Nuclear Chemistry Section 24 1 Nuclear Radiation Section





































- Slides: 120


Nuclear Chemistry Section 24. 1 Nuclear Radiation Section 24. 2 Radioactive Decay Section 24. 3 Nuclear Reactions Section 24. 4 Applications and Effects of Nuclear Reactions Click a hyperlink or folder tab to view the corresponding slides. Exit

Section 24. 1 Nuclear Radiation • Summarize the events that led to understanding radiation. • Identify alpha, beta, and gamma radiations in terms of composition and key properties. nucleus: the extremely small, positively charged, dense center of an atom that contains positively charged protons, neutral neutrons, and is surrounded by empty space through which one or more negatively charged electrons move

Section 24. 1 Nuclear Radiation (cont. ) radioisotope X ray penetrating power Under certain conditions, some nuclei can emit alpha, beta, or gamma radiation.

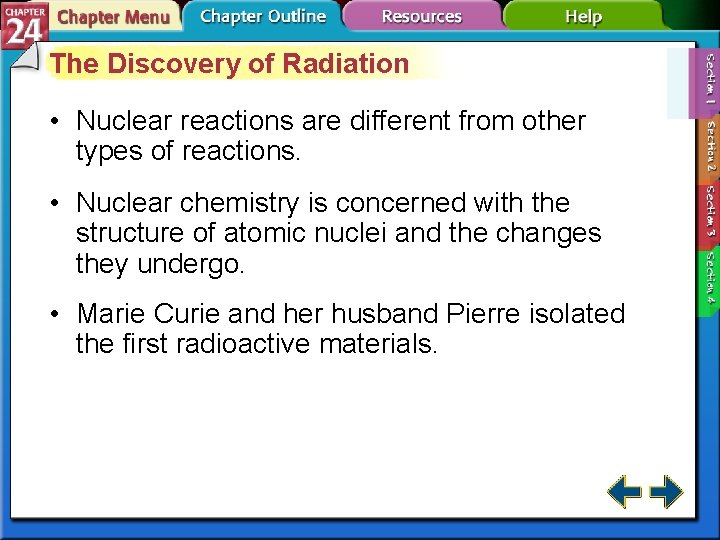
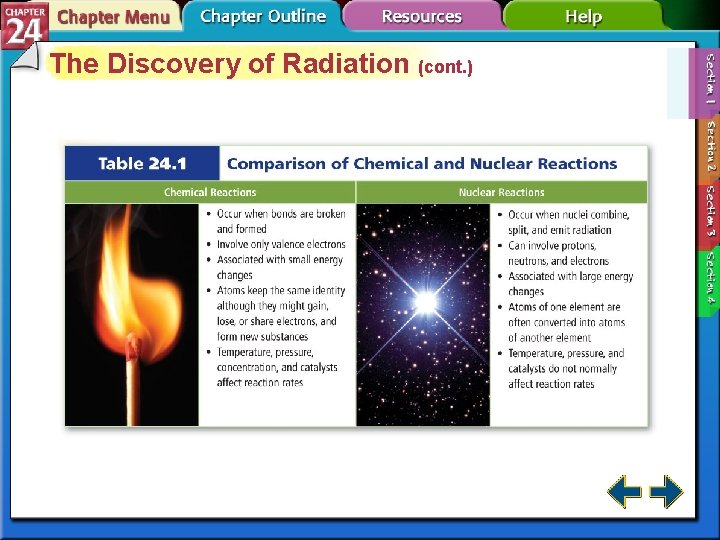
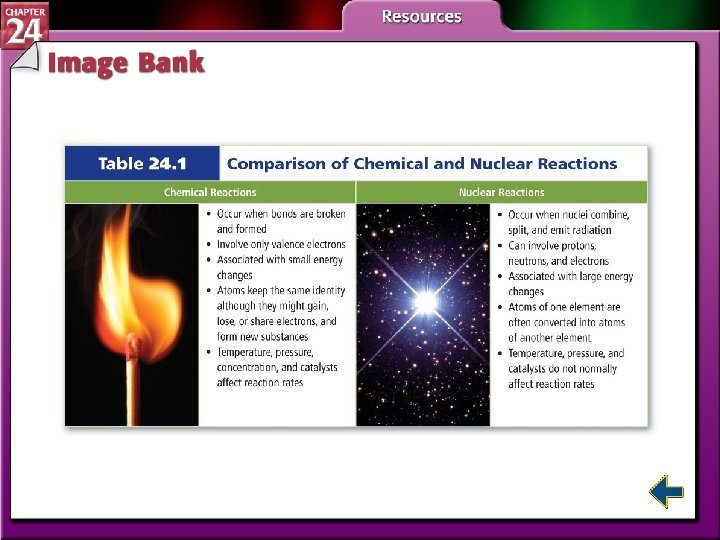
The Discovery of Radiation • Nuclear reactions are different from other types of reactions. • Nuclear chemistry is concerned with the structure of atomic nuclei and the changes they undergo. • Marie Curie and her husband Pierre isolated the first radioactive materials.

The Discovery of Radiation (cont. )

Types of Radiation • Isotopes of atoms with unstable nuclei are called radioisotopes. • Unstable nuclei emit radiation to attain more stable atomic configurations in a process called radioactive decay. • The three most common types of radiation are alpha, beta, and gamma.

Types of Radiation (cont. )

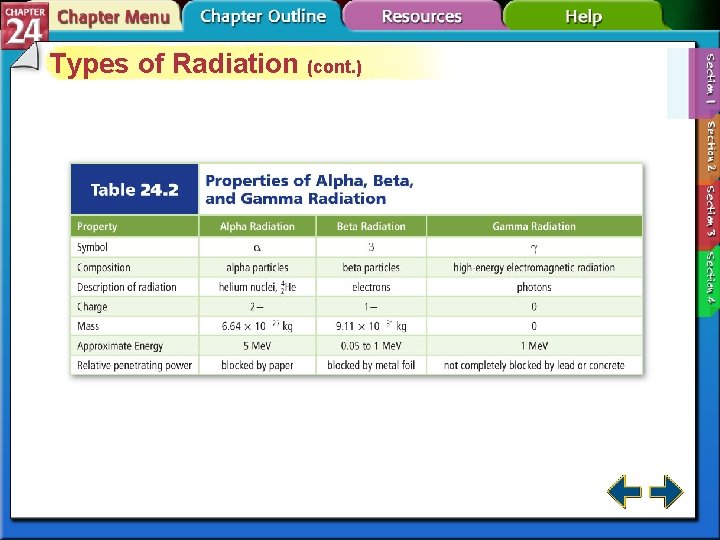
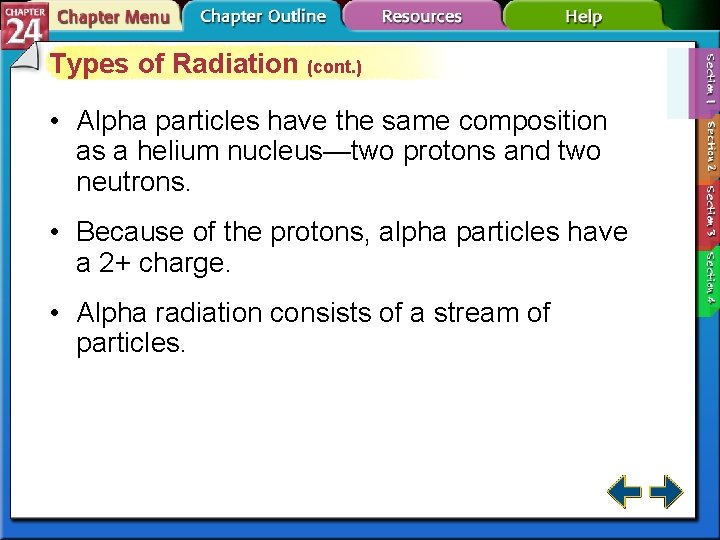
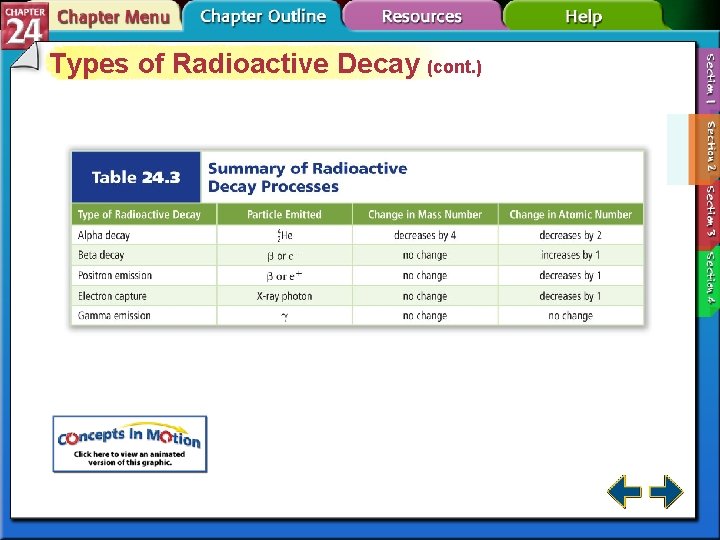
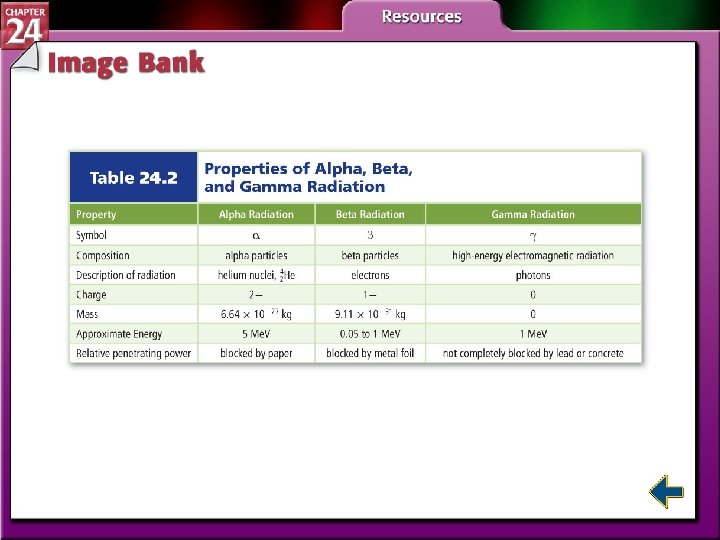
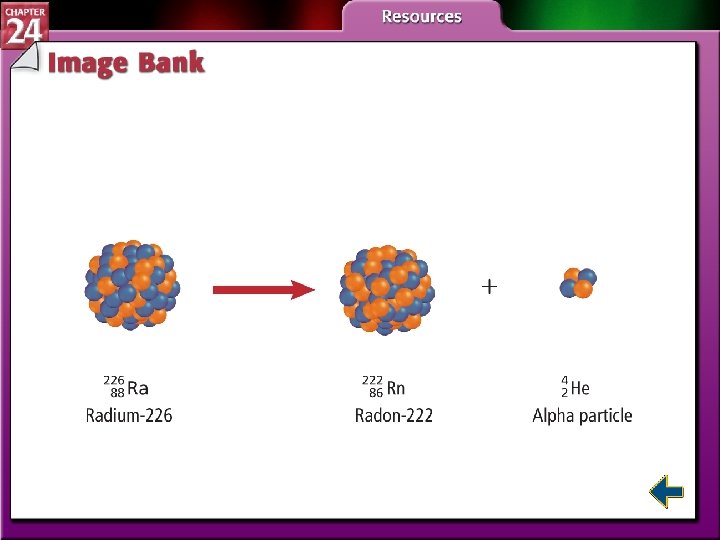
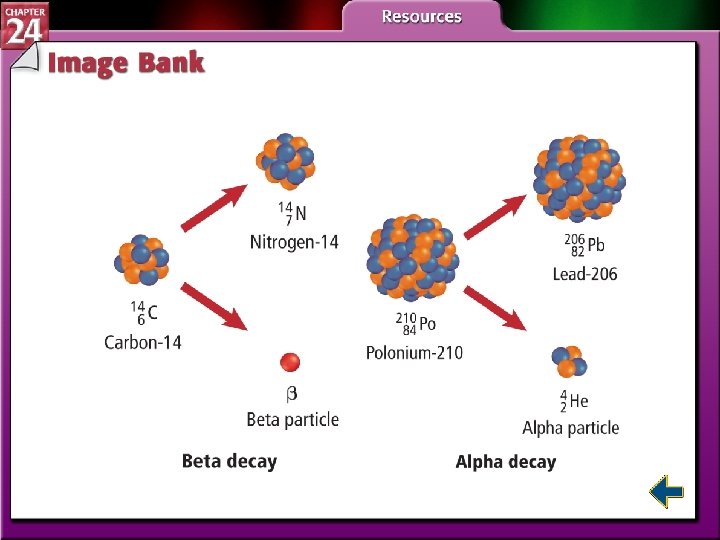
Types of Radiation (cont. ) • Alpha particles have the same composition as a helium nucleus—two protons and two neutrons. • Because of the protons, alpha particles have a 2+ charge. • Alpha radiation consists of a stream of particles.

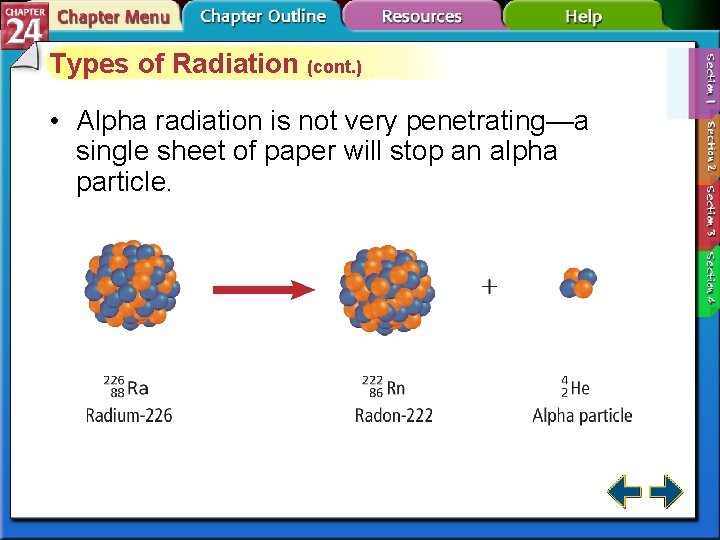
Types of Radiation (cont. ) • Alpha radiation is not very penetrating—a single sheet of paper will stop an alpha particle.

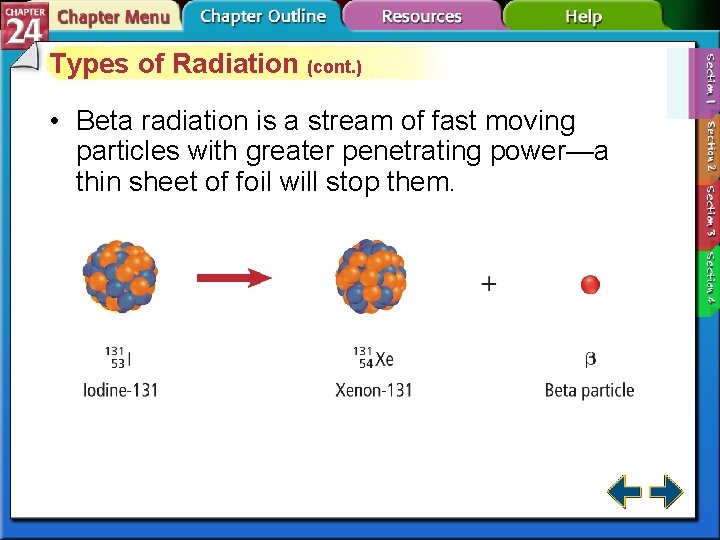
Types of Radiation (cont. ) • Beta particles are very fast-moving electrons emitted when a neutron is converted to a proton. • Beta particles have insignificant mass and a 1 – charge.

Types of Radiation (cont. ) • Beta radiation is a stream of fast moving particles with greater penetrating power—a thin sheet of foil will stop them.

Types of Radiation (cont. ) • Gamma rays are high-energy electromagnetic radiation. • Gamma rays have no mass or charge. • Gamma rays almost always accompany alpha and beta radiation. • X rays are a form of high-energy electromagnetic radiation emitted from certain materials in an excited state.

Types of Radiation (cont. ) • The ability of radiation to pass through matter is called its penetrating power. • Gamma rays are highly penetrating because they have no charge and no mass.

Section 24. 1 Assessment Why do radioisotopes emit radiation? A. to balance charges in the nucleus B. to release energy C. to attain more stable atomic configurations D. to gain energy A. B. C. D. A B C D

Section 24. 1 Assessment X rays are most similar to what type of nuclear emissions? A. gamma rays B. alpha particles C. beta particles D. delta waves A. B. C. D. A B C D


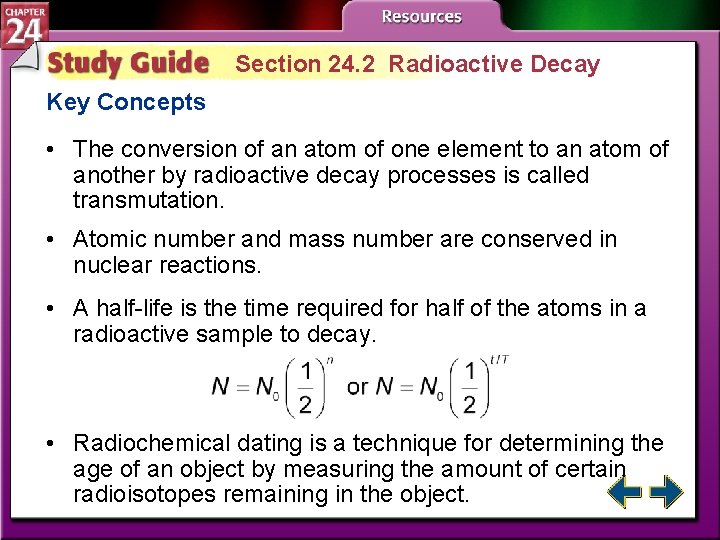
Section 24. 2 Radioactive Decay • Explain why certain nuclei are radioactive. • Apply your knowledge of radioactive decay to write balanced nuclear equations. • Solve problems involving radioactive decay rates. radioactivity: the process by which some substances spontaneously emit radiation

Section 24. 2 Radioactive Decay (cont. ) transmutation positron nucleon electron capture strong nuclear force radioactive decay series band of stability half-life positron emission radiochemical dating Unstable nuclei can break apart spontaneously, changing the identity of atoms.

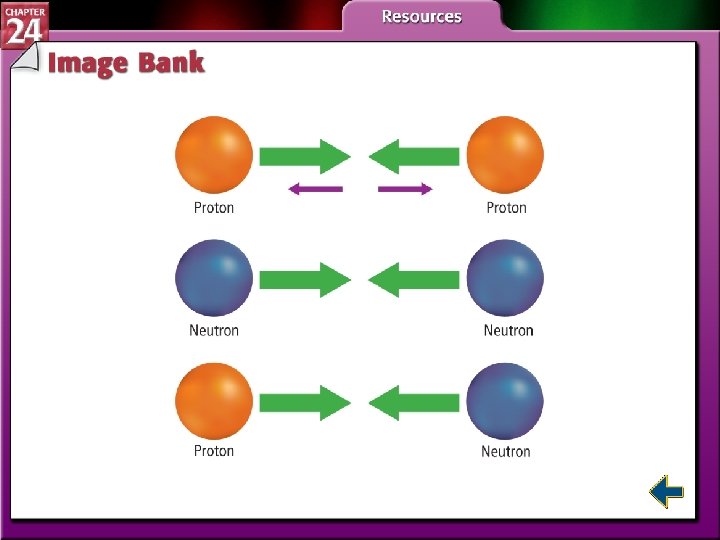
Nuclear Stability • Except for gamma radiation, radioactive decay involves transmutation, or the conversion of an element into another element. • Protons and neutrons are referred to as nucleons. • All nucleons remain in the dense nucleus because of the strong nuclear force.

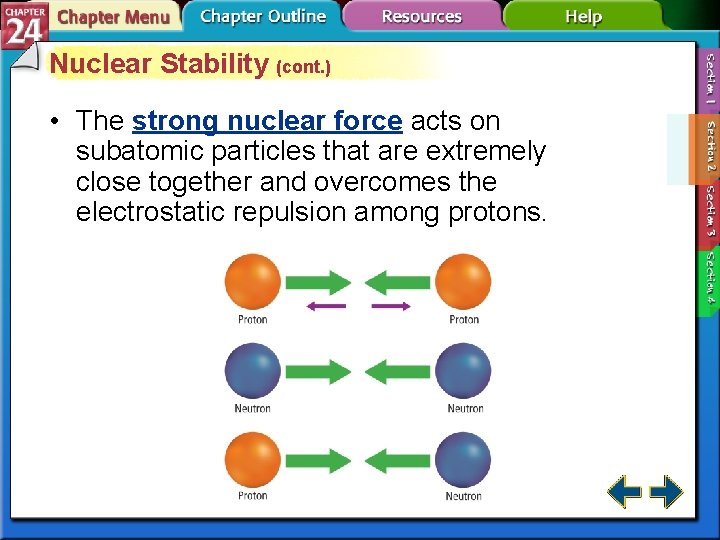
Nuclear Stability (cont. ) • The strong nuclear force acts on subatomic particles that are extremely close together and overcomes the electrostatic repulsion among protons.

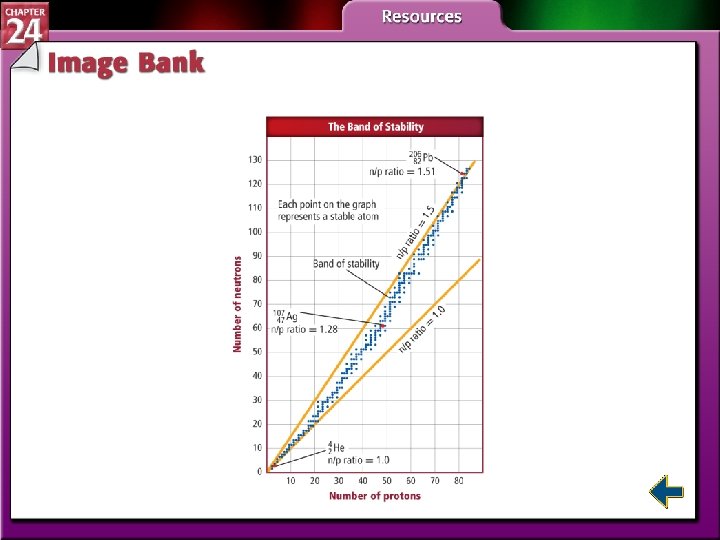
Nuclear Stability (cont. ) • As atomic number increases, more and more neutrons are needed to produce a strong nuclear force that is sufficient to balance the electrostatic repulsion between protons. • Neutron to proton ratio increases gradually to about 1. 5: 1.

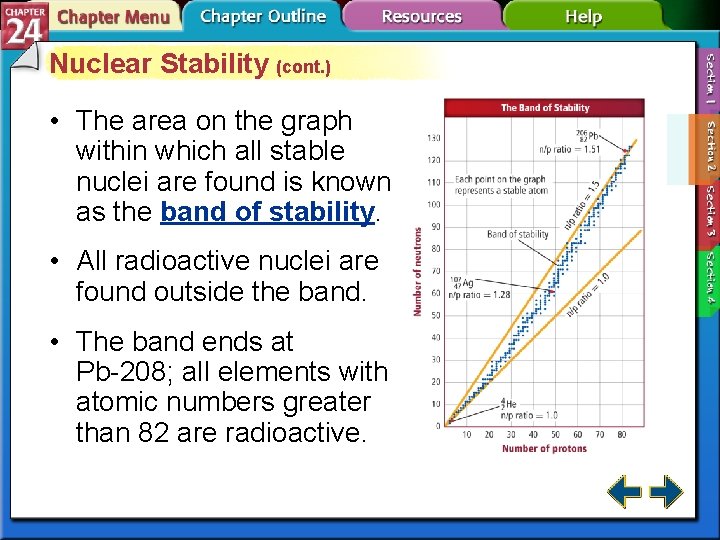
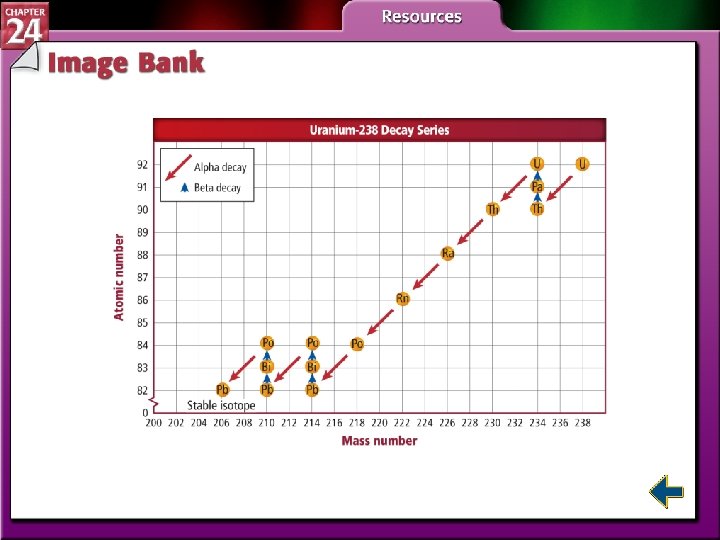
Nuclear Stability (cont. ) • The area on the graph within which all stable nuclei are found is known as the band of stability. • All radioactive nuclei are found outside the band. • The band ends at Pb-208; all elements with atomic numbers greater than 82 are radioactive.

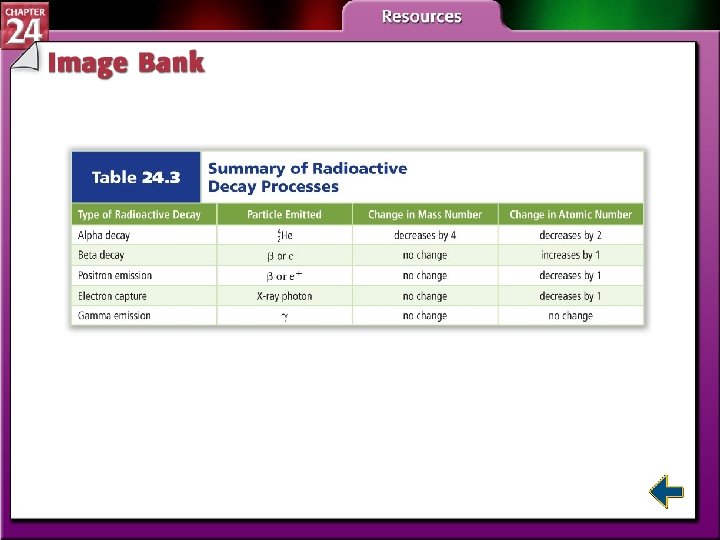
Types of Radioactive Decay • Atoms can undergo different types of decay —beta decay, alpha decay, positron emission, or electron captures—to gain stability.

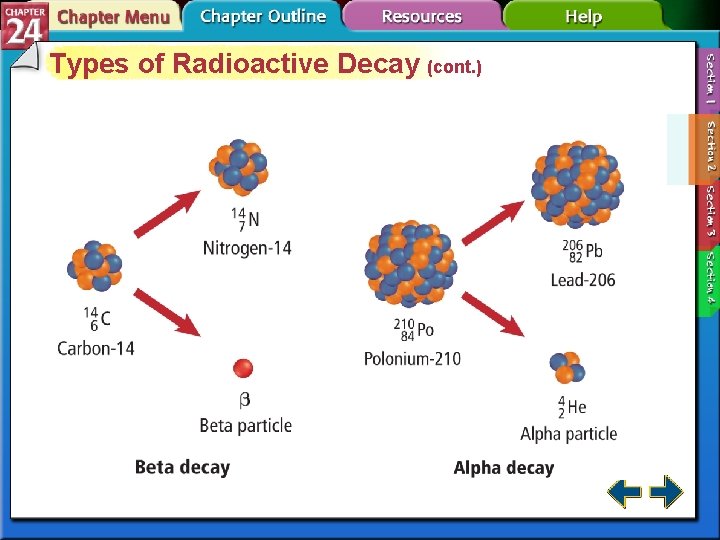
Types of Radioactive Decay (cont. ) • In beta decay, radioisotopes above the band of stability have too many neutrons to be stable. • Beta decay decreases the number of neutrons in the nucleus by converting one to a proton and emitting a beta particle.

Types of Radioactive Decay (cont. ) • In alpha decay, nuclei with more than 82 protons are radioactive and decay spontaneously. • Both neutrons and protons must be reduced. • Emitting alpha particles reduces both neutrons and protons.

Types of Radioactive Decay (cont. )

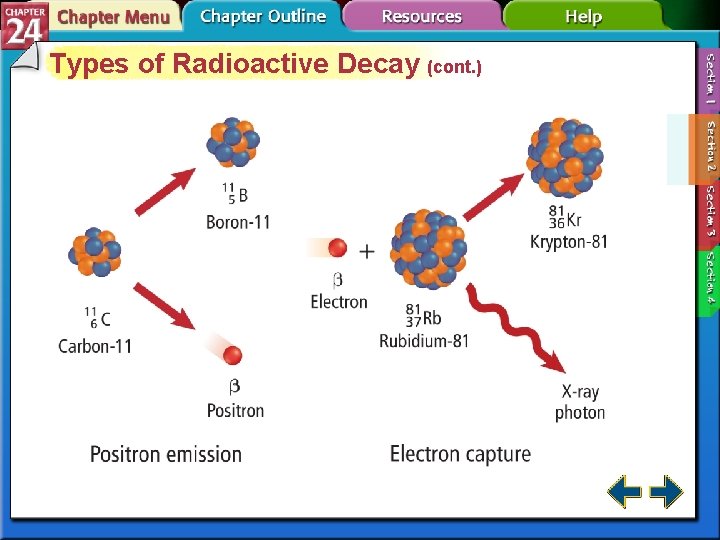
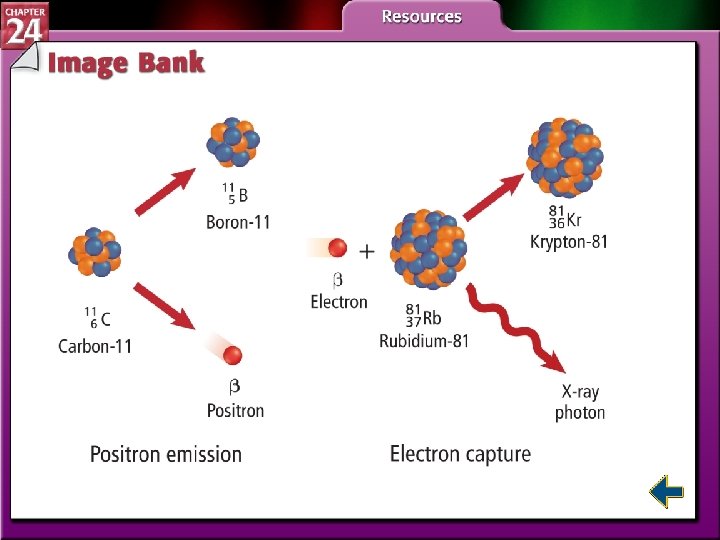
Types of Radioactive Decay (cont. ) • Nuclei with low neutron to proton ratios have two common decay processes. • Positron emission is a radioactive decay process that involves the emission of a positron from the nucleus. • A positron is a particle with the same mass as an electron but opposite charge.

Types of Radioactive Decay (cont. ) • During positron emission, a proton in the nucleus is converted to a neutron and a positron, and the positron is then emitted. • Electron capture occurs when the nucleus of an atom draws in a surrounding electron and combines with a proton to form a neutron.

Types of Radioactive Decay (cont. )

Types of Radioactive Decay (cont. )

Writing and Balancing Nuclear Equations • Nuclear reactions are expressed by balanced nuclear equations. • In balanced nuclear equations, mass numbers and charges are conserved.

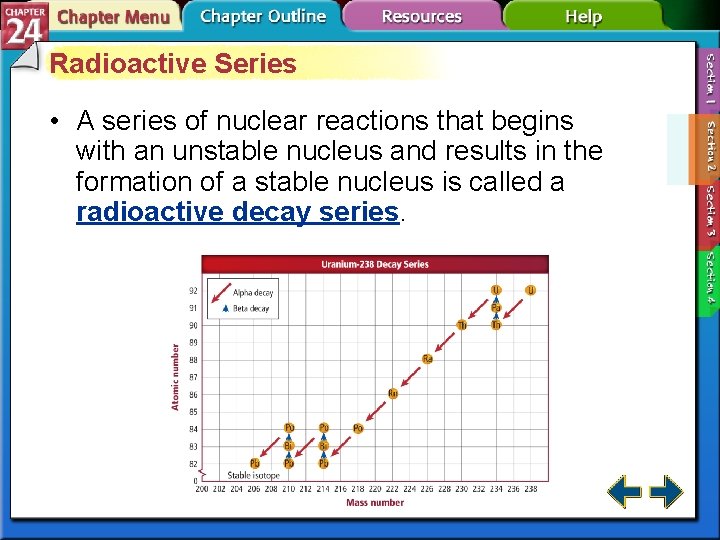
Radioactive Series • A series of nuclear reactions that begins with an unstable nucleus and results in the formation of a stable nucleus is called a radioactive decay series.

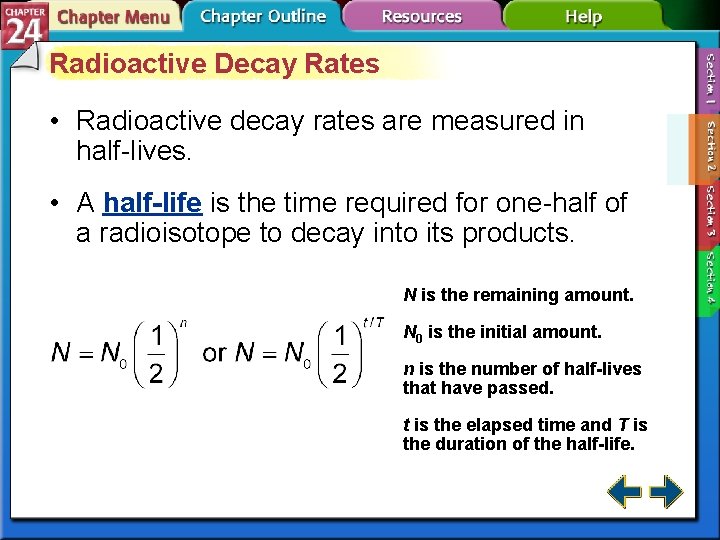
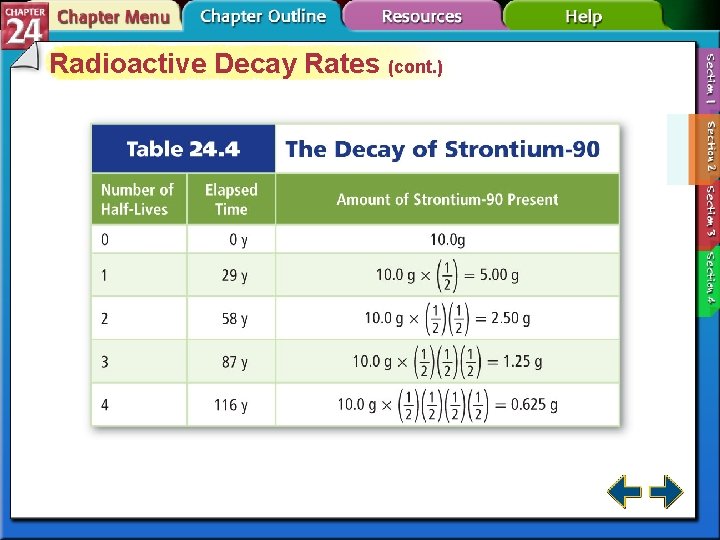
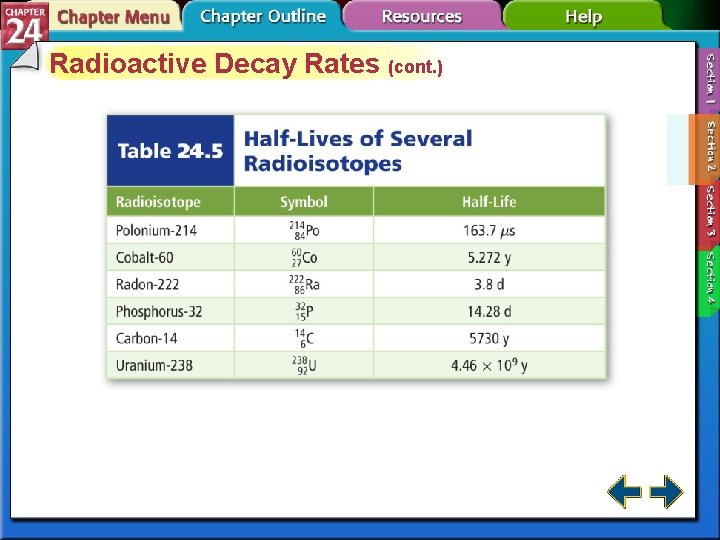
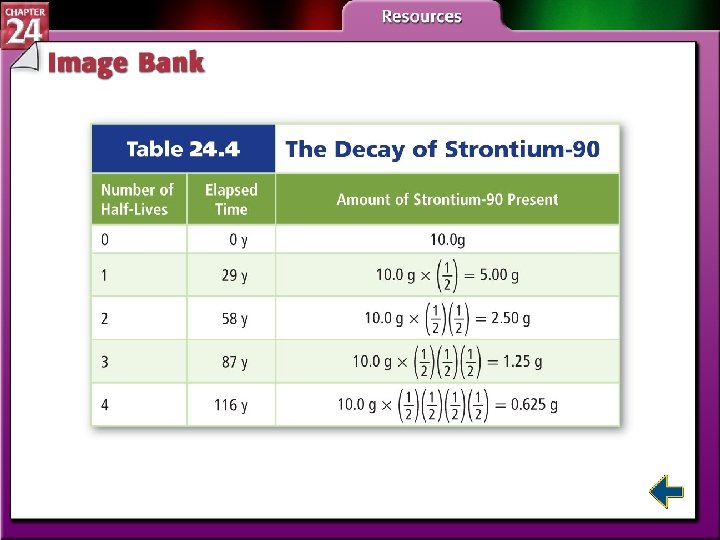
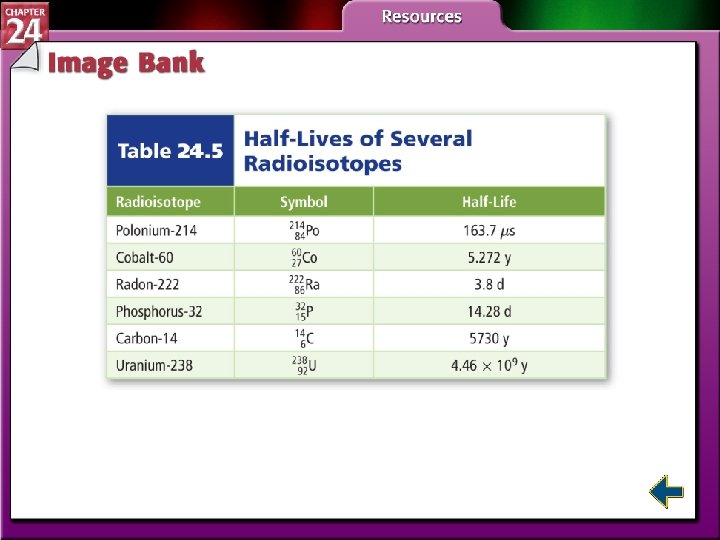
Radioactive Decay Rates • Radioactive decay rates are measured in half-lives. • A half-life is the time required for one-half of a radioisotope to decay into its products. N is the remaining amount. N 0 is the initial amount. n is the number of half-lives that have passed. t is the elapsed time and T is the duration of the half-life.

Radioactive Decay Rates (cont. )

Radioactive Decay Rates (cont. )

Radioactive Decay Rates (cont. ) • The process of determining the age of an object by measuring the amount of certain isotopes is called radiochemical dating. • Carbon-dating is used to measure the age of artifacts that were once part of a living organism.

Section 24. 2 Assessment The process of converting one element into another by radioactive decay is called ____. A. half-life B. nuclear conversion C. transmutation D. trans-decay A. B. C. D. A B C D

Section 24. 2 Assessment An unknown element has a half-life of 40 years. How much of a 20. 0 g sample will be left after 120 years? A. 0. 00 g B. 2. 50 g C. 5. 00 g D. 7. 50 g A. B. C. D. A B C D


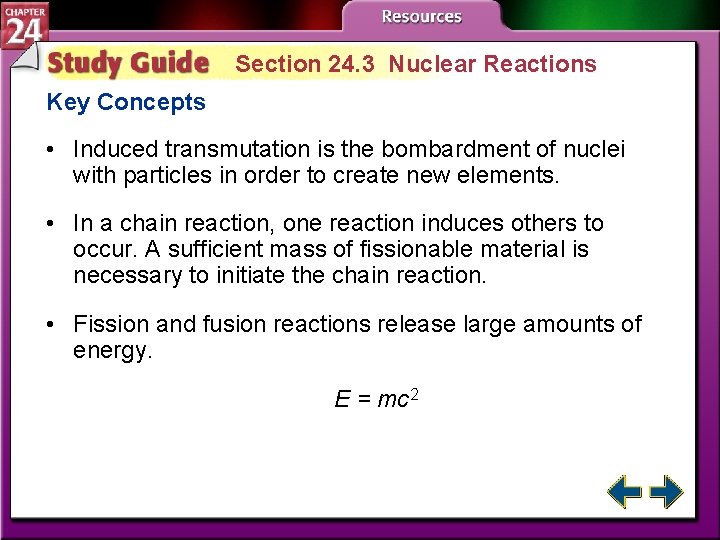
Section 24. 3 Nuclear Reactions • Understand that mass and energy are related. • Compare and contrast nuclear fission and nuclear fusion. • Explain the process by which nuclear reactors generate electricity. mass number: the number after an element’s name, representing the sum of its protons and neutrons

Section 24. 3 Nuclear Reactions (cont. ) induced transmutation critical mass transuranium element breeder reactor mass defect nuclear fusion nuclear fission thermonuclear reaction Fission, the splitting of nuclei, and fusion, the combining of nuclei, release tremendous amounts of energy.

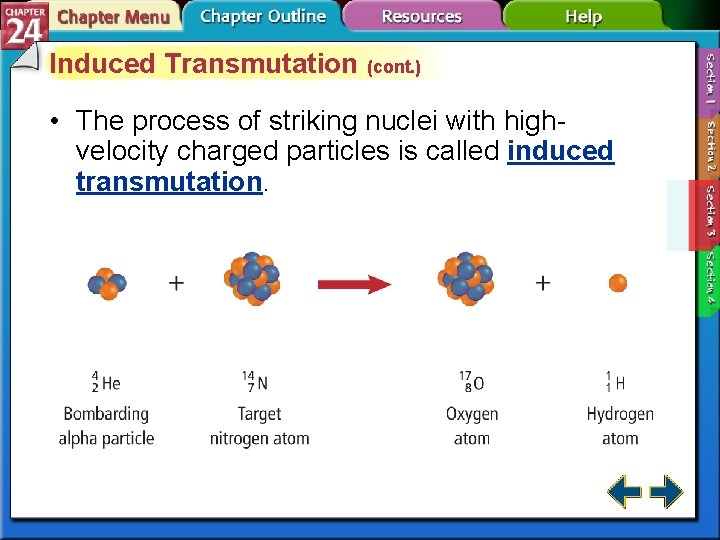
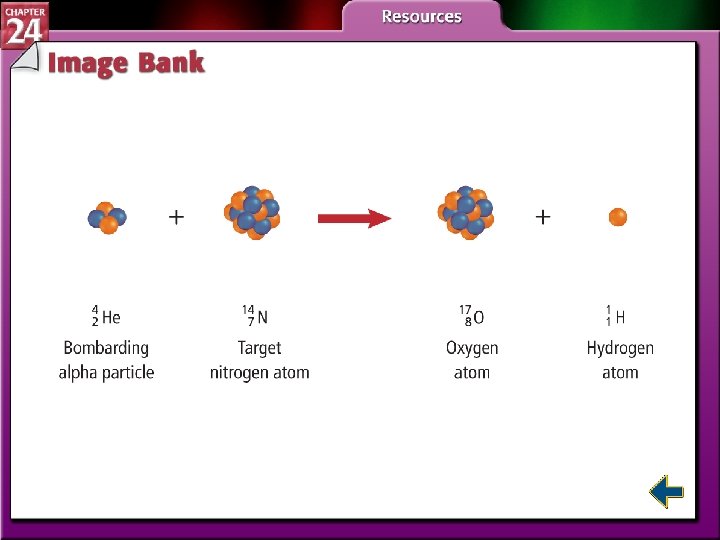
Induced Transmutation • One element can be converted into another by spontaneous emission of radiation. • Elements can also be forced to transmutate by bombarding them with high-energy alpha, beta, or gamma radiation.

Induced Transmutation (cont. ) • The process of striking nuclei with highvelocity charged particles is called induced transmutation.

Induced Transmutation (cont. ) • Particle accelerators used electrostatic and magnetic fields to accelerate charged particles to very high speed. • Transuranium elements are the elements with atomic numbers 93 and higher, immediately following uranium.

Nuclear Reactions and Energy • Mass and energy are related. • Loss or gain in mass accompanies any reaction that produces or consumes energy. • ΔE = Δmc 2 where E represents energy in Joules, m mass in kg, and c the speed of light.

Nuclear Reactions and Energy (cont. ) • Most chemical reactions produce or consume so little energy that the accompanying changes in mass are negligible. • Energy released from nuclear reactions have significant mass changes.

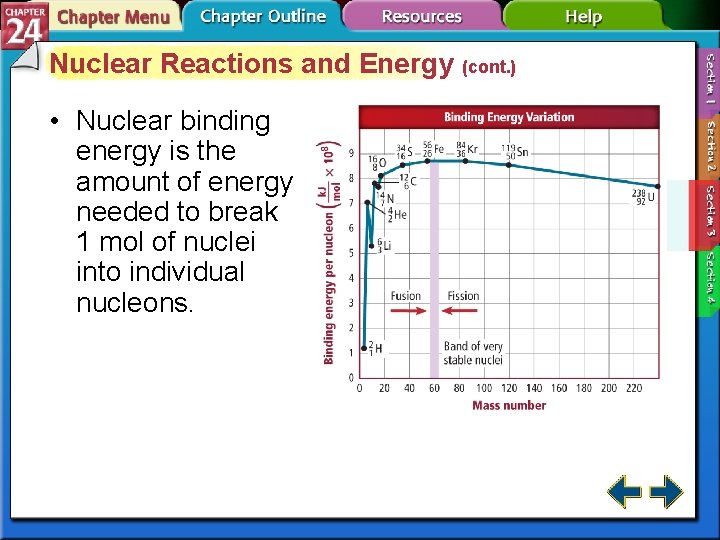
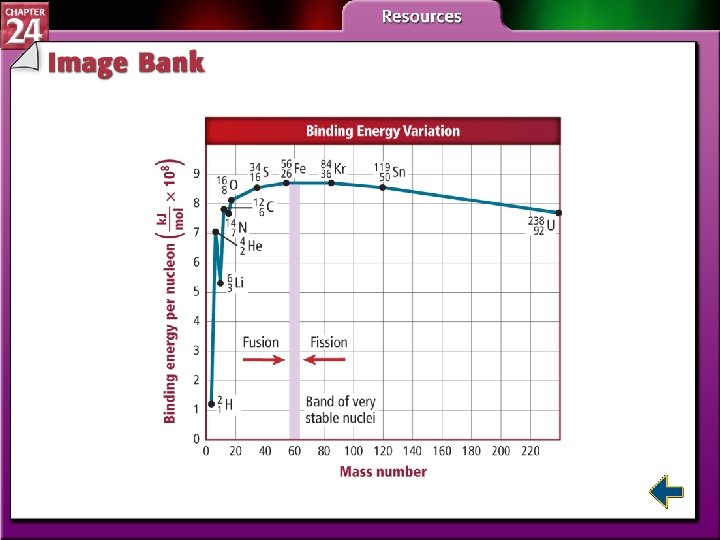
Nuclear Reactions and Energy (cont. ) • The mass of a nucleus is always less than the sum of the masses of the individual protons and neutrons that comprise it. • The difference between a nucleus and its component nucleons is called the mass defect. • Binding together or breaking an atom’s nucleons involves energy changes.

Nuclear Reactions and Energy (cont. ) • Nuclear binding energy is the amount of energy needed to break 1 mol of nuclei into individual nucleons.

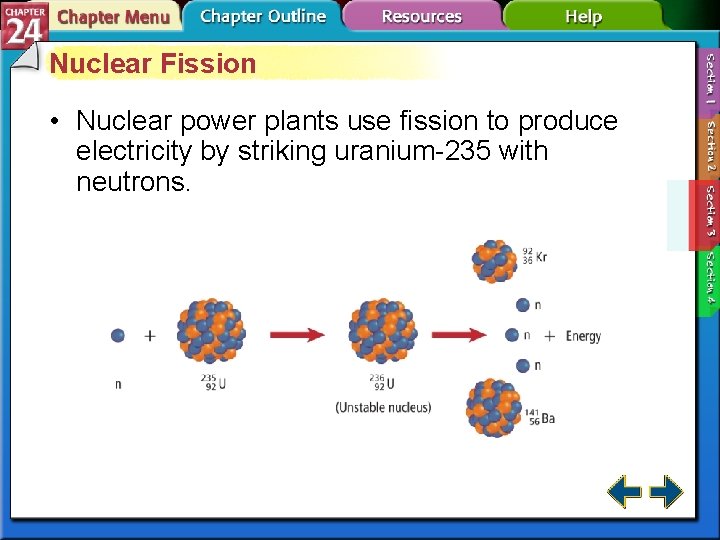
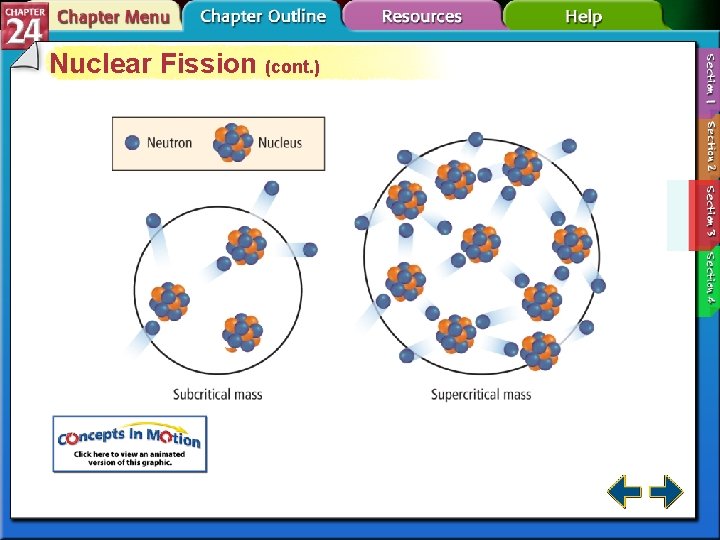
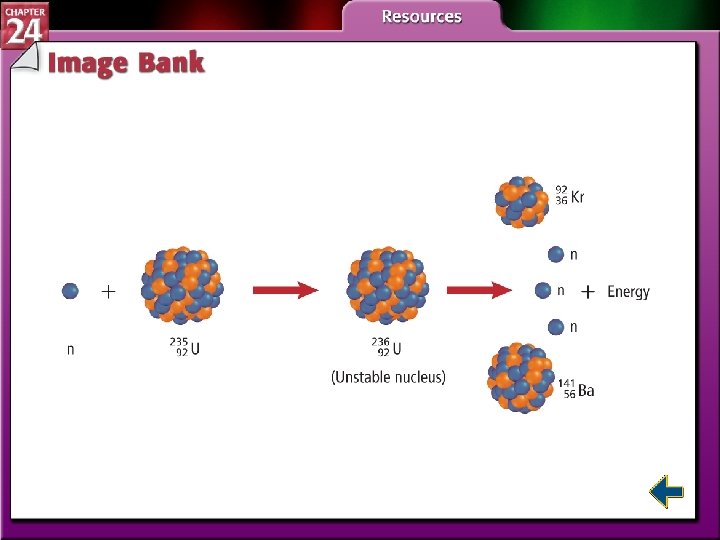
Nuclear Fission • The splitting of nuclei into fragments is known as nuclear fission. • Fission is accompanied with a very large release of energy.

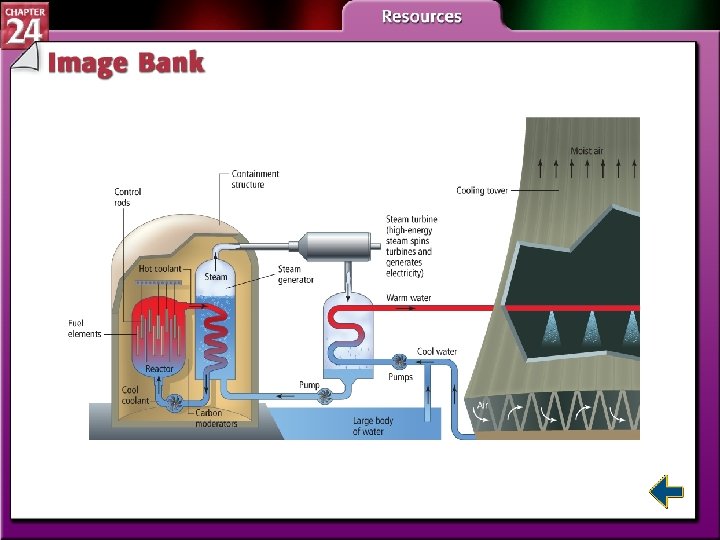
Nuclear Fission • Nuclear power plants use fission to produce electricity by striking uranium-235 with neutrons.

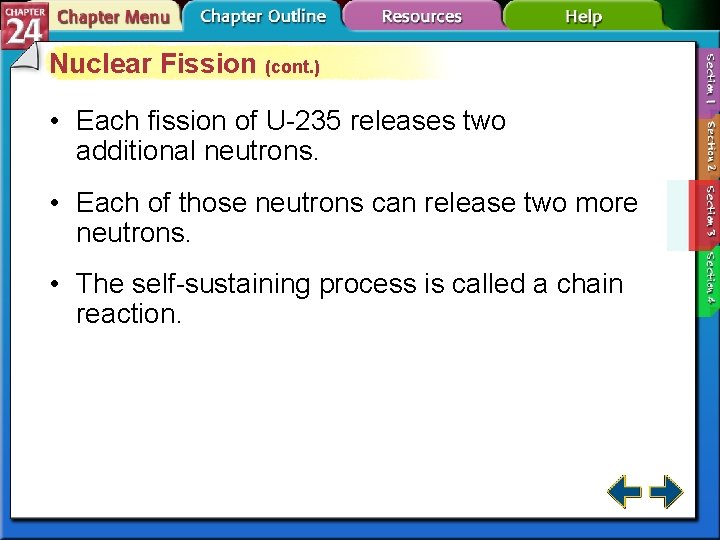
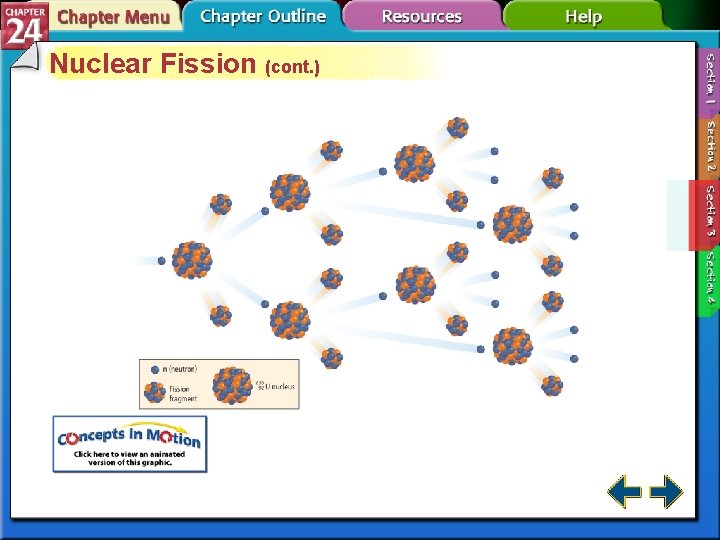
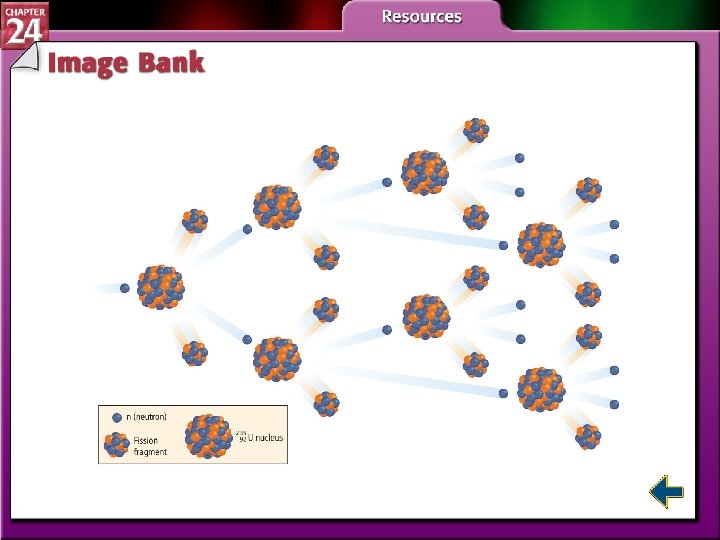
Nuclear Fission (cont. ) • Each fission of U-235 releases two additional neutrons. • Each of those neutrons can release two more neutrons. • The self-sustaining process is called a chain reaction.

Nuclear Fission (cont. )

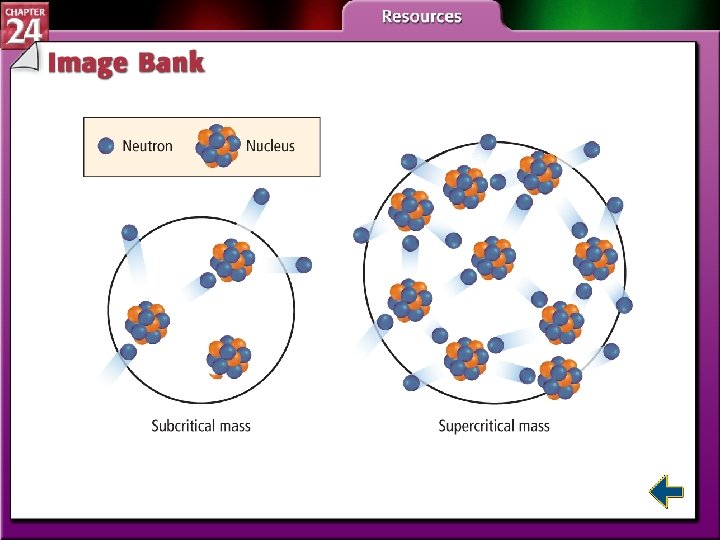
Nuclear Fission (cont. ) • Without sufficient mass, neutrons escape from the sample before starting a chain reaction. • Samples with enough mass to sustain a chain reaction are said to have critical mass.

Nuclear Fission (cont. )

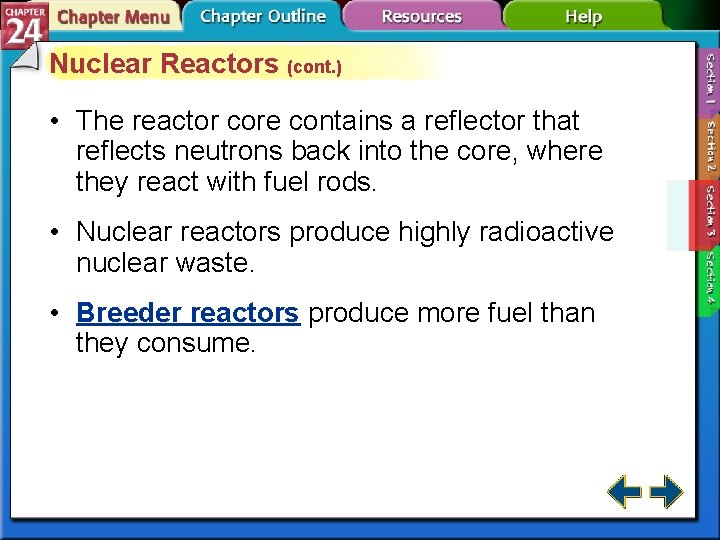
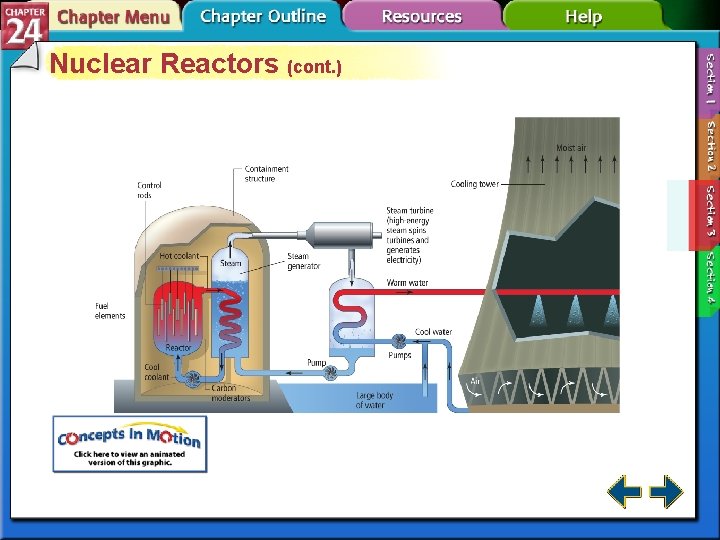
Nuclear Reactors • Nuclear fission produces the energy generated by nuclear reactors. • The fission within a reactor is started by a neutron-emitting source and is stopped by positioning the control rods to absorb virtually all of the neutrons produced in the reaction.

Nuclear Reactors (cont. ) • The reactor core contains a reflector that reflects neutrons back into the core, where they react with fuel rods. • Nuclear reactors produce highly radioactive nuclear waste. • Breeder reactors produce more fuel than they consume.

Nuclear Reactors (cont. )

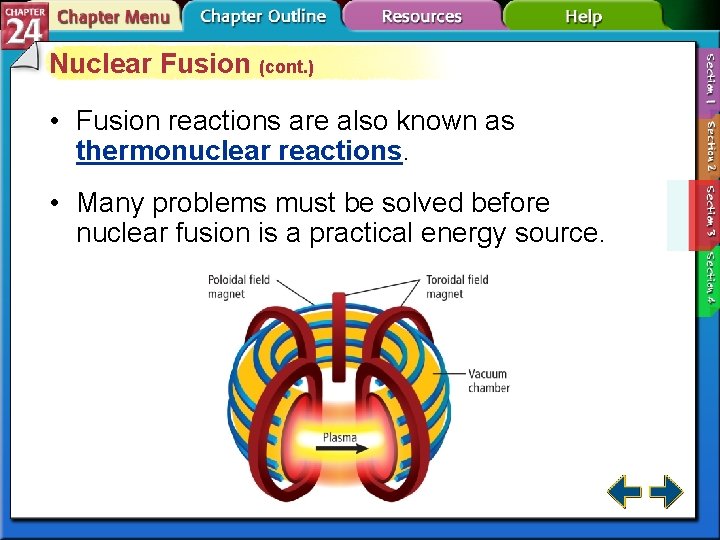
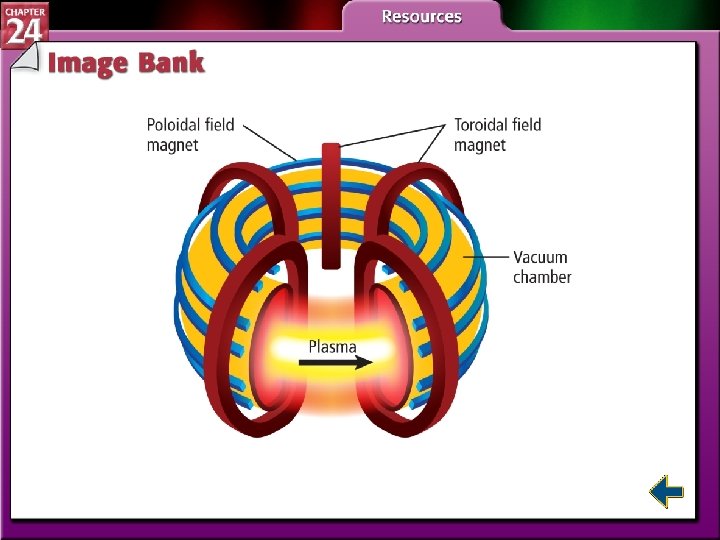
Nuclear Fusion • It is possible to bind together two or more lighter elements (mass number less than 60). • The combining of atomic nuclei is called nuclear fusion. • Nuclear fusion is capable of releasing very large amounts of energy.

Nuclear Fusion (cont. ) • Fusion has several advantages over fission. − Lightweight isotopes are abundant. − Fusion products are not radioactive. − However, fusion requires extremely high energies to initiate and sustain a reaction.

Nuclear Fusion (cont. ) • Fusion reactions are also known as thermonuclear reactions. • Many problems must be solved before nuclear fusion is a practical energy source.

Section 24. 3 Assessment Bombarding a nuclei with charged particle in order to create new elements is called ____. A. nuclear conversion B. nuclear decay C. induced decay D. induced transmutation A. B. C. D. A B C D

Section 24. 3 Assessment Thermonuclear reactions involve: A. splitting nuclei into smaller fragments B. fusing nuclei together to form larger particles C. bombarding nuclei with charged particles D. generating electricity in a nuclear reactor A. B. C. D. A B C D


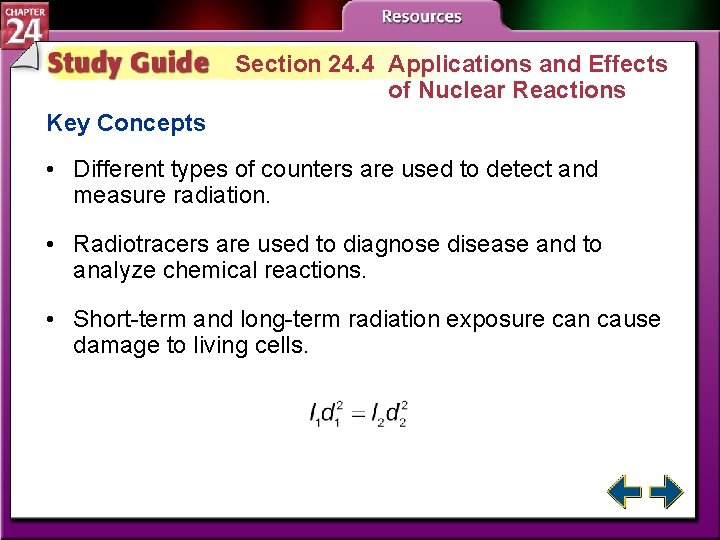
Section 24. 4 Applications and Effects of Nuclear Reactions • Describe several methods used to detect and measure radiation. • Explain an application of radiation used in the treatment of disease. • Describe some of the damaging effects of radiation on biological systems. isotope: an atom of the same element with the same number of protons but different number of neutrons

Section 24. 4 Applications and Effects of Nuclear Reactions (cont. ) ionizing radiation radiotracer Nuclear reactions have many useful applications, but they also have harmful biological effects.

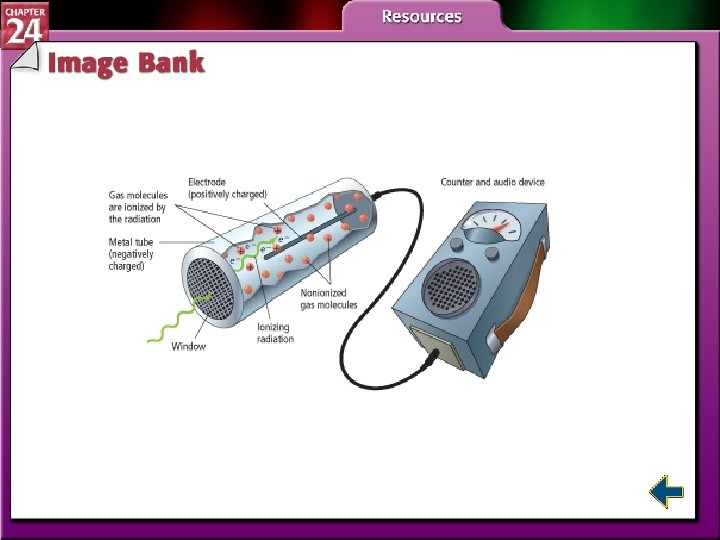
Detecting Radioactivity • Radiation with enough energy to ionize matter it collides with is called ionizing radiation. • The Geiger counter uses ionizing radiation to detect radiation.

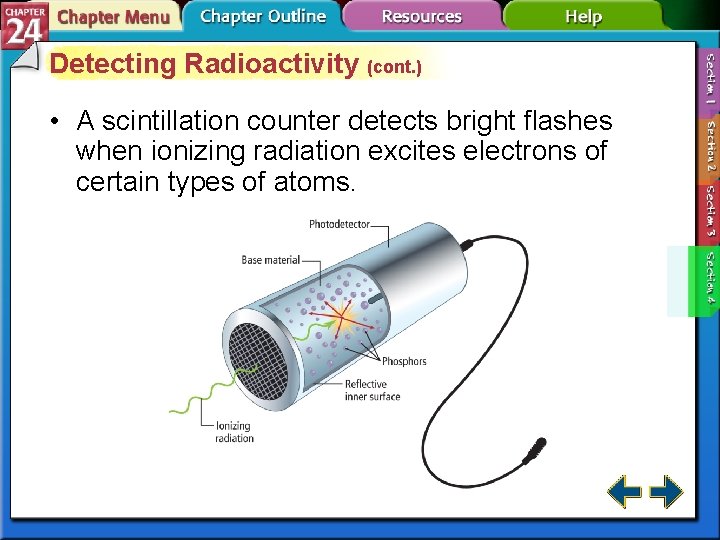
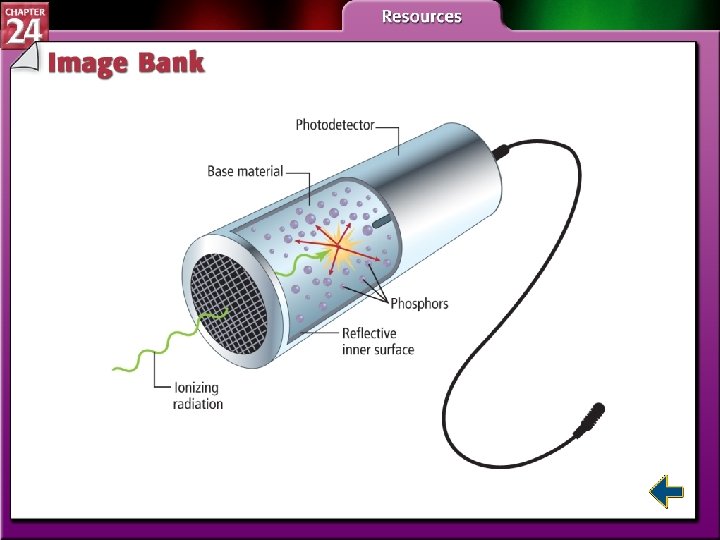
Detecting Radioactivity (cont. ) • A scintillation counter detects bright flashes when ionizing radiation excites electrons of certain types of atoms.

Uses of Radiation • When used safely, radiation can be very useful. • A radiotracer is a radioactive isotope that emits non-ionizing radiation and is used to signal the presence of an element or specific substrate.

Uses of Radiation (cont. ) • Radiation can damage or destroy healthy cells. • Radiation can also destroy unhealthy cells, such as cancer cells. • Unfortunately, radiation therapy also destroys healthy cells in the process of destroying cancerous cells.

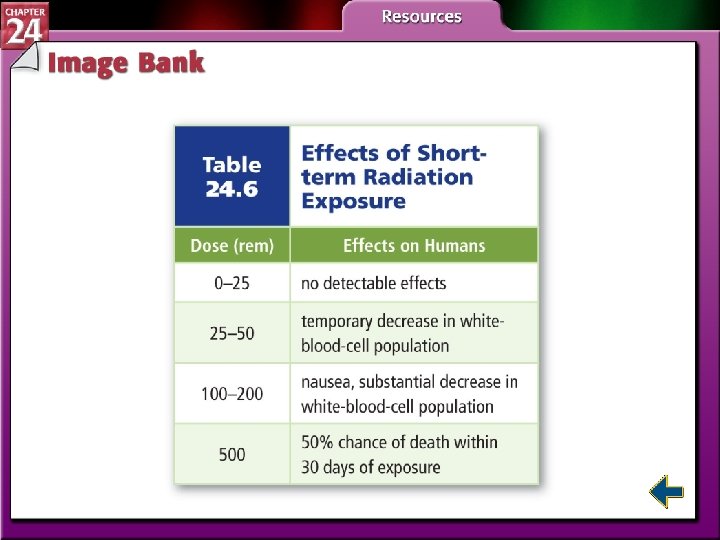
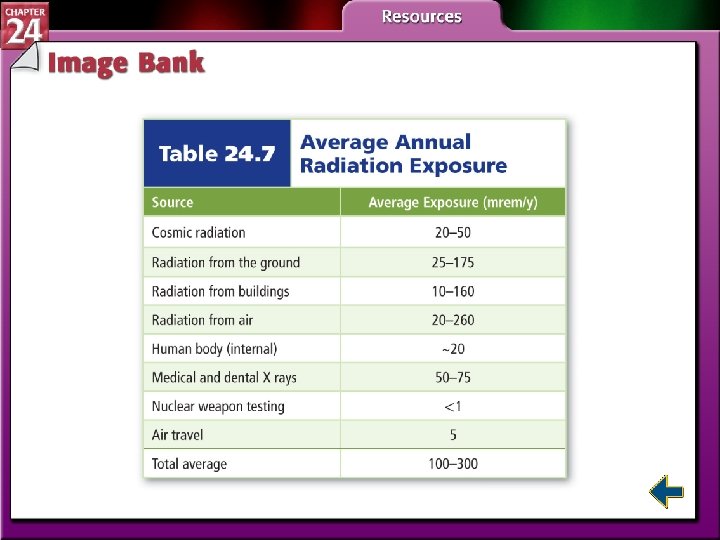
Biological Effects of Radiation • Radiation can be very harmful. • The damage depends on type of radiation, type of tissue, penetrating power, and distance from the source.

Biological Effects of Radiation (cont. ) • High energy radiation is dangerous because it produces free radicals. • Free radicals are atoms or molecules that contain one or more unpaired electrons. • Free radicals are highly reactive.

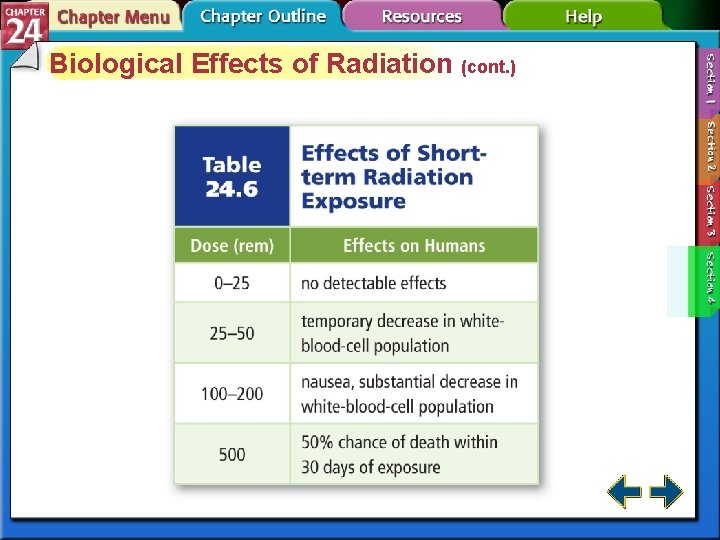
Biological Effects of Radiation (cont. ) • Two units measure doses of radiation. • The rad stands for Radiation-Absorbed Dose, which is the amount of radiation that results in 0. 01 J of energy per kilogram of tissue. • The rad does not account for the type of tissue that is absorbing the radiation. • The rad is multiplied by a factor related to its effect on the tissue involved and is called the rem, Roentgen Equivalent for Man.

Biological Effects of Radiation (cont. )

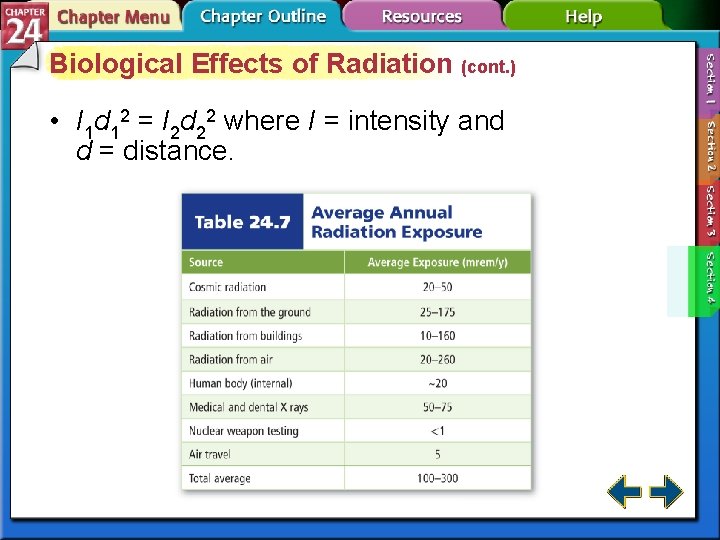
Biological Effects of Radiation (cont. ) • I 1 d 12 = I 2 d 22 where I = intensity and d = distance.

Section 24. 4 Assessment What is a radioisotope that emits nonionizing radiation and is used to signal the presence of certain elements called? A. rad B. rem C. radiotracer D. free radical A. B. C. D. A B C D

Section 24. 4 Assessment Radiation with enough energy to cause tissue damage by ionizing the particles it collides with is called ____. A. alpha decay B. beta decay C. gamma radiation D. ionizing radiation A. B. C. D. A B C D


Chemistry Online Study Guide Chapter Assessment Standardized Test Practice Image Bank Concepts in Motion

Section 24. 1 Nuclear Radiation Key Concepts • Wilhelm Roentgen discovered X rays in 1895. • Henri Becquerel, Marie Curie, and Pierre Curie pioneered the fields of radioactivity and nuclear chemistry. • Radioisotopes emit radiation to attain more-stable atomic configurations.

Section 24. 2 Radioactive Decay Key Concepts • The conversion of an atom of one element to an atom of another by radioactive decay processes is called transmutation. • Atomic number and mass number are conserved in nuclear reactions. • A half-life is the time required for half of the atoms in a radioactive sample to decay. • Radiochemical dating is a technique for determining the age of an object by measuring the amount of certain radioisotopes remaining in the object.

Section 24. 3 Nuclear Reactions Key Concepts • Induced transmutation is the bombardment of nuclei with particles in order to create new elements. • In a chain reaction, one reaction induces others to occur. A sufficient mass of fissionable material is necessary to initiate the chain reaction. • Fission and fusion reactions release large amounts of energy. E = mc 2

Section 24. 4 Applications and Effects of Nuclear Reactions Key Concepts • Different types of counters are used to detect and measure radiation. • Radiotracers are used to diagnose disease and to analyze chemical reactions. • Short-term and long-term radiation exposure can cause damage to living cells.

The half-life of a radioisotope is: A. one-half its total life B. 2500 years C. the amount of time it takes to completely decay D. the amount of time it takes for one-half to decay A. B. C. D. A B C D

What is a positron? A. a nucleon with the same mass as a neutron and a positive charge B. a nucleon with the same mass as a proton and a negative charge C. a nucleon with the same mass as an electron and a positive charge D. a type of radioactive emission with a negative charge A. B. C. D. A B C D

What is the force that holds the protons and neutrons together in the nucleus of an atom? A. nuclear magnetic force B. strong nuclear force C. ionic bonding D. nuclear bond A. B. C. D. A B C D

During positron emission, a proton is converted to: A. a neutron and electron B. an electron and positron C. a proton and neutron D. a neutron and positron A. B. C. D. A B C D

A thermonuclear reaction is also called ____. A. nuclear fission B. nuclear fusion C. mass defect D. critical mass A. B. C. D. A B C D

Which statement is NOT true of beta particles? A. They have the same mass as an electron. B. They have a charge of 1+. C. They are less penetrating than alpha particles. D. They are represented by 0 -1β. A. B. C. D. A B C D

The site that oxidation occurs at in a battery is called ____. A. anode B. cathode C. nothode D. salt bridge A. B. C. D. A B C D

A solution of 0. 500 M HCl is used to titrate 15. 00 m. L if KOH solution. The end point of the titration is reached after 25. 00 m. L of HCl is added. What is the concentration of KOH? A. 9. 00 M B. 1. 09 M C. 0. 833 M D. 0. 015 M A. B. C. D. A B C D

The half-life of K-40 is 1. 26 × 109 years. How much of a 10. 0 g sample will be left after 200 million years? A. 8. 96 g B. 8. 03 g C. 7. 75 g D. 4. 99 g A. B. C. D. A B C D

Elements above the band of stability are radioactive and decay by ____. A. alpha decay B. beta decay C. positron emission D. electron capture A. B. C. D. A B C D

Click on an image to enlarge.
























Table 24. 3 Radioactive Decay Processes Figure 24. 16 Chain Reactions Figure 24. 17 Critical Mass Figure 24. 20 Nuclear Power Plants

Click any of the background top tabs to display the respective folder. Within the Chapter Outline, clicking a section tab on the right side of the screen will bring you to the first slide in each respective section. Simple navigation buttons will allow you to progress to the next slide or the previous slide. The Chapter Resources Menu will allow you to access chapter specific resources from the Chapter Menu or any Chapter Outline slide. From within any feature, click the Resources tab to return to this slide. The “Return” button will allow you to return to the slide that you were viewing when you clicked either the Resources or Help tab. To exit the presentation, click the Exit button on the Chapter Menu slide or hit Escape [Esc] on your keyboards while viewing any Chapter Outline slide.

This slide is intentionally blank.