Nuclear Model and Nuclear Stability by Two Models

Nuclear Model and Nuclear Stability by
Two Models were given in order to explain to different aspects of structure of Nuclei and their behaviour ØThe “Liquid Drop” Model ØThe “Nuclear Shell” Model
The Liquid Drop Model The main assumptions regarding this model are • The nuclei of all elements are considered to be behave like a liquid drop of incompressible liquid of very high density. • In an equilibrium state the nuclei of atoms remain spherically symmetric under the action of strong attractive nuclear forces just like the drop of a liquid which is spherical due to surface tension. • The density of a nucleus is independent of its size just like the density of liquid which is also independent of its size.
• The nucleons of the nucleus move about within a spherical enclosure called the nuclear potential barrier just like the movement of the molecules of a liquid within a spherical drop of liquid. • The binding energy per nucleon of a nucleus is constant just like the latent heat of vaporization of a liquid.

Main Achievements of liquid drop model Ø It explains binding energy of large number of nuclei. Ø It explains the fusion and fission processes nicely. Ø Explains energies of radioactive decays, fission and fusion. Drawback of the liquid drop model Ø It is not able to explain the magic numbers. Ø is not able to explain excited states. Ø It is not able to calculate the nuclear spin.

The Shell Model The Important assumptions of the shell model are as under • The basic assumption of the liquid drop model is that each nucleon in a nucleus interact only with its nearest neighbors, like a molecule in a liquid. • In shell model each nucleon interact chiefly with a general force field produced by all the other nucleons. • The atoms with 2, 10, 18, 36, 54 and 86 electrons have all their electron shell completely filled. • In the same way, nuclei that have 2, 8, 20, 28, 50, 82 and 126 neutrons and protons are more abundant than other nuclei of similar mass numbers, suggesting their structures are more stable. These Numbers are called Magic Numbers

Main Achievements the shell model Ø It explains Magic numbers. Ø It explains the magnetic moment of some nuclei nicely. Ø It explains successfully the ground state spin. Ø It explains the great stability and high binding energy. Ø It explains the phenomenon of nuclear isomerism. Drawback of Shell model Ø It fail to explain the stability of four stable nuclei 1 H 2, 3 Li 6, 5 B 10 7 N 14. Ø It does not predict correct values of nuclear spin for certain nuclei

Nuclear Stability Nuclear stability is a concept that helps to identify the stability of an isotope. The main factor to determine the stability of an isotope is ratio of neutrons to protons (N/P Ratio) Ø When the N/P ratio is unity or closer to unity the atom is more stable Ø When the N/P ratio is above or below the unity the atom is less stable
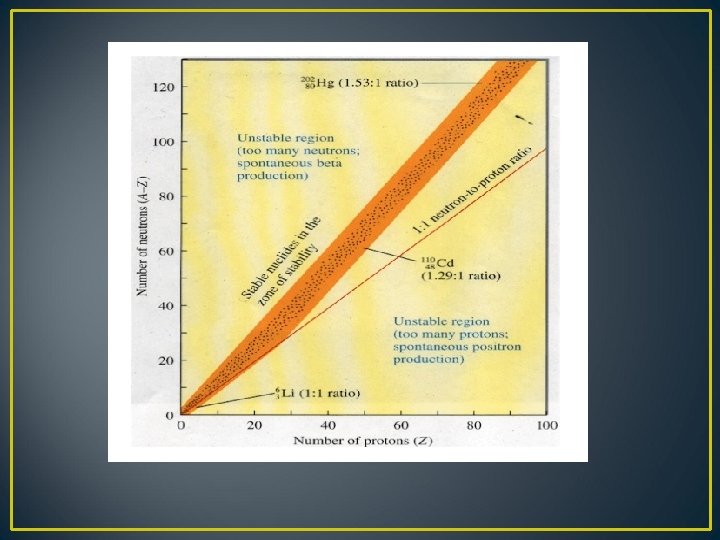
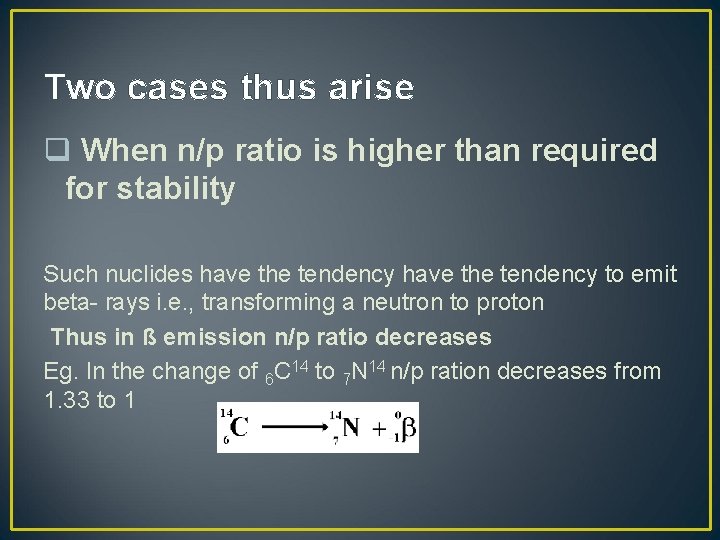
Two cases thus arise q When n/p ratio is higher than required for stability Such nuclides have the tendency to emit beta- rays i. e. , transforming a neutron to proton Thus in ß emission n/p ratio decreases Eg. In the change of 6 C 14 to 7 N 14 n/p ration decreases from 1. 33 to 1
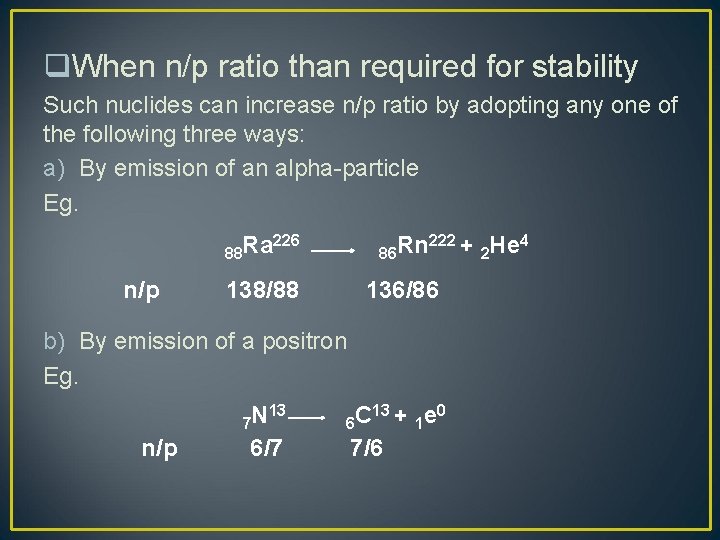
q. When n/p ratio than required for stability Such nuclides can increase n/p ratio by adopting any one of the following three ways: a) By emission of an alpha-particle Eg. 88 Ra n/p 86 Rn 226 138/88 222 + 136/86 b) By emission of a positron Eg. n/p 13 N 7 6/7 13 + e 0 C 6 1 7/6 2 He 4
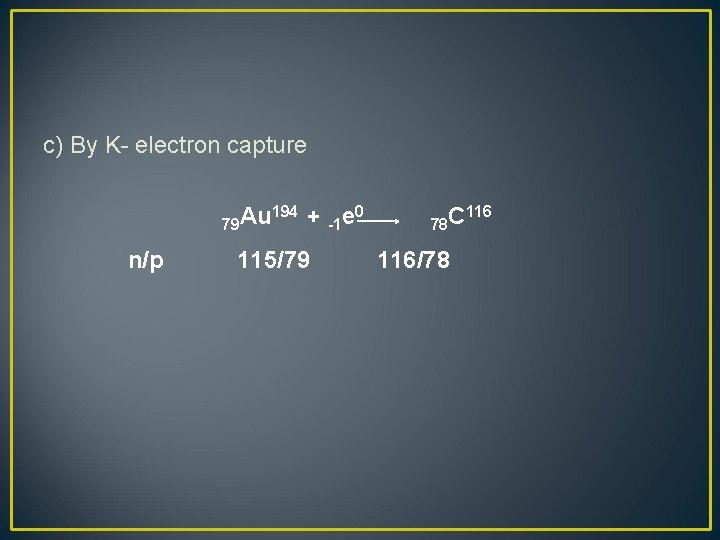
c) By K- electron capture 79 Au n/p 194 + -1 e 0 115/79 78 C 116/78 116

REFERENCES • Nuclear and Radio Chemistry: John Wiley. Friedlander, Kennedy and Miller • Nuclear Chemistry- B. G. Harvey, • Essentials of Nuclear Chemistry, H. J. Arnikar, John Wiley • Nuclear and Radiation Chemistry: B. K. Sharma, Krishna Publication
- Slides: 13