NUCLEAR MEDICINE Introduction to Nuclear Medicine By Dr

NUCLEAR MEDICINE Introduction to Nuclear Medicine By: Dr. Abdulrahman M. M. khair
What means the Nuclear Medicine ?

NUCLEAR MEDICINE Diagnosis and therapy with unsealed sources Clinical problem Radiopharmaceutical Instrumentation Part 0. Introduction to Nuclear Medicine 3

Table of Contents Objectives Introduction Types of Diagnostic Procedures Nuclear Medicine Detection Equipment Personnel Monitoring Safety Equipment Nuclear Medicine Facility Nuclear Medicine Process

Objective Upon completion of these lectures , the student will have an understanding of the procedures, equipment and processes of Diagnostic Nuclear Medicine

Introduction Nuclear Medicine? Nuclear Medicine is a medical specialty that is used to diagnose diseases in a safe and painless way. Nuclear medicine procedures permit the acquisition of medical information that may otherwise be unavailable, obtained only by surgery, or through more expensive and invasive diagnostic tests. The procedures very often identify abnormalities very early in the progression of a disease.

Introduction Nuclear Medicine? A diagnostic nuclear medicine study is one that is useful in the determination of the cause, nature, or manifestations of a disease or condition. It may include monitoring the progression or regression of a disease or injury in response to therapeutic regimens. A diagnostic nuclear medicine study can reveal structure or anatomy, and/or function, or physiology and metabolism.

Introduction Nuclear Medicine? The uniqueness of nuclear medicine studies lies in their ability to demonstrate function, physiology, and metabolism.
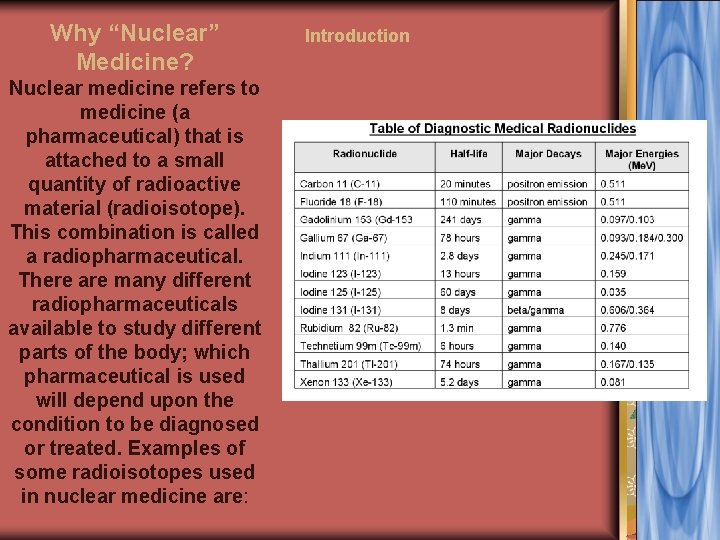
Why “Nuclear” Medicine? Nuclear medicine refers to medicine (a pharmaceutical) that is attached to a small quantity of radioactive material (radioisotope). This combination is called a radiopharmaceutical. There are many different radiopharmaceuticals available to study different parts of the body; which pharmaceutical is used will depend upon the condition to be diagnosed or treated. Examples of some radioisotopes used in nuclear medicine are: Introduction

Nuclear Medicine Detection Equipment PET/CT scanner Dual head scanner Single head SPECT scanner
GAMMA CAMERA Part 0. Introduction to Nuclear Medicine 11
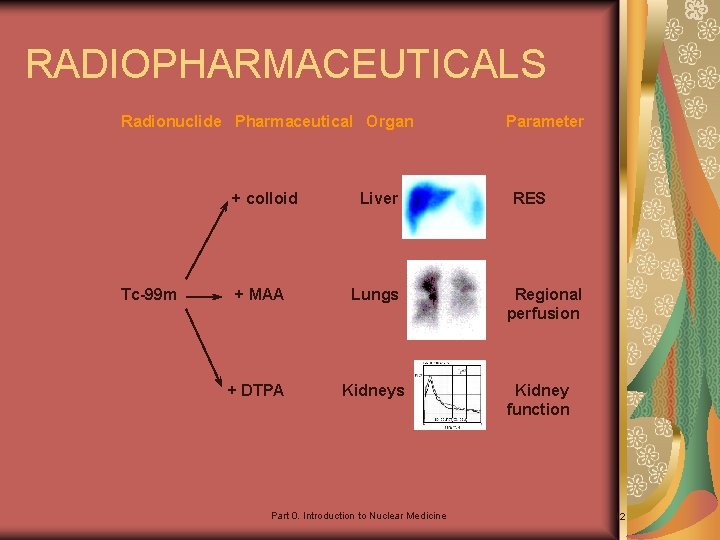
RADIOPHARMACEUTICALS Radionuclide Pharmaceutical Organ + colloid Tc-99 m Liver + MAA Lungs + DTPA Kidneys Part 0. Introduction to Nuclear Medicine Parameter RES Regional perfusion Kidney function 12

PIONEERS Henri Becquerel Ernest Rutherford Maria Curie Frederique Joliot-Irene Curie Part 0. Introduction to Nuclear Medicine 13
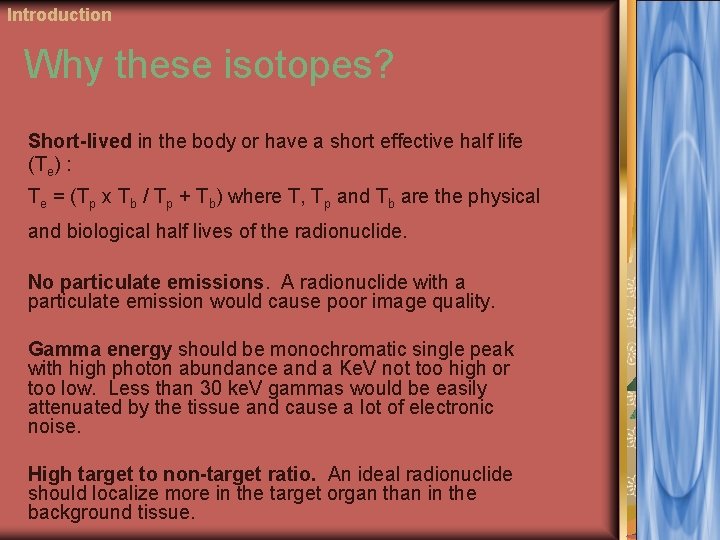
Introduction Why these isotopes? Short-lived in the body or have a short effective half life (Te) : Te = (Tp x Tb / Tp + Tb) where T, Tp and Tb are the physical and biological half lives of the radionuclide. No particulate emissions. A radionuclide with a particulate emission would cause poor image quality. Gamma energy should be monochromatic single peak with high photon abundance and a Ke. V not too high or too low. Less than 30 ke. V gammas would be easily attenuated by the tissue and cause a lot of electronic noise. High target to non-target ratio. An ideal radionuclide should localize more in the target organ than in the background tissue.

Thyroid scan the thyroid is normally a bilobed or a butterfly shaped organ with each lobe typically measuring 4– 5 cm by 1. 5– 2. 0 cm. The right side is slightly larger than the left. There is extreme variability in the appearance of the isthmus. The thyroid lies superior to the suprasternal notch, though this is dependent on the degree of neck extension present at the time of imaging.
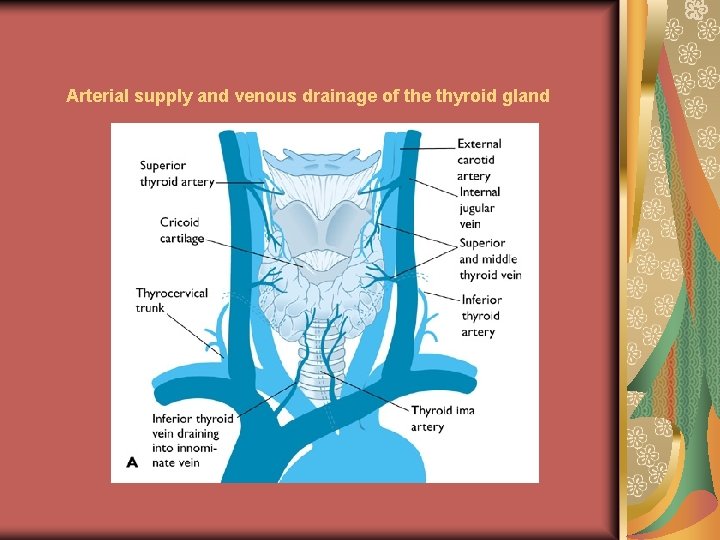
Arterial supply and venous drainage of the thyroid gland

Thyroid scintigraphy is based on iodide physiology involving the following: iodine ingestion, trapping and concentration in the thyroid to produce thyroid hormones.
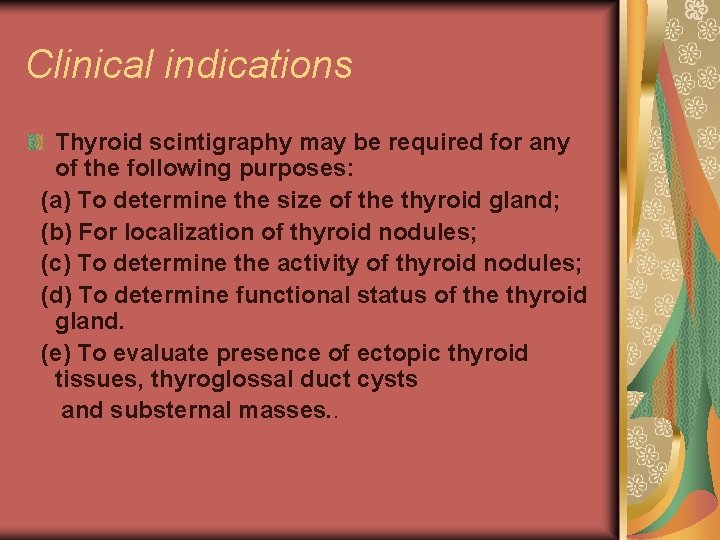
Clinical indications Thyroid scintigraphy may be required for any of the following purposes: (a) To determine the size of the thyroid gland; (b) For localization of thyroid nodules; (c) To determine the activity of thyroid nodules; (d) To determine functional status of the thyroid gland. (e) To evaluate presence of ectopic thyroid tissues, thyroglossal duct cysts and substernal masses. .

Radiopharmaceuticals Details of the radiopharmaceuticals used in thyroid scintigraphy are given below: CHARACTERISTICS OF THE RADIOPHARMACEUTICALS USED IN THYROID SCINTIGRAPHY
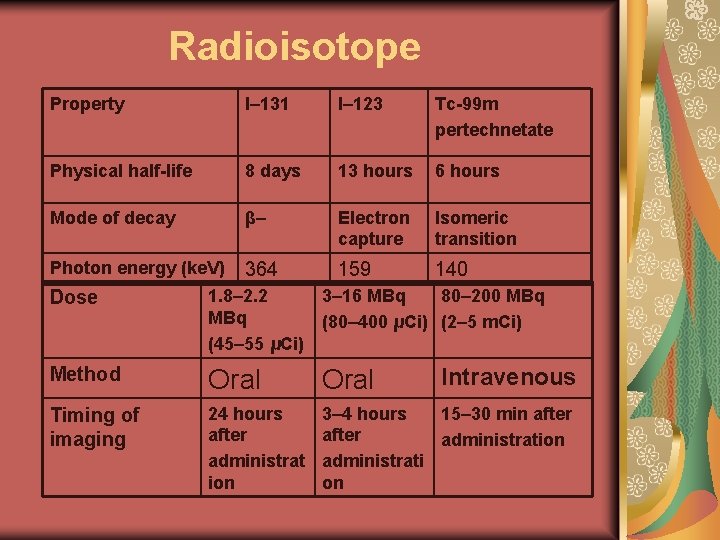
Radioisotope Property I– 131 I– 123 Tc-99 m pertechnetate Physical half-life 8 days 13 hours 6 hours Mode of decay β– Electron capture Isomeric transition Photon energy (ke. V) 364 159 140 Dose 1. 8– 2. 2 3– 16 MBq 80– 200 MBq (80– 400 μCi) (2– 5 m. Ci) (45– 55 μCi) Method Oral Timing of imaging 24 hours after administrat ion 3– 4 hours 15– 30 min after administration administrati on Intravenous

Equipment A gamma camera with a pinhole or high- resolution parallel hole collimator is preferred, to allow multiple views of the thyroid and better resolution of thyroid nodules.

Patient preparation Stop: thyroid hormones(T 3 and T 4 for at least two and four weeks, respectively) —Anti-thyroid agents (for at least one week); —Iodine-containing food, for example kelp (for at least one week); —Iodine-containing medications (e. g. iodinated contrast agents for weeks, and lipid soluble media and amiodarone for months).

Clinical contraindications Radiopharmaceuticals are contraindicated in pregnant Discontinuation of breast feeding for nursing mothers (12 hours for 99 m. Tc, permanently for current child with 131 I).

Procedure The following procedure should be adopted: (a) Patient position: Supine with neck extended to elevate thyroid. (b) Timing of imaging: —For 123 I: Imaging can be done 3– 4 hours after oral administration. Delayed images at 24 hours have lower body background but with a lower count rate. —For 131 I: Images are obtained 24 hours postadministration. —For 99 m. Tc-pertechnetate: Images are obtained 15– 30 min after intravenous administration «.
Procedure (c) Acquisition parameters: —Obtain 100 000 counts or 5 min observation time, whichever occurs first, with 99 m. Tc, 20 000 counts or 10 min with 131 I, 50 000 counts or 10 min with 123 I for images in the following projections: ● Anterior view; ● 45° right anterior oblique view; ● 45° left anterior oblique view. —Note that the image of the thyroid should occupy at least two thirds of the FOV, necessitating adjustments in the distance between the pinhole aperture and the neck. —Radioactive markers may be used to identify anatomical landmarks (e. g. sternal notches). —Note the position of and mark the palpable nodules and surgical scars. —In cases of oesophageal activity in 99 m. Tc-pertechnetate or 123 I due to salivary excretion, ask the patient to rinse their mouth and drink a glass of water.

Thyroid treatment We used IODINE-131 THERAPY for Thyroid cancer: or Hyperthyroidism:
Iodine-131 therapy is beneficial in therapy of thyroid remnants or of metastatic thyroid cancer. The primary treatment for thyroid carcinoma is thyroidectomy. Following thyroidectomy the Iodine-131 therapy.

Indications The indications are iodine-avid thyroid remnants or metastatic disease in patients with thyroid carcinoma, usually papillary or follicular.

Contraindications The contraindications are: (a) Absolute: pregnancy and/or lactation; (b) Relative: patient on thyroid hormone replacement therapy

Radiopharmaceuticals Iodine-131, in the form of sodium iodide, is administered orally.

Action prior to 131 I therapy Patients at intermediate or high risk of thyroid cancer usually receive 131 I therapy after definitive thyroid surgery (usually total or radical thyroidectomy, for thyroid surgery must not use an iodine containing compound. Patients must not receive thyroid hormone replacement for at least four weeks prior to 131 I therapy.

Therapy Ablative therapy is defined as that given immediately following definitive surgery.

Therapy dose In most centres, a fixed dose between 1 and 4 GBq (25– 100 m. Ci) of 131 I is given.

Treatment of Hyperthyroidism: The most common form of hyperthyroidism is Graves’ disease, which accounts for 60– 90% of all cases of thyrotoxicosis. Other causes of thyrotoxicosis include toxic adenoma and toxic multinodular goitre. Elevated T 4 and suppressed TSH are the biochemical hallmarks of thyrotoxicosis.

Dose and administration Administration of an ablative dose up to (15 m. Ci)
Types of acquisitions Static imaging: this done after injection of tracer or given orally after period of time.
Whole body imaging : done after period of time after injection of tracer or given orally radioactive material. (e. g bone scan) or immediately (e. g lymphscintiography)
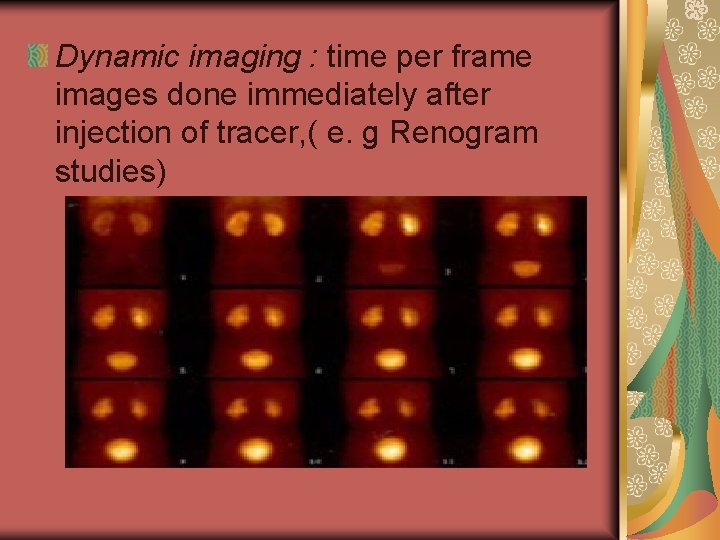
Dynamic imaging : time per frame images done immediately after injection of tracer, ( e. g Renogram studies)
Renal radionuclide studies Dynamic renal radionuclide studies This procedure is also called renography ( renogram) The uptake by the kidneys of a tracer substance, its transit through the nephrons and its excretion into the pelvis of kidney and then through the urethers into the bladder.
Clinical indications Renography can be used for any of the following purposes: (a) Measurement of the contribution renal function of each kidney. (b) Evaluation of obstructive nephropathy and obstructing uropathy. (c) Determination of the presence of renovascular disorder as a cause of hypertension. (d) Evaluation of renal transplantation. (e) Investigation of lumbar pain. (f) Investigation of acute or chronic renal failure. (g) Investigation of renal disorder in patients who are allergic to contrast media. (h) Renal trauma.

Radiopharmaceuticals (a) Technetium-99 m DTPA: (diethylenetriamine-pentaacetic acid) Technetium-99 m DTPA is no longer the agent of choice for renal radionuclide studies except when GFR measurements are required. Although it is low cost and readily available. It should be prepared according to the manufacturer’s instructions.

(b) Technetium-99 m MAG 3: This is currently the radiopharmaceutical of choice for radionuclide renography. It should be prepared according to the manufacturer’s recommendations.
Equipment A large FOV gamma camera is required so that the left ventricle of the heart, the kidneys, the pelvis and the proximal ureters are in the FOV. A low energy, parallel hole collimator with high resolution is preferred for the most widely used 99 m. Tc agents. The data are transferred to a computer online in a 128 × 128 or 64 × 64 matrix. Electronic zoom should be used for small children.

Procedure The procedure should be explained to the patient or parents before entering the gamma camera room. An anaesthetic cream can be applied to relieve discomfort of the venipuncture. The patient should be hydrated before the study. Normally, 500 m. L of water is given to the patient half an hour before the procedure so that the patient is hydrated or slightly overhydrated.

The bladder should be emptied before entering the camera room and the time should be noted. The patient should lie in the supine position on the couch with a camera positioned below or preferably reclining against the camera face, so that the kidneys drop back. This is the most comfortable position

An image of the pelvis and bladder before and after micturition and/or after five minutes in the upright position to ensure gravitational drainage is recommended in the event of pelvic retention at the end of the study.
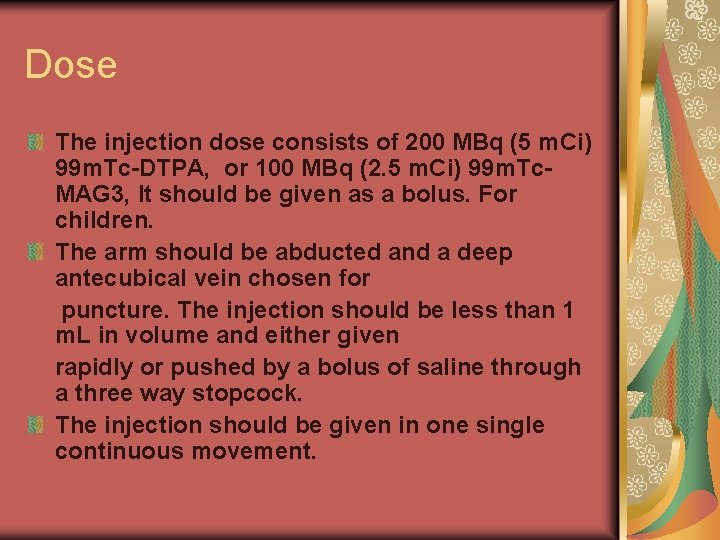
Dose The injection dose consists of 200 MBq (5 m. Ci) 99 m. Tc-DTPA, or 100 MBq (2. 5 m. Ci) 99 m. Tc. MAG 3, It should be given as a bolus. For children. The arm should be abducted and a deep antecubical vein chosen for puncture. The injection should be less than 1 m. L in volume and either given rapidly or pushed by a bolus of saline through a three way stopcock. The injection should be given in one single continuous movement.

Acquisition protocol Acquisition should last for a minimum of 30 min. A flow study is not generally useful unless vascular problems are suspected. If it is performed, one frame per 2 s for the first 60 s is suggested. The rest of the study should be recorded at one frame per 10 s.
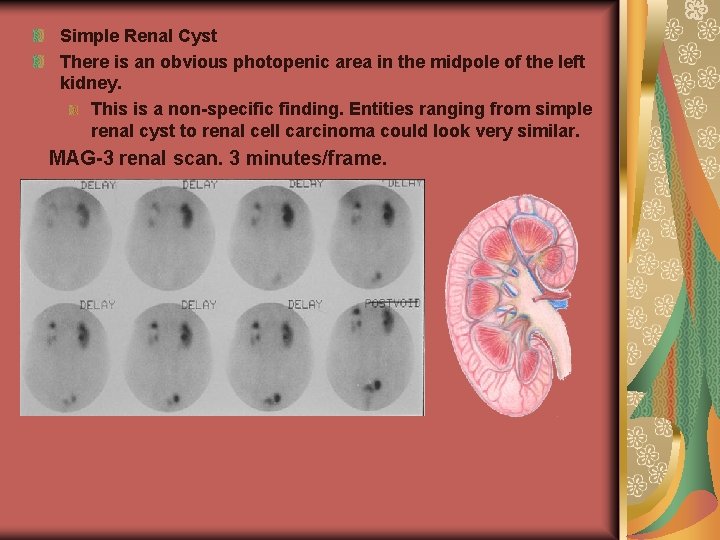
Simple Renal Cyst There is an obvious photopenic area in the midpole of the left kidney. This is a non-specific finding. Entities ranging from simple renal cyst to renal cell carcinoma could look very similar. MAG-3 renal scan. 3 minutes/frame.
Renal transplantation can be performed from either a live donor or a cadaver. Both types of transplant may suffer rejection, which usually starts at about seven days and is associated with a progressive reduction in blood flow.
Clinical indications Radiography studies may be made after a renal transplantation for the following purposes: (a) Evaluation of the progress of the transplant shortly after the operation; (b) Evaluation of the transplant for chronic rejection, drug toxicity or renovascular hypertension.
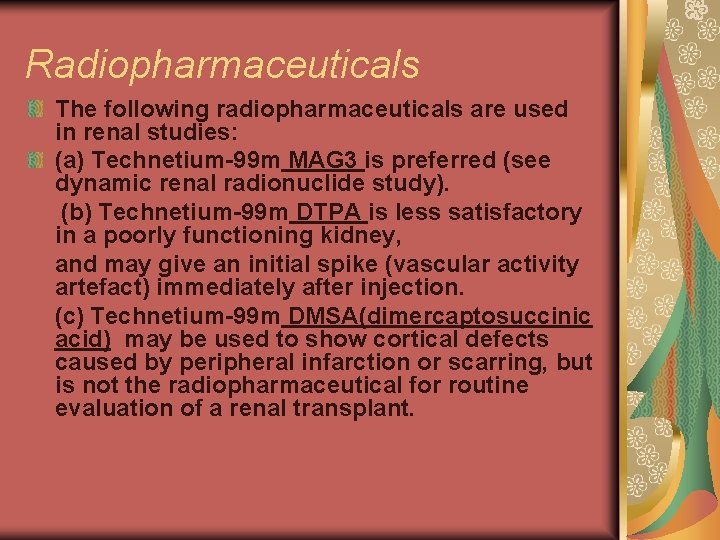
Radiopharmaceuticals The following radiopharmaceuticals are used in renal studies: (a) Technetium-99 m MAG 3 is preferred (see dynamic renal radionuclide study). (b) Technetium-99 m DTPA is less satisfactory in a poorly functioning kidney, and may give an initial spike (vascular activity artefact) immediately after injection. (c) Technetium-99 m DMSA(dimercaptosuccinic acid) may be used to show cortical defects caused by peripheral infarction or scarring, but is not the radiopharmaceutical for routine evaluation of a renal transplant.

Procedure and equipment It is important to place the gamma camera over the correct side of the transplant. The gamma camera is set up as for a renal dynamic radionuclide study. Imaging should be performed within the first 24– 48 hours of the transplant, to verify perfusion and to serve as a baseline study. «

The data are recorded at one frame per second for 60 s, followed by one frame per 10 s up to 30 min. The data are collected in a 64 × 64 or 128 × 128 matrix. Dose is 300 MBq (8 m. Ci) of 99 m. Tc. MAG 3. (mercaptoacetyltriglycine)
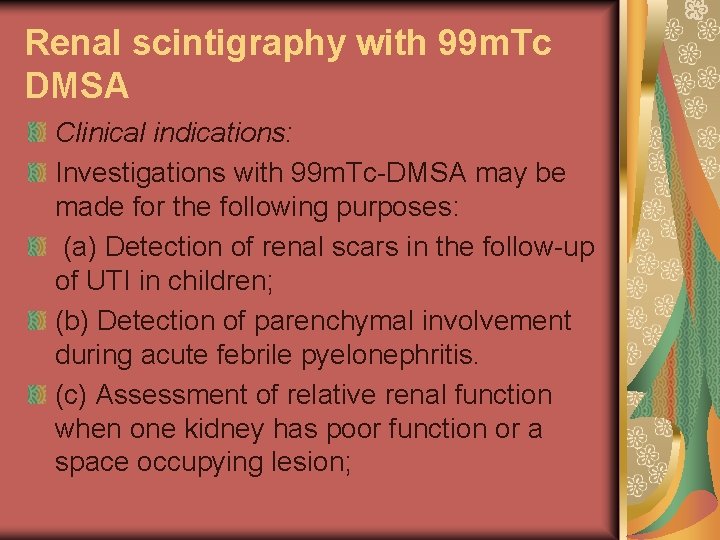
Renal scintigraphy with 99 m. Tc DMSA Clinical indications: Investigations with 99 m. Tc-DMSA may be made for the following purposes: (a) Detection of renal scars in the follow-up of UTI in children; (b) Detection of parenchymal involvement during acute febrile pyelonephritis. (c) Assessment of relative renal function when one kidney has poor function or a space occupying lesion;

(d) Detection of associated abnormalities: abnormal duplex kidneys, small kidneys, dysplastic kidneys and horseshoe kidneys; (e) Distinguishing pseudotumours from tumours; (f) Ectopic kidneys; (g) Allergy to iodine contrast agents precluding radiological investigations.

Radiopharmaceuticals Technetium-99 m DMSA should be reconstituted with 99 m. Tcpertechnetate and used within 30 min. prevent oxidationto DMSA agent. The agent should be prepared according to the manufacturer’s instructions for kidney studies.

Equipment A single- or double-headed gamma camera is required with a low energy, high resolution (LEHR) parallel hole collimator, or a pinhole collimator for infants. Data should be recorded in a 256 × 256 matrix into a computer for subsequent analysis. Alternatively the electronic zoom (factor 2) can be used for recording in a 128 × 128 matrix.

Patient preparation An explanation to the patient and/or the child’s parents should be given before the start of the study. For adults, no preparation is necessary. Infants should be fed before imaging. Anaesthetic skin preparation before an injection is advised for children.

Dose An intravenous injection of 80– 100 MBq (2– 2. 5 m. Ci) of 99 m. Tc-DMSA is given for adults. For children and infants, the dose is scaled down according to the body surface area; however, a minimum of 15 MBq (0. 4 m. Ci) should be administered.
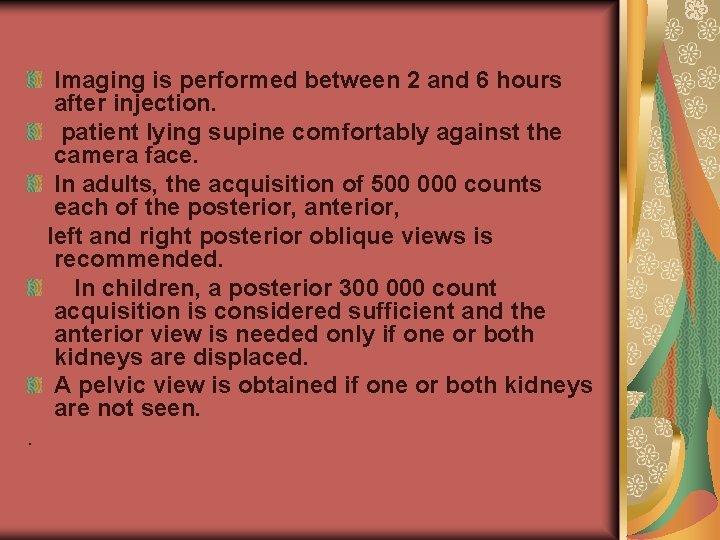
Imaging is performed between 2 and 6 hours after injection. patient lying supine comfortably against the camera face. In adults, the acquisition of 500 000 counts each of the posterior, anterior, left and right posterior oblique views is recommended. In children, a posterior 300 000 count acquisition is considered sufficient and the anterior view is needed only if one or both kidneys are displaced. A pelvic view is obtained if one or both kidneys are not seen. .
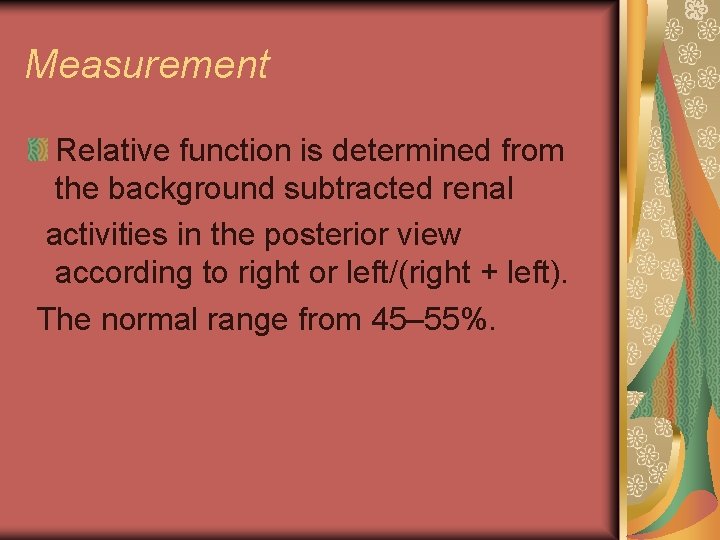
Measurement Relative function is determined from the background subtracted renal activities in the posterior view according to right or left/(right + left). The normal range from 45– 55%.
Pulmonary scintigraphy (Lung scan) Radionuclide methods are available for the study of lung ventilation and perfusion. Indication: The main indication for lung scintigraphy is suspected pulmonary embolism. Other indications are: for assessment of residual lung function if surgery is planned for lung tumours.
Types of scanning: (a) Ventilation or inhalation studies are performed using radioactive gases (133 Xe, 127 Xe and 81 m. Kr) or (99 m. Tc. DTPA or colloid). The images obtained show the distribution of gases in the lungs. (b) Perfusion lung imaging permits an evaluation of the pulmonary arterial blood flow. Usually 99 m. Tc macroaggregated albumin (MAA) is employed.

Dose: For lung perfusion scan: The usual adult administered activity is 40– 150 MBq (1– 4 m. Ci). The usual paediatric administered activity is 0. 5– 2. 0 MBq/kg (20– 80 m. Ci/kg), with a minimum of 7– 8 MBq (» 200 m. Ci). For lung ventilation scan: Technetium-99 m( diethylene pentaacetic acid DTPA) triamine is the preferred radiopharmaceutical. The usual administered activity of 99 m. Tc. DTPA is 900– 1300 MBq (25– 35 m. Ci) in the nebulizer, from which approximately 20– 40 MBq (0. 5– 1. 0 m. Ci) reach the patient’s lungs.

Equipment: The following equipment is useful for pulmonary scintigraphy studies: (a) Large FOV gamma cameras with low energy, general purpose (LEGP) collimators or LEHR collimators. (b) Large FOV gamma cameras with a rotation capability, as well as a computer with appropriate software, for SPECT imaging. An LEGP collimator is recommended for SPECT in order to minimize acquisition time. .
Preparation and procedure Patient preparation: Before intravenous administration of the pulmonary perfusion radiopharmaceutical, the patient should be instructed to cough and to take several deep breaths. The patient should be in a supine position during injection or, in the case of a patient with orthopnea, as close to the supine position as possible, since particle distribution is affected by gravity.
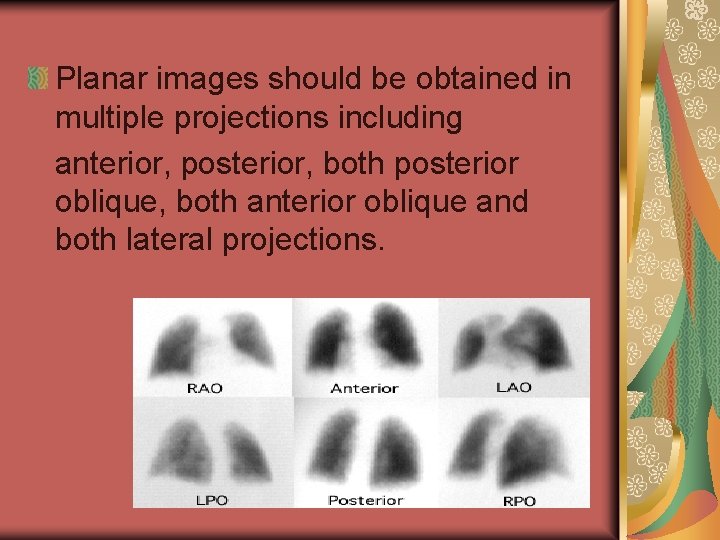
Planar images should be obtained in multiple projections including anterior, posterior, both posterior oblique, both anterior oblique and both lateral projections.
Bone scintigraphy: For nuclear imaging of the Bone scintigraphy using 99 m. Tc-methylene-diphosphonate (MDP) or hydroxyethylene diphosphonate (HEDP) in a broad spectrum of diseases of the bone. Bone infections can additionally be imaged using 111 In-leucocytes and 67 Ga. Bone scan modes include whole body planar scintigraphy, planar spot scintigraphy, SPECT, planar pinhole scintigraphy and pinhole SPECT.

Dose The usual dose for adults is 740– 1110 MBq (20– 30 m. Ci) injected intravenously. For markedly obese adults, the administered dose may be increased to 11– 13 MBq/kg (0. 3– 0. 35 m. Ci/kg). For children, the administered dose is 9– 11 MBq/kg (0. 25– 0. 3 m. Ci/kg), with a minimum of 40– 90 MBq (1. 1– 2. 4 m. Ci).
Equipment: Gamma camera system A large FOV gamma camera is best suited for bone scintigraphy. A low energy, high resolution collimator and a pinhole collimator are the two most widely used collimators for bone scanning. For SPECT, a high resolution parallel hole should be used but some systems also accept pinhole collimators.

Modes of bone scintigraphy Scintigraphic modes for the imaging of bone include planar whole body scintigraphy, planar spot scintigraphy, three phase planar scintigraphy, planar pinhole scintigraphy, conventional SPECT and pinhole SPECT.
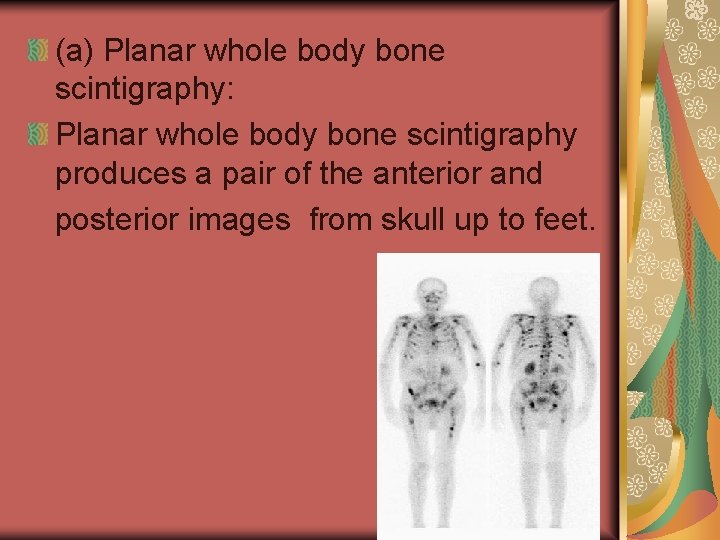
(a) Planar whole body bone scintigraphy: Planar whole body bone scintigraphy produces a pair of the anterior and posterior images from skull up to feet.
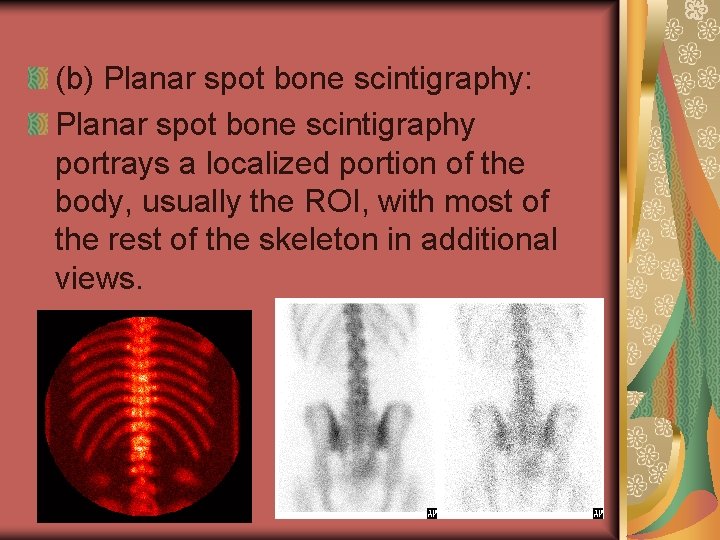
(b) Planar spot bone scintigraphy: Planar spot bone scintigraphy portrays a localized portion of the body, usually the ROI, with most of the rest of the skeleton in additional views.
(c) Three phase planar bone scintigraphy: Three phase planar bone scintigraphy consists of dynamic arterial blood flow images, static blood pool images and delayed static bone images. The blood flow phase is obtained in sequence as the tracer is injected in a bolus. Sixteen to 20 frames are taken, with the acquisition time per frame varying from 2 to 4 seconds according to the site imaged. The static images of the blood pool, one or two in number, are taken within 10 min of the injection. «

The delayed planar images are taken 2– 3 hours after injection. Scanning can be started as early as 1. 5 hours after injection when HEDP is used. (C) Conventional single photon computed tomography of bone : (SPECT cameras can be single, dual or triple headed. ) Bone SPECT produces sectional images of a portion of the skeleton. The standard projections are transverse, coronal and sagittal.

Patient preparation: the procedures should be explained to patients in details. patients should be well hydrated by drinking at least two glasses (500 m. L) of water. Patients should be instructed to urinate immediately prior to delayed imaging.

Clinical applications 1) Acute infective diseases of bone; (2) Tuberculosis of bone; (3) Non-infective inflammations of bone; (4) Indium-111 and 99 m. Tc labelled leucocytes and 67 Ga scans in bone infection. (5) Osteoarthritis; (6) Rheumatoid arthritis (7) Avascular necrosis of bone; (8) Metabolic diseases of bone; (9) Benign and primary malignant bone tumours; (10) Metastatic bone tumours. (

Clinical contraindications: Absolute pregnancy. breast feeding should be discontinued for 24 hours after the injection of the radiopharmaceutical.
Liver spleen scan Liver–spleen imaging is performed following the injection of a 99 m. Tc labelled colloid, which is rapidly absorbed by the reticuloendothelial cells of the liver, spleen and bone marrow.
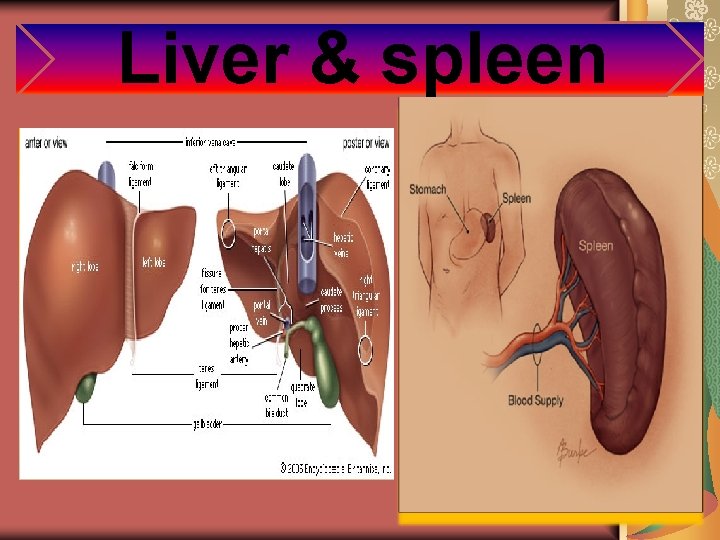
Liver & spleen

Clinical indications These studies can be used for determining the size and shape of the liver and spleen as well as for detecting functional abnormalities of the reticuloendothelial cells of these organs. Specifically, these studies are occasionally performed for:
(1) Suspected focal nodular hyperplasia of the liver. (2) Assessment of reticuloendothelial system (RES) function in patients with suspected liver disease. (3) for the diagnosis of cavernous hemangiomas of the liver. The sensitivity for detecting large lesions (more than 2– 3 cm) is very high, but hemangiomas as small as 0. 5 cm may be detected with SPECT.
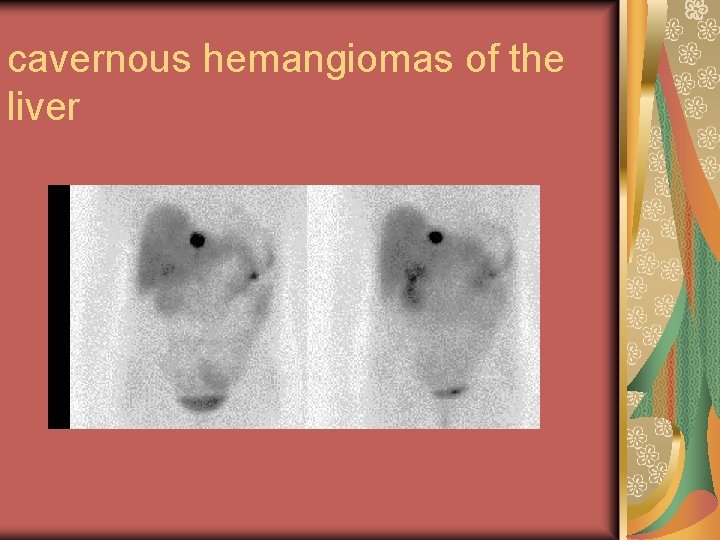
cavernous hemangiomas of the liver
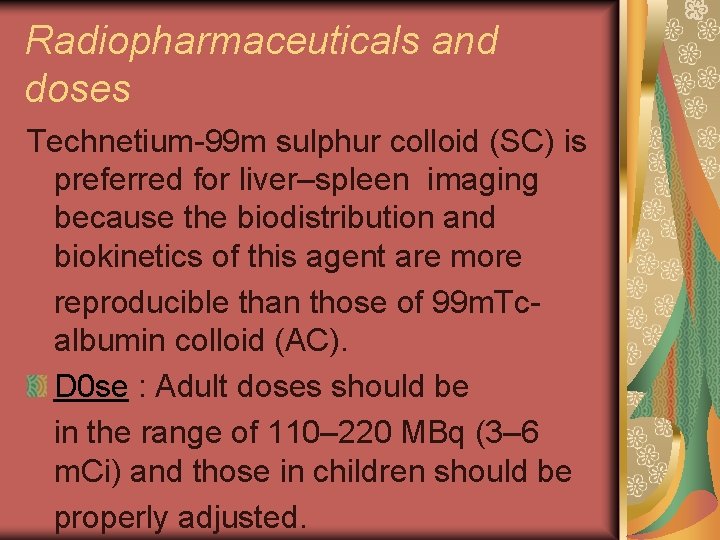
Radiopharmaceuticals and doses Technetium-99 m sulphur colloid (SC) is preferred for liver–spleen imaging because the biodistribution and biokinetics of this agent are more reproducible than those of 99 m. Tcalbumin colloid (AC). D 0 se : Adult doses should be in the range of 110– 220 MBq (3– 6 m. Ci) and those in children should be properly adjusted.

Equipment A large FOV gamma camera equipped with a low energy, all purpose or high resolution collimator is usually used.

Preparation No special patient preparation is required.
Procedures: Patient lie in supine position. Imaging is begun 10– 15 min or longer after the intravenous administration of 99 m. Tc-colloid. Anterior, posterior, right lateral, right anterior oblique and right posterior oblique images of the liver are commonly obtained. «

Left posterior oblique and left lateral views are added to evaluate the spleen. For large FOV gamma cameras, an anterior image is usually acquired for 500 000– 1 000 counts. Subsequent images are then obtained for the same length of time as for the anterior image. A size marker and a costal margin marker are needed for measuring liver and spleen size and for identifying anatomical landmarks.
NUCLEAR CARDIOLOGY Nuclear cardiology is a super specialty, in which nuclear physicians use nuclear imaging technology to investigate a variety of physiological and pathological aspects of the cardiovascular system. The major techniques used in nuclear cardiology can be categorized as: «
1 - Equilibrium radionuclide angiography ( ERNA) or multigated blood pool imaging, 2 - Myocardial perfusion imaging,
Equilibrium radionuclide angiography ( ERNA) or Multigated blood pool imaging Equilibrium radionuclide angiography (ERNA) is a non-invasive means of quantitatively assessing cardiac function. It has been demonstrated to be a highly accurate and reproducible technique, capable of assessing left and right ventricular function even if infarction, hypertrophy or dilation has distorted the shape of the ventricle.
hypertrophy or dilation has distorted the shape of the ventricle. This imaging modality makes use of an intravenously injected radionuclide that remains in the cardiac chambers in a concentration directly proportional to the blood volume. It can be used to assess regional wall motion, chamber size , and ventricular function including ejection fraction. Acquisitions are made at rest or during exercise, or under pharmacological.

The procedure is also known as gated cardiac blood pool imaging, multigated acquisition (MUGA) or radionuclide ventriculography (RVG).

MUGA with normal EF
Clinical indications (a) Coronary artery disease (b) Congestive heart failure (c) Valvular heart disease (d) Doxorubicin cardiotoxicity (e) Other indications ( e. g. ERNA may be helpful in cardiomyopathy, asymmetrical septal hypertrophy and chronic obstructive pulmonary disease (COPD).

Contraindications The following conditions are contraindications for ERNA: —Severe arrhythmia; —Uncontrolled unstable angina; —Decompensated congestive heart failure; —Uncontrolled hypertension (blood pressure more than 200/120 mm Hg); —Acute myocardial infarction of less than two days evolution.

Note Stress testing should be avoided in cases of particular contraindications for exercise, pharmacological procedures or other forms of cardiac challenge.

Radiopharmaceuticals Technetium-99 m is the only radionuclide that has been used for ERNA studies. Historically, 99 m. Tc human serum albumin was the agent of choice for ERNA, but image quality was poor due to albumin trapping in the pulmonary arterial tree. This was then replaced by 99 m. Tc labelled red blood cells (RBCs) ( called stannous).
Equipment Cameras Both large and small FOV cameras may be used for the procedure. The more frequently used large FOV camera provides diagnostic quality images. Small FOV cameras provide higher resolution images and are easily manipulated into the required position. With either type of camera, The detector must be positioned as close as possible to the patient’s chest during acquisition. Multicrystal cameras are not recommended due to their lower spatial resolution. An ECG gating device should be interfaced with the camera.
(b) Collimator A standard parallel hole, low energy, all-purpose collimator is sufficient for most ERNA studies. High resolution collimators improve image quality but require longer imaging times. (c) Computer systems Current nuclear imaging computers are capable of acquiring ERNA data.

The software should be capable of handling 64 ¥ 64 and 128 ¥ 128 acquisitions at rates of 8– 32 frames per cycle in frame and list mode. Patient preparation Resting ERNA studies require no special preparation. For exercise studies, 3– 4 h fasting prior to the procedure is recommended, and the patient should be haemodynamically and clinically stable. «

Pharmacological stress is recommended for patients unable to exercise. Cardiac medication, particularly that affecting heart rate, should be withheld unless contraindicated by the patient’s medical condition or if there is interest in testing the efficacy of the drug.
Procedure (a) Positioning The patient should lie down comfortably to prevent movement during the procedure. Three standard projections — left anterior oblique (LAO), anterior and left lateral views — are acquired for 10– 15 min each. The best septal view is taken with the detector in a 40– 50 o LAO position.
(b) Acquisition parameters An adequate study contains 250 000– 500 000 counts per frame, acquired in approximately 5 min from 300– 400 heart beats. (c) Stress protocols Treadmill exercise is inappropriate for ERNA because of chest motion. Bicycle exercise is preferred and can be performed in both the upright and supine positions.
A resting ERNA is usually performed first and in the same position that will be used in the exercise study so that any changes reflect true cardiac conditions rather than positional changes. (d) Data processing Processing begins with a review on a cinematic display to evaluate the adequacy of the counting statistics,

The whole activity from the left ventricle must be encompassed by the ROI, drawn either manually or automatically.
Myocardial perfusion scan Myocardial perfusion scintigraphy uses perfusion radiotracers that are distributed in the myocardium (primarily the left ventricle) in proportion to coronary blood flow. Areas of normal flow a relatively high level of tracer uptake. while ischaemic regions present a relatively low uptake.
Clinical indications The clinical indications for myocardial perfusion tomography are: (a) Detection of myocardial ischaemia and myocardium at risk. (b) Assessment of myocardial viability. (c) Assessment of ventricular function.
Radiopharmaceuticals A number of single photon emitting radiopharmaceuticals may be used for imaging myocardial perfusion. The three most commonly used at present are 201 Tl and the 99 m. Tc labelled tracers sestamibi and tetrofosmin. (a) Thallium-201 It is used in the chemical form of thallous chloride. «

It will emit mostly mercury X rays of 68– 80 ke. V. And also has gamma rays of 135 and 167 ke. V, which contribute little to the total image counts. Physical half-life 73 h. (b) Technetium-99 m labelled perfusion agents: 99 m. Tc labelled tracers Sestamibi (MIBI) and Tetrofosmin. Notes: The major route of excretion mibi is through the hepatobiliary «

liver and intestinal activity occasionally causes significant interference with image quality. Protocols employing 99 m. Tc-sestamibi involve post-injection waiting times of 45– 90 min, to allow for adequate clearance of subdiaphragmatic activity. The main advantages of Tetrofosmin are ease of preparation and faster hepatic clearance, allowing shorter post-injection waiting times of 20– 30 min.

Equipment (a) Cameras A single-crystal gamma camera is the basic piece of equipment required for myocardial perfusion imaging using both 201 Tl and 99 m. Tc agents. Most commercial models are equipped with a rotating gantry for SPECT imaging. (b) Collimators A general purpose collimator, a high resolution

(c) Gating devices An ECG or gating device is required for gated cardiac SPECT if one is not already included with the camera.
Procedure: Thallium rest only; patient wait 5 -20 minutes after injection before imaging. Sestamibi: patient wait 45 -60 minutes after injection before imaging. Give patient a glass of cold water before imaging to clear thyroid , liver and bowel. Tetrofosmin : patient waits 5 -30 minutes after injection before imaging. ( Give water). Position patient supine with heart in

center field of view and left arm up over head if possible, if arm is down at side because of problems with shoulder joint or recent surgery , both rest and stress images should be taken the same way. - Images may include a static anterior picture first ( 300 second ). Start SPECT images with camera right anterior oblique to left posterior oblique. «
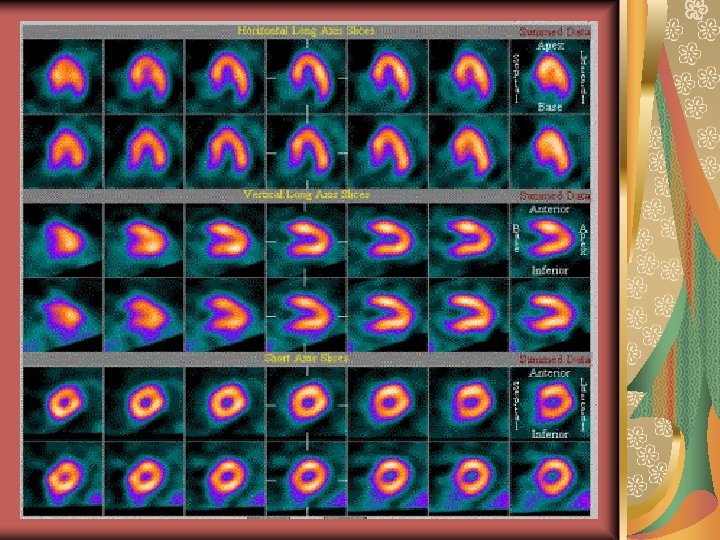
Processing computers An automated reconstruction and reorientation program is a requirement. Most manufacturers, however, provide for automated endocardial border ROI placement for quantitation of ventricular function. Computer analysis of left ventricle showing the ventrical long axis, horizontal long axis and sort axis.

Stress protocol Treadmill or Bicycle ergometry is the method of choice for exercise studies. Done by cardiologist and nuclear medicine staff. Inject the tracer during the exercise when the heart beats reach the maximum rate. After 10 minutes from treadmill the scan should be start. Do all procedures as rest protocol.
Hepatobiliary scintigraphy HIDA Scan Hepatobiliary scintigraphy is a diagnostic imaging study that evaluates hepatocellular function and patency of the biliary system by tracing the production and flow of bile from the liver through the biliary system into the small intestine.

Clinical indications (a) Functional assessment of the hepatobiliary system. (b) Evaluation of integrity of the hepatobiliary tree. These include investigation of: —Suspected acute cholecystitis; —Suspected chronic biliary tract disorders; —Common bile duct obstruction; —Bile extravasation;

Radiopharmaceuticals Technetium-99 m labelled disofenin (iminodiacetic acid (HIDA)) Dose Adult dose range from 50– 200 MBq (1. 5– 5 m. Ci) is administered intravenously For infants and children, the administered activity is 2– 7 MBq/kg (0. 05– 0. 2 m. Ci/kg), with a minimum of 15– 20 MBq (0. 3– 0. 5 m. Ci).

Equipment A large FOV gamma camera equipped with a low energy, all purpose or high resolution collimator is generally used. Whenever possible, continuous computer acquisition should be performed (1 frame/min for 30– 60 min). «
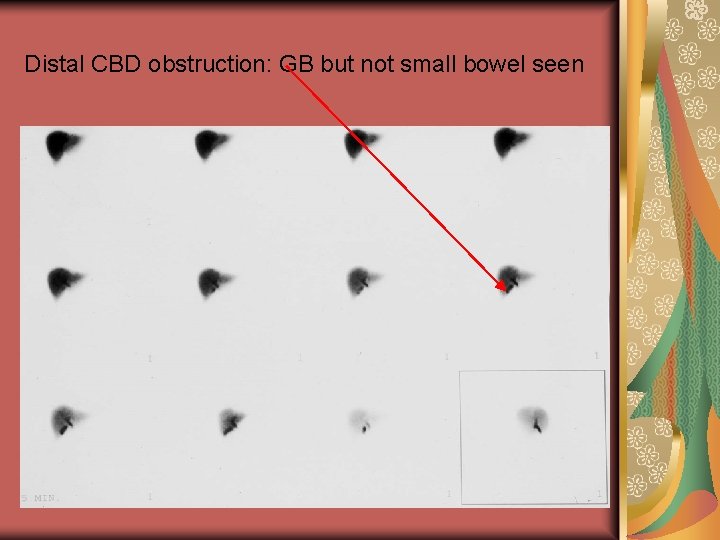
Distal CBD obstruction: GB but not small bowel seen

Patient preparation To permit gall bladder visualization, the patient must have fasted for a minimum of two and preferably four hours prior to administration of the radiopharmaceutical.

Information to performing the procedure The physician or technologist should give full information prior to the study to the patient.

Procedure imaging should commence at injection and continue serially for 60 min or until activity is seen in both the gall bladder (which confirms the patency of the cystic duct) and the small bowel (which confirms the patency of the common bile duct). Additional views (e. g. right lateral, left or right anterior oblique) may be obtained as needed to clarify the anatomy.
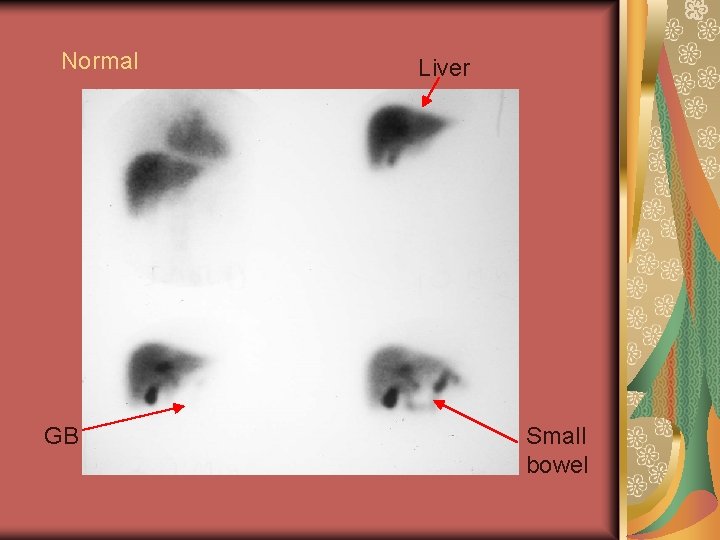
Normal GB Liver Small bowel

Gall bladder contractility may be evaluated by determining the gall bladder ejection fraction (GBEF) response to fatty meal Gall bladder ejection fraction ROIs are drawn around the gall bladder (taking into account patient motion) and adjacent liver (background) using any standard nuclear medicine software package.

The liver background ROI is selected taking care to exclude ductal activity. GBEF is calculated from the gall bladder time–activity curve as: GBEF (%) = (net GB counts max ) - (net GB counts min ) x 100 ——————— (net GB counts max ) where GB stands for gall bladder.
Brain scan is an old term referring to scintigraphic detection of the integrity of the BBB. Dynamic imaging of the head immediately after tracer injection, referred to as radionuclide cerebral angiography, depicts the cerebral vasculature.
Clinical indications (a) To detect the existence, location, extent and distribution of brain lesions tumour, infarct, inflammation, haemorrhage or trauma) or intracraniallesions; ( (b) To assess the infection, (c) To detect the patency and morphology of major intracranial vessels; (d) To diagnose brain death.

Radiopharmaceuticals 99 m. Tc-DTPA, 99 m. Tcpertechnetate and 99 m. Tc-glucoheptonate, are widely used for brain scans

Protocols —The patient should rest quietly for a few minutes before the study. Sedation is indicated for those unable to cooperate. —Potassium perchlorate should be given orally, if indicated, 30 min before injection. —The patient is positioned for cerebral angiography, usually in the anterior view. —The selected radiopharmaceutical is then given as a bolus into a peripheral vein. —Data acquisition is started immediately (angiography) or 30– 90 min (static scan) after injection.

Acquisition Brain scans are usually performed in the anterior, posterior, and both lateral projections, but occasionally additional views may help delineate a lesion better. Cerebral angiography is acquired in dynamic mode at 1– 2 s/frame for 20– 40 s, using a 64 × 64 matrix, with or without zoom. —Brain scans are usually started in the anterior view, using a 256 × 256 matrix, for 600 000 counts. The acquisition time is used to determine the time for the other views, for comparison purposes. —Tomography (SPECT) might be used, in which case acquisition is carried out 40– 60 min after injection, using a 360° orbit and preferably in a 128 × 128 matrix.
- Slides: 135