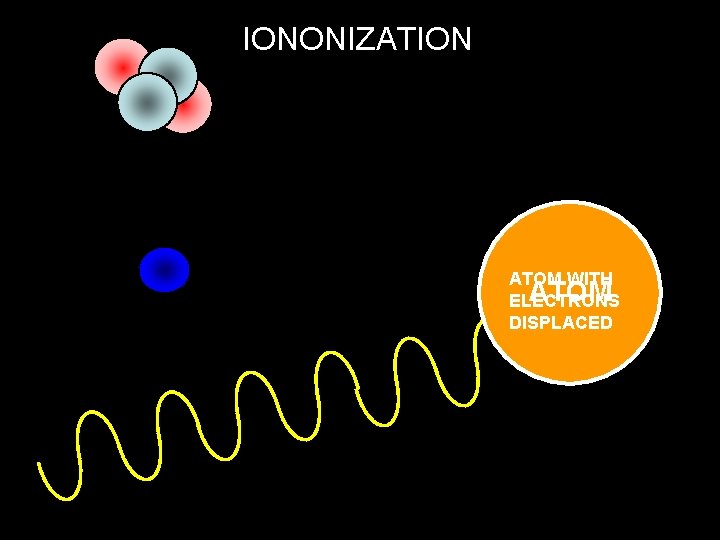
Ionisation Powers of Radiation Prolonged exposure to ionising radiation is dangerous to humans. Ionisation is the ability of radiation to remove electrons from atoms which will then change their structure

IONONIZATION ATOM WITH ATOM ELECTRONS DISPLACED

Type of Radiation Ionizing power Alpha particle High Beta particle Low Gamma rays Extremely low

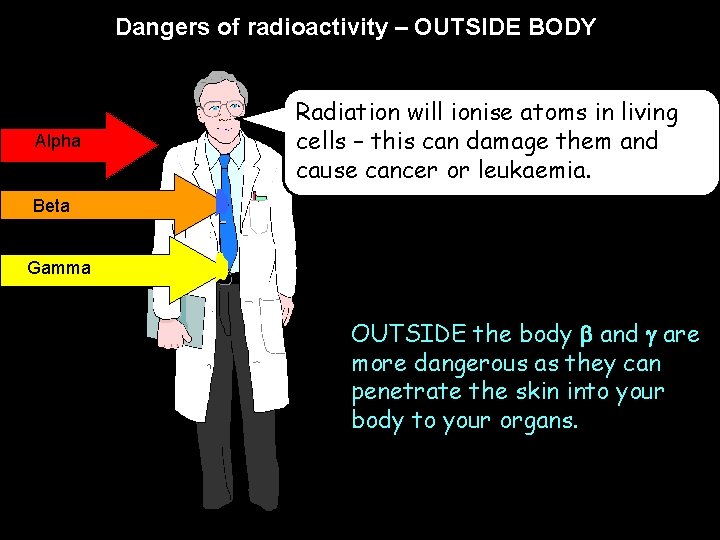
Dangers of radioactivity – OUTSIDE BODY Alpha Radiation will ionise atoms in living cells – this can damage them and cause cancer or leukaemia. Beta Gamma OUTSIDE the body and are more dangerous as they can penetrate the skin into your body to your organs.

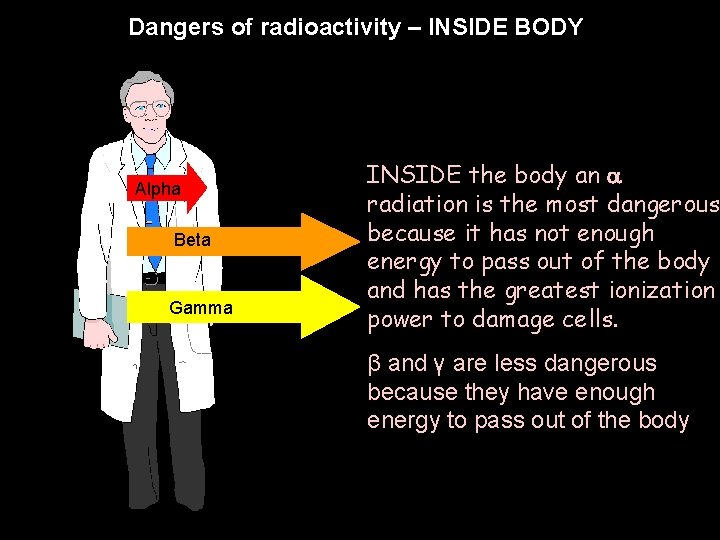
Dangers of radioactivity – INSIDE BODY Dangers of radioactivity (INSIDE BODY) Alpha Beta Gamma INSIDE the body an radiation is the most dangerous because it has not enough energy to pass out of the body and has the greatest ionization power to damage cells. β and γ are less dangerous because they have enough energy to pass out of the body

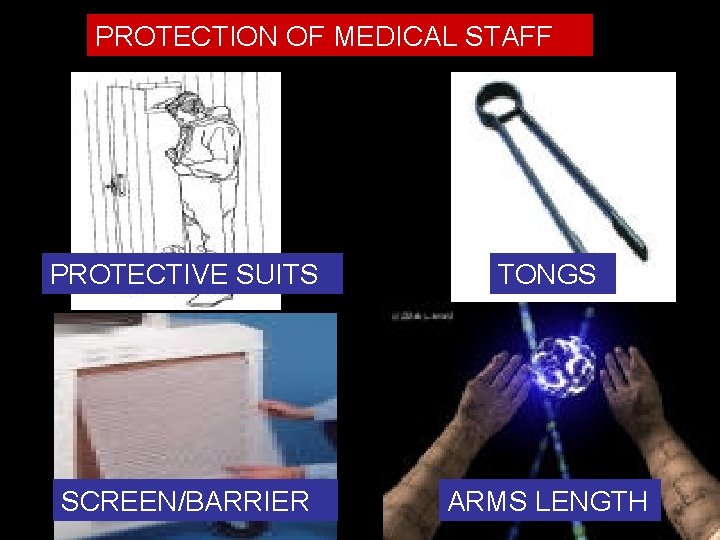
PROTECTION OF MEDICAL STAFF PROTECTIVE SUITS TONGS SCREEN/BARRIER ARMS LENGTH

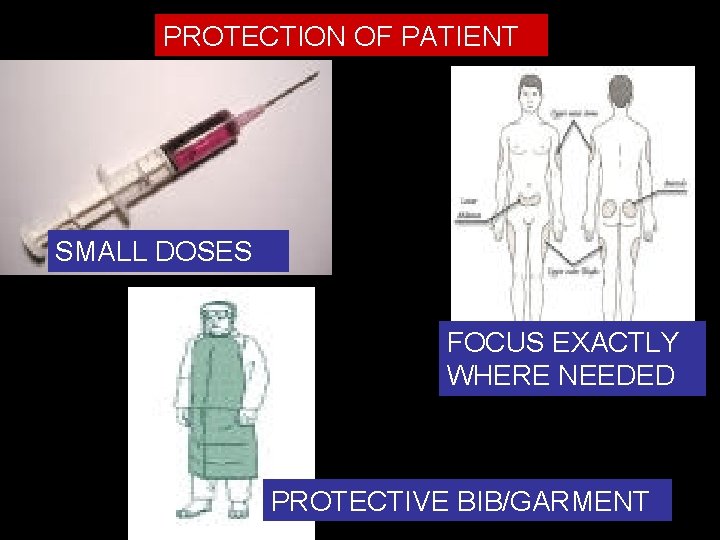
PROTECTION OF PATIENT SMALL DOSES FOCUS EXACTLY WHERE NEEDED PROTECTIVE BIB/GARMENT

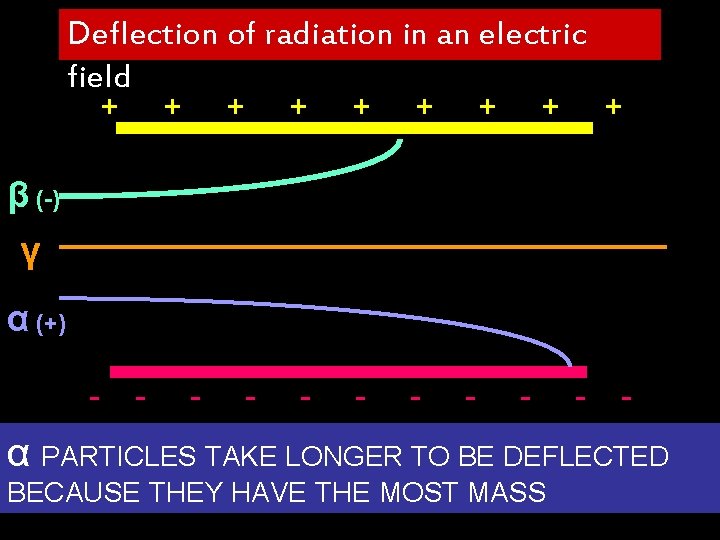
Deflection of radiation in an electric field + + + - - - + + + β (-) γ α (+) - - - - α PARTICLES TAKE LONGER TO BE DEFLECTED BECAUSE THEY HAVE THE MOST MASS

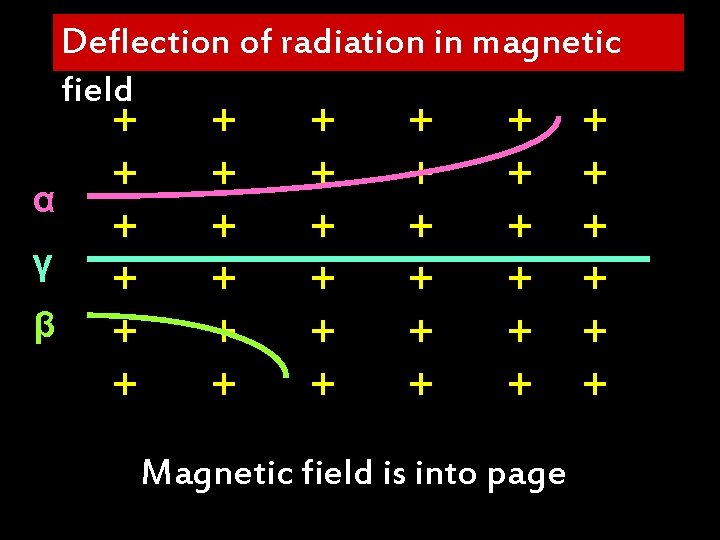
Deflection of radiation in magnetic field α γ β + + + + + + + + Magnetic field is into page + + +

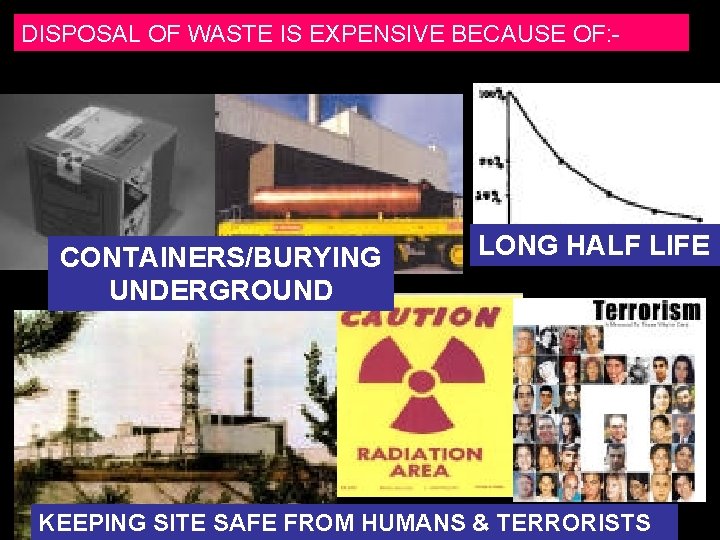
DISPOSAL OF WASTE IS EXPENSIVE BECAUSE OF: - CONTAINERS/BURYING UNDERGROUND LONG HALF LIFE KEEPING SITE SAFE FROM HUMANS & TERRORISTS

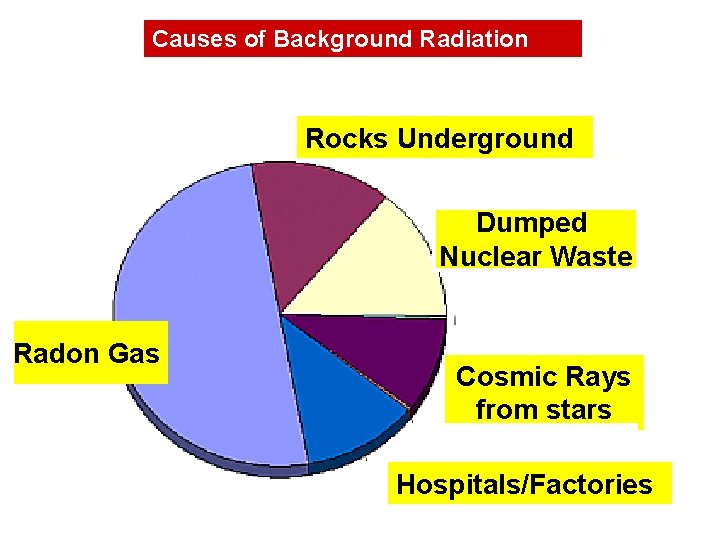
Causes of Background Radiation Rocks Underground Dumped Nuclear Waste Radon Gas Cosmic Rays from stars Hospitals/Factories