Nuclear Fission Through the 1930s higher mass elements





















- Slides: 45

Nuclear Fission • Through the 1930’s higher mass elements could be created by bombarding nuclei with neutrons, followed by beta decay • Attempts to create transuranic elements failed, however. Instead, Barium and other lighter elements were identified in the reaction products. • (1939) Meiner and Frisch proposed that Uranium undergoes fission, or splits into fragments, after neutron absorption • Fission represents a competition between nuclear binding and Coulomb repulsion Nuclear Binding ~ A Coulomb Repulsion ~ Z 2 10/19/20 21 Physics 590 B - Fall 2014 1

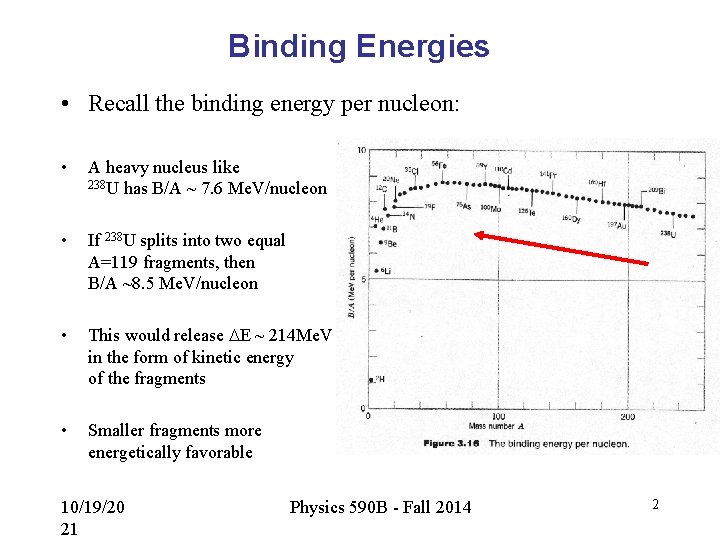
Binding Energies • Recall the binding energy per nucleon: • A heavy nucleus like 238 U has B/A ~ 7. 6 Me. V/nucleon • If 238 U splits into two equal A=119 fragments, then B/A ~8. 5 Me. V/nucleon • This would release DE ~ 214 Me. V in the form of kinetic energy of the fragments • Smaller fragments more energetically favorable 10/19/20 21 Physics 590 B - Fall 2014 2

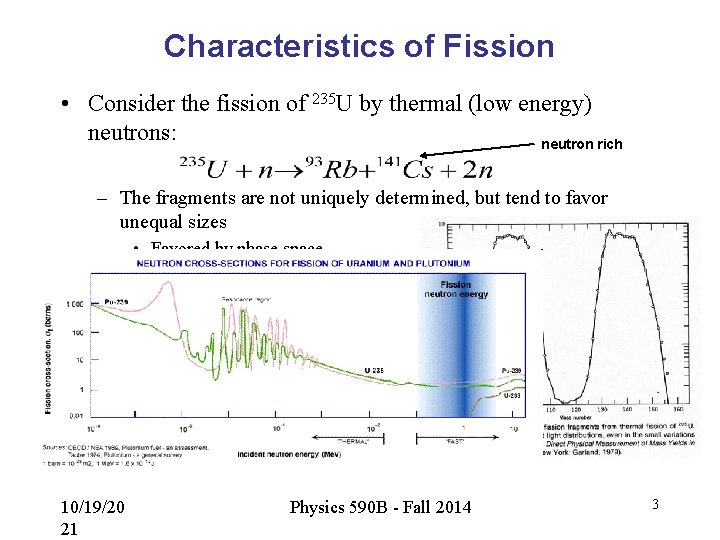
Characteristics of Fission • Consider the fission of 235 U by thermal (low energy) neutrons: neutron rich – The fragments are not uniquely determined, but tend to favor unequal sizes • Favored by phase space arguments, both nuclei closer to stability – “Fast” neutrons favor more equal mass fragments 10/19/20 21 Physics 590 B - Fall 2014 3

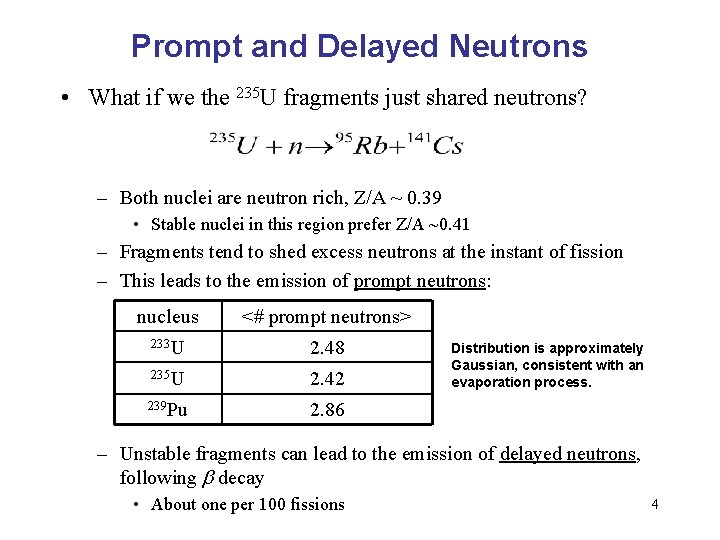
Prompt and Delayed Neutrons • What if we the 235 U fragments just shared neutrons? – Both nuclei are neutron rich, Z/A ~ 0. 39 • Stable nuclei in this region prefer Z/A ~0. 41 – Fragments tend to shed excess neutrons at the instant of fission – This leads to the emission of prompt neutrons: nucleus <# prompt neutrons> 233 U 2. 48 235 U 2. 42 239 Pu 2. 86 Distribution is approximately Gaussian, consistent with an evaporation process. – Unstable fragments can lead to the emission of delayed neutrons, following b decay • About one per 100 fissions 4

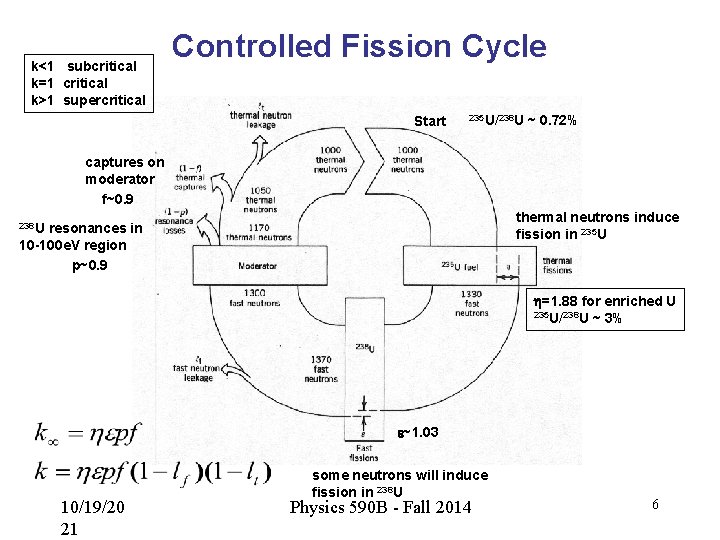
Controlled Fission • The neutrons produced in a fission reaction are fast (few Me. V) • If we can moderate or slow down the neutrons, then they can initiate additional fission reactions – Slower neutrons have higher capture cross sections – This is the idea behind a controlled chain reaction used in nuclear production • E. Fermi (1942) – Early reactors used carbon as a moderator • Light nucleus, large energy transfer in collision • Interleaved U and C blocks formed a pile (238 U capture resonances) • Neutron Reproduction Factor k: – The change in the number of thermal neutrons from one generation of reactions to the next 10/19/20 21 Physics 590 B - Fall 2014 5

k<1 subcritical k=1 critical k>1 supercritical Controlled Fission Cycle Start 235 U/238 U ~ 0. 72% captures on moderator f~0. 9 thermal neutrons induce fission in 235 U 238 U resonances in 10 -100 e. V region p~0. 9 h=1. 88 for enriched U 235 U/238 U ~ 3% e~1. 03 10/19/20 21 some neutrons will induce fission in 238 U Physics 590 B - Fall 2014 6

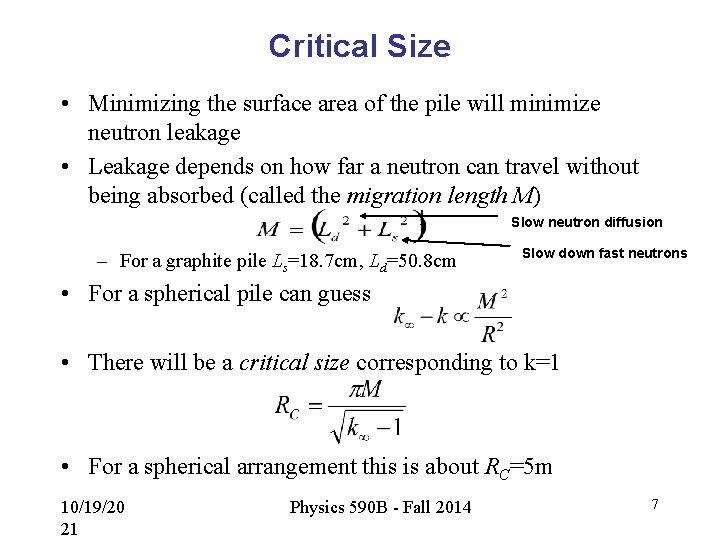
Critical Size • Minimizing the surface area of the pile will minimize neutron leakage • Leakage depends on how far a neutron can travel without being absorbed (called the migration length M) Slow neutron diffusion – For a graphite pile Ls=18. 7 cm, Ld=50. 8 cm Slow down fast neutrons • For a spherical pile can guess • There will be a critical size corresponding to k=1 • For a spherical arrangement this is about RC=5 m 10/19/20 21 Physics 590 B - Fall 2014 7

Timescales and Control • The neutrons are characterized by a time constant t that involves both moderation (10 -6 s) and absorption (10 -3 s) • If you have N neutrons at t=0, you have k. N at t=t • So if k>1 the number of neutrons will grow exponentially with a timescale of order ms…. • Solution is to use Cd control rods to absorb neutrons – Reactor is subcritical for prompt neutrons – Delayed neutrons (with longer timescale) make it critical 10/19/20 21 Physics 590 B - Fall 2014 8

A Natural Fission Reactor • The natural abundance of 235 U relative to 238 U is about 0. 72% – Moon rocks show same abundance • Oklo mine (Gabon, Africa) shows an unusually low abundance of 235 U (3 s below the mean), some places as low as 0. 44%! – No known chemical process should change the natural ratio like this • About 2 x 109 years ago, the natural abundance of 235 U relative to 238 U was about 3% – A “natural” fission reactor could have operated, using groundwater as a moderator – Low power (0. 01 MW) or it would boil away the groundwater – Burned for about 106 years – Consumed about 5 tons of 235 U 10/19/20 21 Physics 590 B - Fall 2014 9

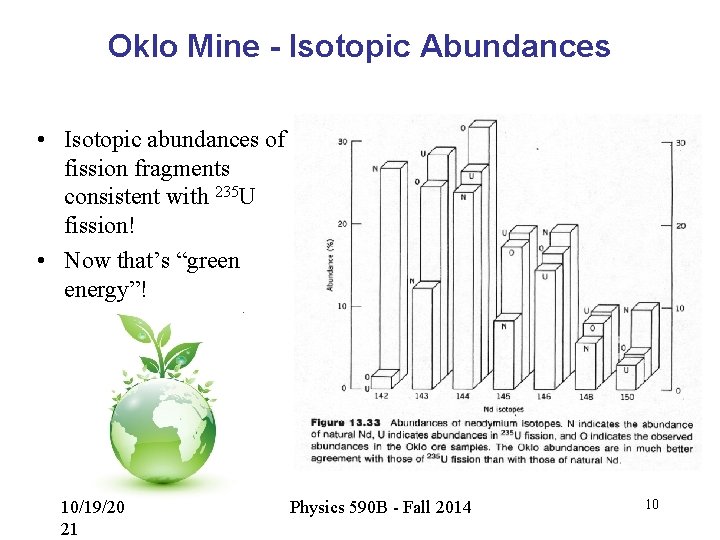
Oklo Mine - Isotopic Abundances • Isotopic abundances of fission fragments consistent with 235 U fission! • Now that’s “green energy”! 10/19/20 21 Physics 590 B - Fall 2014 10

Fission Reactor Technology (I) • Classify fission reactors by MODERATOR – Graphite moderated • Older design, safety issues (Chernobyl) – Heavy water (D 20) moderated • Can use unenriched Uranium – Bypass international restrictions on enriching Uranium – Produce more Pu as a byproduct – Light water moderated • Require enriched Uranium • Negative feedback stabilizes reactor – Density of water falls as temperature increases – Molten Salt Reactors (Li, Be) • Very compact design (aircraft) • Simple design, low pressure – Liquid Metal (fast reactors, unmoderated) 10/19/20 21 • Soviet nuclear submarines (Alfa class) Physics 590 B - Fall 2014 11

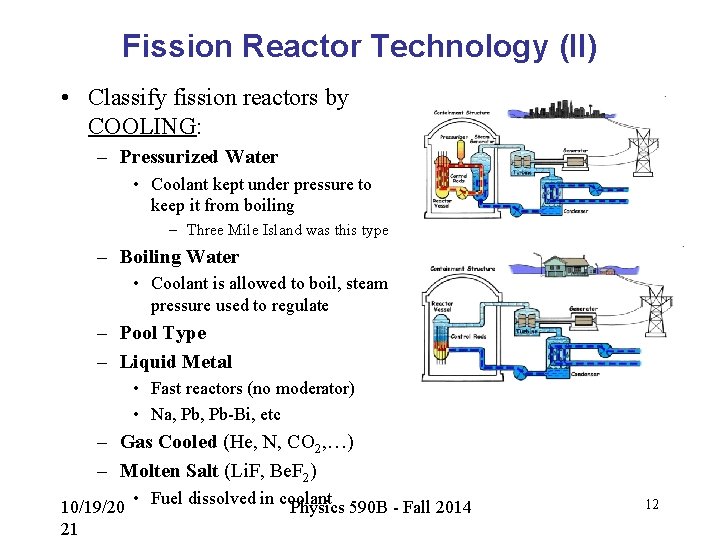
Fission Reactor Technology (II) • Classify fission reactors by COOLING: – Pressurized Water • Coolant kept under pressure to keep it from boiling – Three Mile Island was this type – Boiling Water • Coolant is allowed to boil, steam pressure used to regulate – Pool Type – Liquid Metal • Fast reactors (no moderator) • Na, Pb-Bi, etc – Gas Cooled (He, N, CO 2, …) – Molten Salt (Li. F, Be. F 2) 10/19/20 21 • Fuel dissolved in coolant Physics 590 B - Fall 2014 12

Nuclear Fuel • Uranium and Plutonium are used in a variety of forms as a nuclear fuel – Uranium Oxide • Enrichment varies – MOX Fuel • Mixture of Pu and depleted U • Alternative to LEU for LWR’s • Used by England, France and Russia, India, Japan to a lesser extent • China plans fast breeders with reprocessing – Molten Salts – TRISO • Pebble-bed reactors 10/19/20 21 C Si. C UO Physics 590 B - Fall. X 2014 13

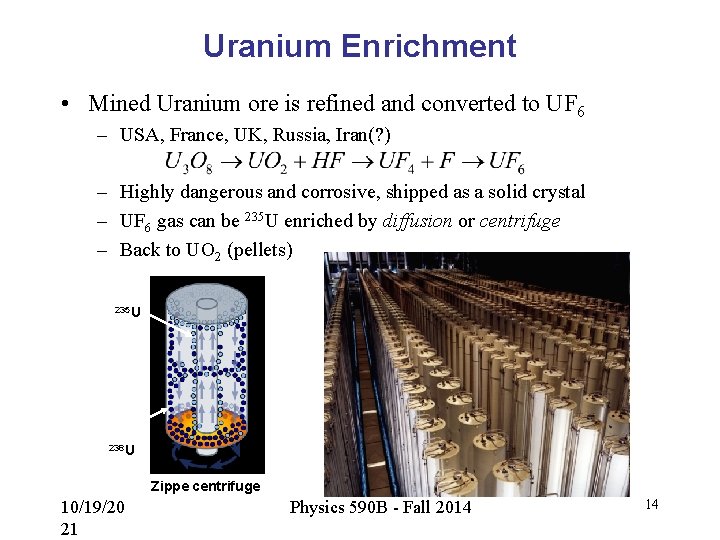
Uranium Enrichment • Mined Uranium ore is refined and converted to UF 6 – USA, France, UK, Russia, Iran(? ) – Highly dangerous and corrosive, shipped as a solid crystal – UF 6 gas can be 235 U enriched by diffusion or centrifuge – Back to UO 2 (pellets) 235 U 238 U Zippe centrifuge 10/19/20 21 Physics 590 B - Fall 2014 14

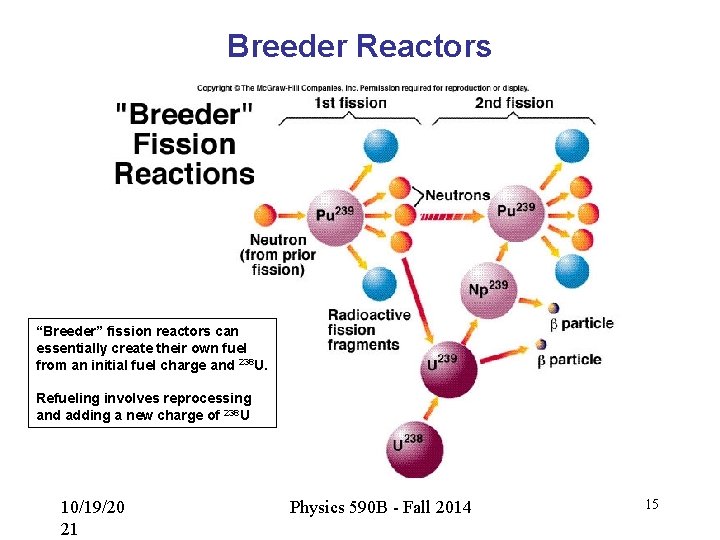
Breeder Reactors “Breeder” fission reactors can essentially create their own fuel from an initial fuel charge and 238 U. Refueling involves reprocessing and adding a new charge of 238 U 10/19/20 21 Physics 590 B - Fall 2014 15

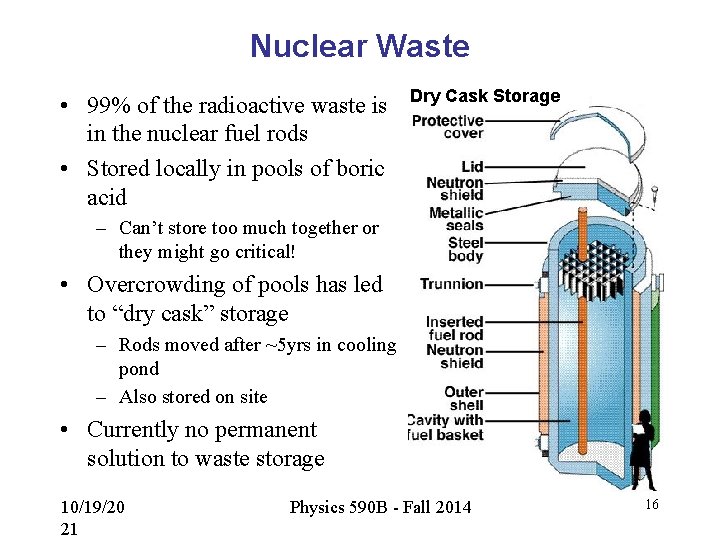
Nuclear Waste • 99% of the radioactive waste is in the nuclear fuel rods • Stored locally in pools of boric acid Dry Cask Storage – Can’t store too much together or they might go critical! • Overcrowding of pools has led to “dry cask” storage – Rods moved after ~5 yrs in cooling pond – Also stored on site • Currently no permanent solution to waste storage 10/19/20 21 Physics 590 B - Fall 2014 16

Yucca Mountain Geologically stable for ~10 k years (expected) Underground storage facility constructed by tunnel boring into the mountain. 10/19/20 21 Physics 590 B - Fall 2014 17

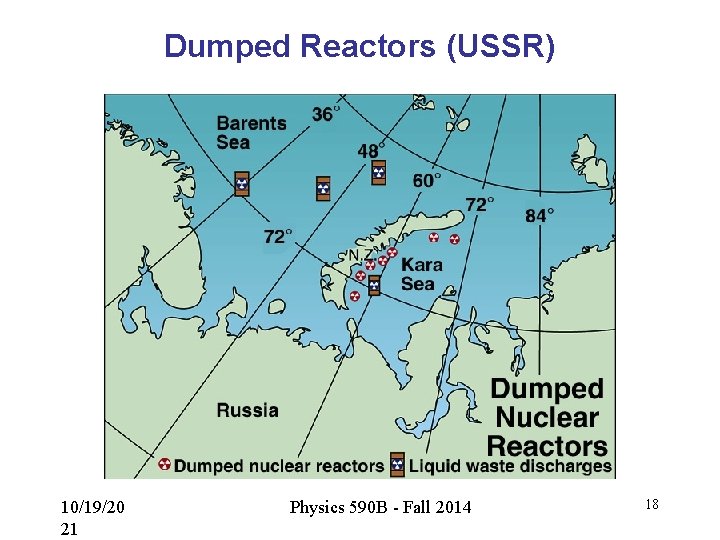
Dumped Reactors (USSR) 10/19/20 21 Physics 590 B - Fall 2014 18

Chernobyl • 26 April 1986: • Explosion and fire in reactor #4 at Chernobyl nuclear facility near Pripyat, Ukraine • 400 times more fallout that Hiroshima • Catastrophic power excursion caused steam explosion • Ironically caused by a failed safety test prior to shutdown for refueling 10/19/20 21 radiation shield and containment building steam separator control rods steam cooling water RMB-1000 Nuclear Reactor Physics 590 B - Fall 2014 19

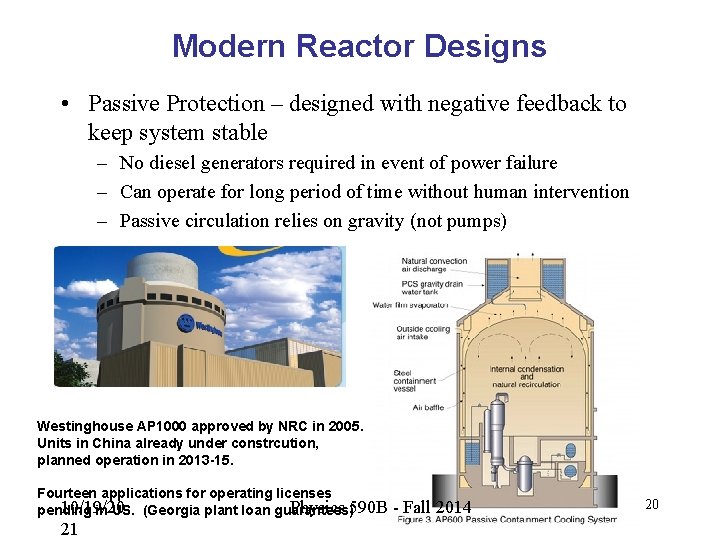
Modern Reactor Designs • Passive Protection – designed with negative feedback to keep system stable – No diesel generators required in event of power failure – Can operate for long period of time without human intervention – Passive circulation relies on gravity (not pumps) Westinghouse AP 1000 approved by NRC in 2005. Units in China already under constrcution, planned operation in 2013 -15. Fourteen applications for operating licenses 10/19/20 Physics 590 B pending in US. (Georgia plant loan guarantees) 21 - Fall 2014 20

Fission Explosives • Nuclear weapons require much more highly enriched Uranium – Need energy release from a supercritical mass faster than the mass is blown apart – A crude device could be built with ~20% 235 U, “modern” weapons use >85% • Collect subcritical pieces into a critical assembly Frank Spedding 10/19/20 21 Physics 590 B - Fall Harley Wilhelm ~2 M lbs of pure Uranium 1942 -45 21 2014

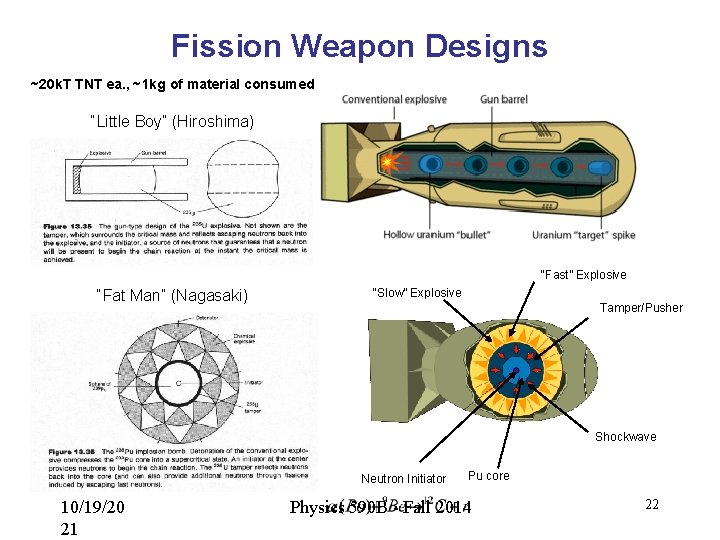
Fission Weapon Designs ~20 k. T TNT ea. , ~1 kg of material consumed “Little Boy” (Hiroshima) “Fast” Explosive “Fat Man” (Nagasaki) “Slow” Explosive Tamper/Pusher Shockwave Neutron Initiator 10/19/20 21 Pu core Physics 590 B - Fall 2014 22

Fusion • Instead of splitting large nuclei, what if we combine light elements • Fusion has many key advantages over fission: – Light nuclei (p, d, t) easy to obtain – End products light and stable • However, in order to get nuclei to fuse you have to overcome the Coulomb barrier between them. 10/19/20 21 Physics 590 B - Fall 2014 23

Coulomb Repulsion (Again!) • Consider: (Q = 20. 7 Me. V) – Using the “two spheres touching” model: – So if we collide a beam of 20 Ne on a 20 Ne target at 21. 2 Me. V we would get back – Almost double our investment! – Why doesn’t this work as a power source? • Doesn’t take inefficiencies into account • High intensity beams difficult to produce • At best you could get a few Watts… 10/19/20 Physics 590 B - Fall 2014 21 24

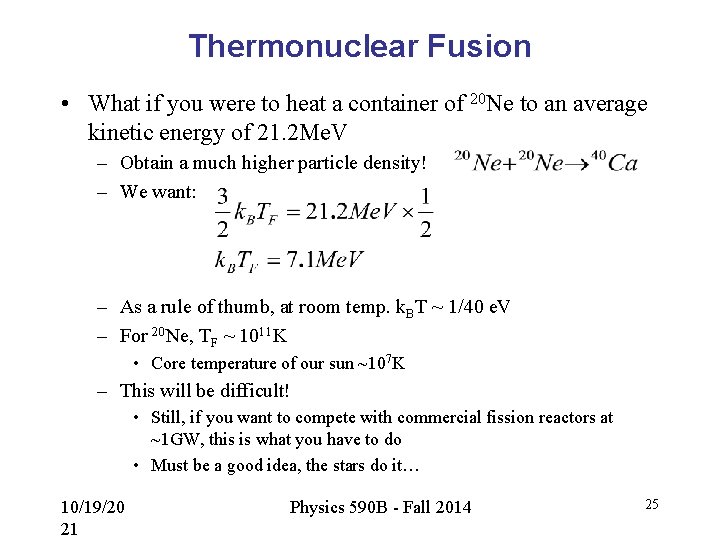
Thermonuclear Fusion • What if you were to heat a container of 20 Ne to an average kinetic energy of 21. 2 Me. V – Obtain a much higher particle density! – We want: – As a rule of thumb, at room temp. k. BT ~ 1/40 e. V – For 20 Ne, TF ~ 1011 K • Core temperature of our sun ~107 K – This will be difficult! • Still, if you want to compete with commercial fission reactors at ~1 GW, this is what you have to do • Must be a good idea, the stars do it… 10/19/20 21 Physics 590 B - Fall 2014 25

Basic Fusion Processes (I) • The most basic fusion process we can think of is: – Not possible! 2 He is unstable… • A possible reaction is: (Q=1. 44 Me. V) – Requires the weak interaction to come into play – This reaction will be rate limiting! • Also possible: (Q=23. 8 Me. V) – 4 He excited state high in energy, so photon necessary for energy balance – Q is greater than the n, p separation energy for 4 He – This reaction is unlikely 10/19/20 21 Physics 590 B - Fall 2014 26

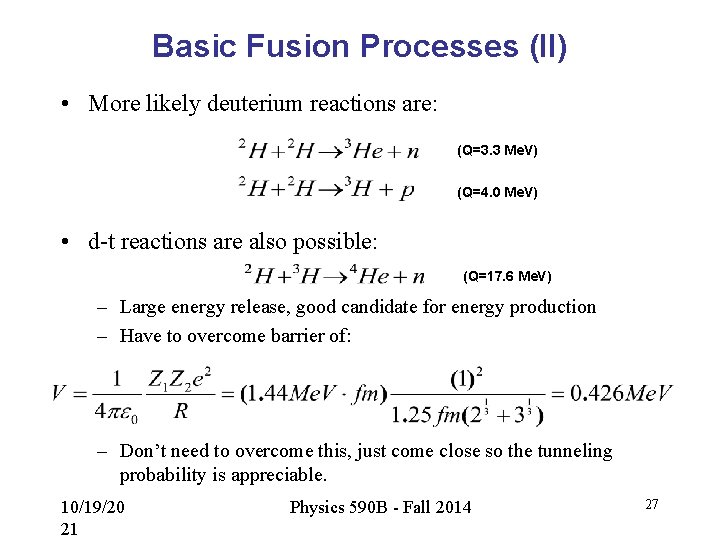
Basic Fusion Processes (II) • More likely deuterium reactions are: (Q=3. 3 Me. V) (Q=4. 0 Me. V) • d-t reactions are also possible: (Q=17. 6 Me. V) – Large energy release, good candidate for energy production – Have to overcome barrier of: – Don’t need to overcome this, just come close so the tunneling probability is appreciable. 10/19/20 21 Physics 590 B - Fall 2014 27

Kinematics • If the initial kinetic energy is low compared to the Q value, so we can write: The lightest particle will carry away most of the Q-value! • For the d-t reaction, <En> ~ 14. 1 Me. V – This energy can be difficult to extract 10/19/20 21 Physics 590 B - Fall 2014 28

Fusion Cross Sections • For particles interacting at thermal energies, the reaction will most likely occur away from any resonances • The basic fusion cross section can be written as: v = relative velocity – Where G is the same Gamow factor we encountered in a decay – For Ek << VB we can approximate: – The proportionality factor will account for statistical factors, spins, etc. 10/19/20 21 Physics 590 B - Fall 2014 29

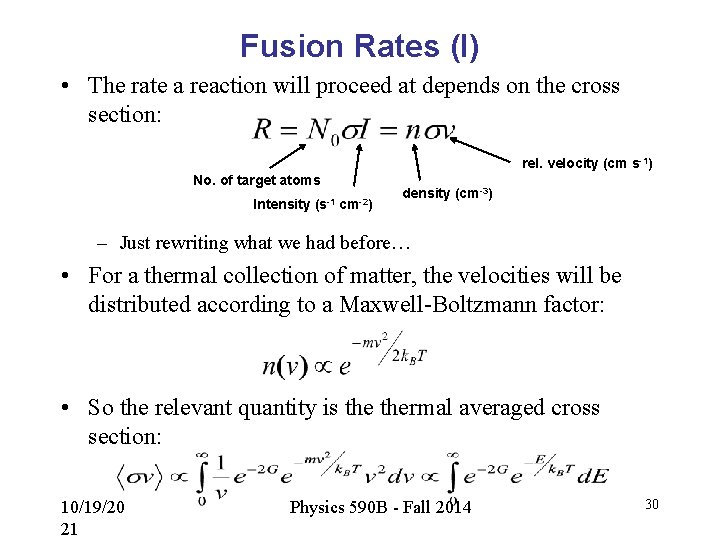
Fusion Rates (I) • The rate a reaction will proceed at depends on the cross section: rel. velocity (cm s-1) No. of target atoms Intensity (s-1 cm-2) density (cm-3) – Just rewriting what we had before… • For a thermal collection of matter, the velocities will be distributed according to a Maxwell-Boltzmann factor: • So the relevant quantity is thermal averaged cross section: 10/19/20 21 Physics 590 B - Fall 2014 30

Fusion Rates (II) • The fusion rate for a process will depend on the interplay between the cross section and the Maxwell. Boltzmann distribution – MB peaked low – sv grows for higher energies (For asymmetric distributions and s as a function of vrel) 10/19/20 systems we need two Boltzmann Physics 590 B - Fall 2014 21 31

Fusion Rates (III) 107 K 10/19/20 21 108 K Physics 590 B - Fall 2014 32

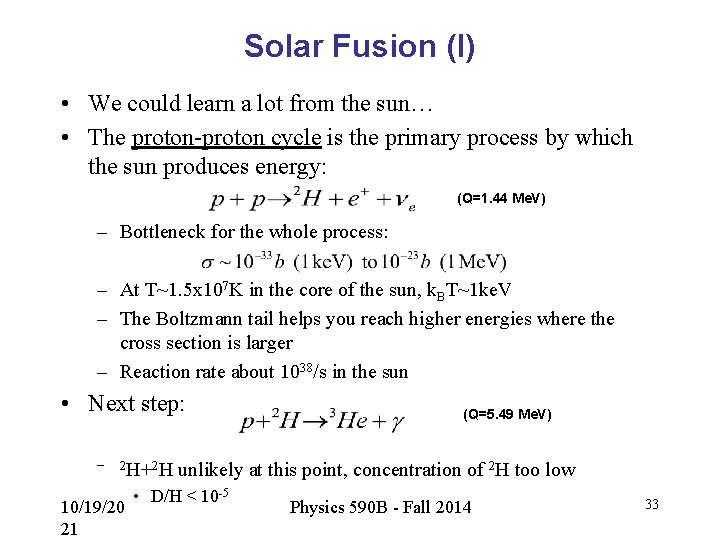
Solar Fusion (I) • We could learn a lot from the sun… • The proton-proton cycle is the primary process by which the sun produces energy: (Q=1. 44 Me. V) – Bottleneck for the whole process: – At T~1. 5 x 107 K in the core of the sun, k. BT~1 ke. V – The Boltzmann tail helps you reach higher energies where the cross section is larger – Reaction rate about 1038/s in the sun • Next step: – 2 H+2 H 10/19/20 21 (Q=5. 49 Me. V) unlikely at this point, concentration of 2 H too low • D/H < 10 -5 Physics 590 B - Fall 2014 33

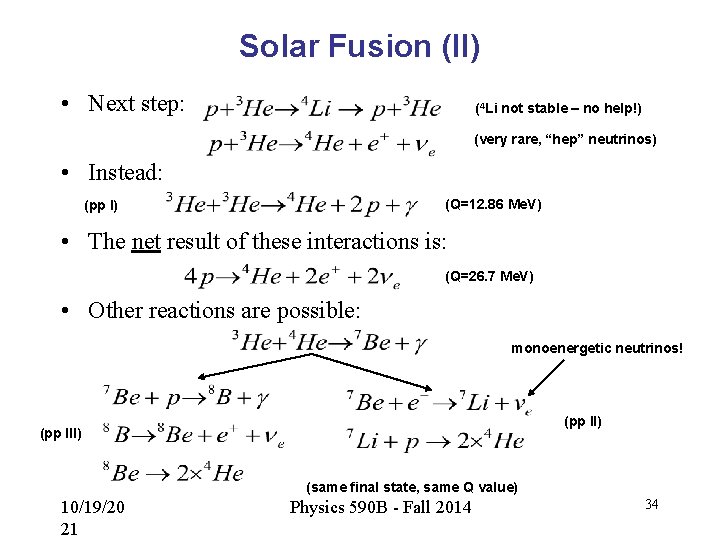
Solar Fusion (II) • Next step: (4 Li not stable – no help!) (very rare, “hep” neutrinos) • Instead: (Q=12. 86 Me. V) (pp I) • The net result of these interactions is: (Q=26. 7 Me. V) • Other reactions are possible: monoenergetic neutrinos! (pp II) (pp III) (same final state, same Q value) 10/19/20 21 Physics 590 B - Fall 2014 34

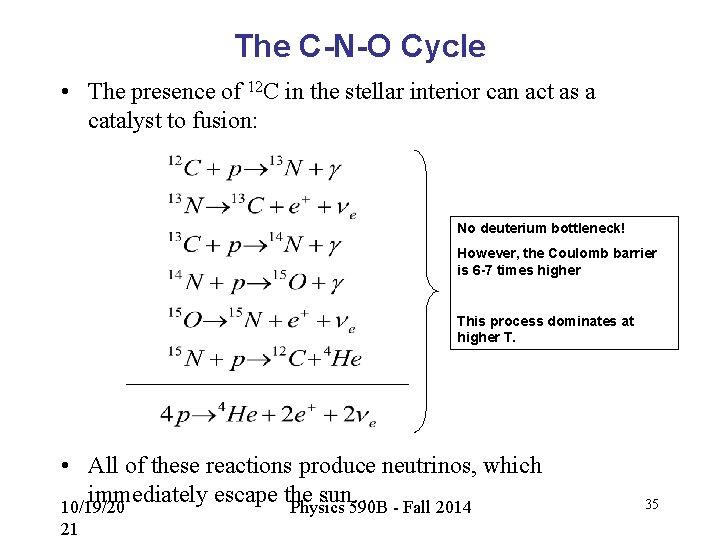
The C-N-O Cycle • The presence of 12 C in the stellar interior can act as a catalyst to fusion: No deuterium bottleneck! However, the Coulomb barrier is 6 -7 times higher This process dominates at higher T. • All of these reactions produce neutrinos, which immediately escape the sun… 10/19/20 Physics 590 B - Fall 2014 21 35

Solar Neutrinos Ray Davies (1964) – deficit of neutrinos from inverse beta process Kamiokande, Gallex, SAGE, etc (80’s-90’s): confirm deficit SNO (2001) – not all neutrinos are electron neutrinos when they reach Earth!! Kamland – verified neutrino oscillations theory. 10/19/20 Physics 590 B - Fall 2014 21 36

Neutrino Oscillations • The neutrino flavor eigenstates are not the same as the mass eigenstates: (E=1 Ge. V, Dm 2=0. 005 e. V 2) • Neutrinos born as ne can be detected as nm 10/19/20 21 Physics 590 B - Fall 2014 37

Fusion Power Magnetic Confinement: Tokamak “Mirror” Inertial Confinement: Atmos. Formation 10/19/20 21 Compression Ignition Physics 590 B - Fall 2014 Burn 38

ITER 24 m Vacuum Vessel Magnet System Blanket 30 m Divertor R=6. 2 m Ip=15 MA Pfus=500 MW 10/19/20 21 Person Physics 590 B - Fall 2014 39

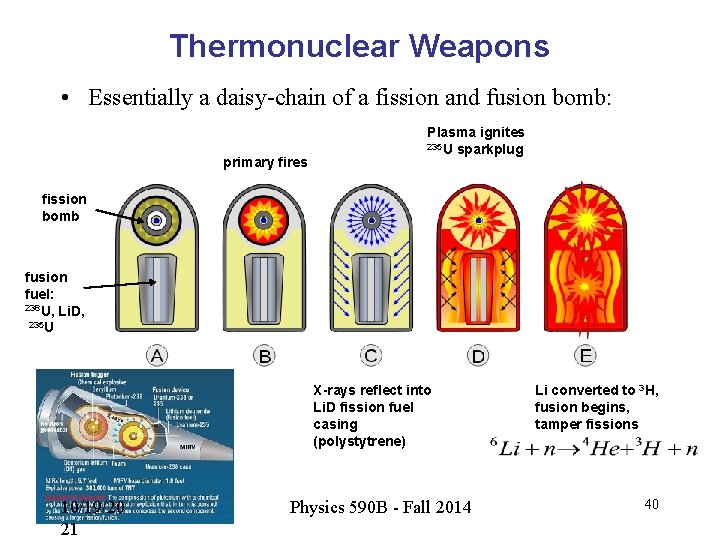
Thermonuclear Weapons • Essentially a daisy-chain of a fission and fusion bomb: primary fires Plasma ignites 235 U sparkplug fission bomb fusion fuel: 238 U, Li. D, 235 U X-rays reflect into Li. D fission fuel casing (polystytrene) 10/19/20 21 Physics 590 B - Fall 2014 Li converted to 3 H, fusion begins, tamper fissions 40

BACKUP 10/19/20 21 Physics 590 B - Fall 2014 41

Fission Lifetimes • If it is energetically favorable, why don’t nuclei spontaneously fall apart? – For 238 U, t 1/2 = 4. 5 x 109 years for a decay, but 1016 years for fission! • The Coulomb barrier inhibits fission in much the same way as for a decay – Barrier height for 238 U decay to 119 Pd estimate: – The 214 Me. V energy release makes many final states available, however the barrier height makes tunneling unlikely! 10/19/20 21 Physics 590 B - Fall 2014 42

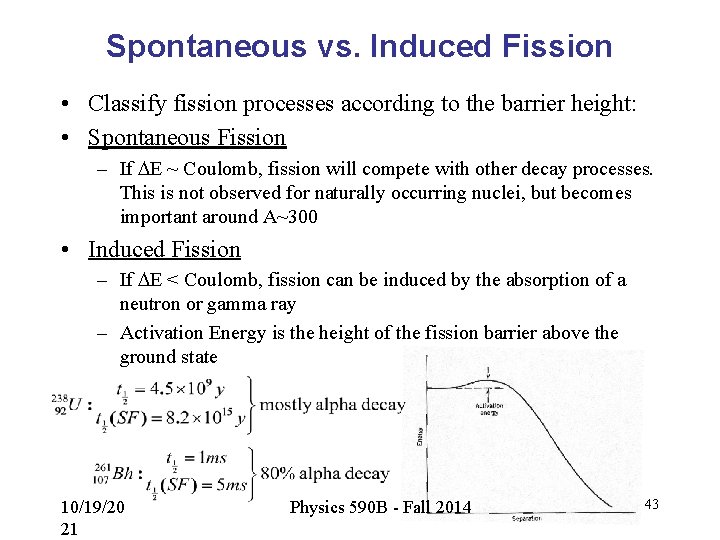
Spontaneous vs. Induced Fission • Classify fission processes according to the barrier height: • Spontaneous Fission – If DE ~ Coulomb, fission will compete with other decay processes. This is not observed for naturally occurring nuclei, but becomes important around A~300 • Induced Fission – If DE < Coulomb, fission can be induced by the absorption of a neutron or gamma ray – Activation Energy is the height of the fission barrier above the ground state 10/19/20 21 Physics 590 B - Fall 2014 43

Activation Energy (I) • The liquid-drop model can predict the average behavior – Of course, shell effects will modify this – Get a quantitative feel for the fission process – Stretch a nucleus, keeping the volume constant b a – As the nucleus is stretched, the surface area changes: – The dominant change in the binding energy comes from the surface area and Coulomb terms: 10/19/20 21 Physics 590 B - Fall 2014 44

Activation Energy (II) • If the second term dominates the first term, we gain energy and the nucleus will be unstable spontaneous fission condition 10/19/20 21 Physics 590 B - Fall 2014 45