Nuclear Chemistry Radioactivity One of the pieces of


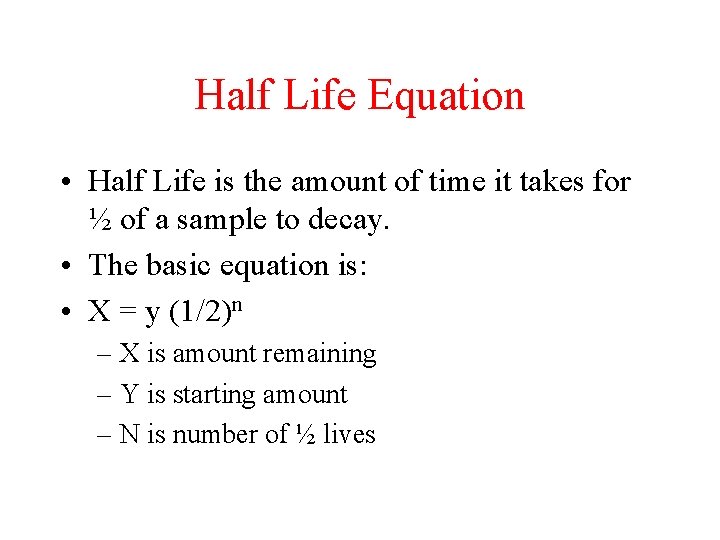





- Slides: 51

Nuclear Chemistry

Radioactivity • One of the pieces of evidence for the fact that atoms are made of smaller particles came from the work of Marie Curie (1876 -1934). • She discovered radioactivity, the spontaneous disintegration of some elements into smaller pieces.

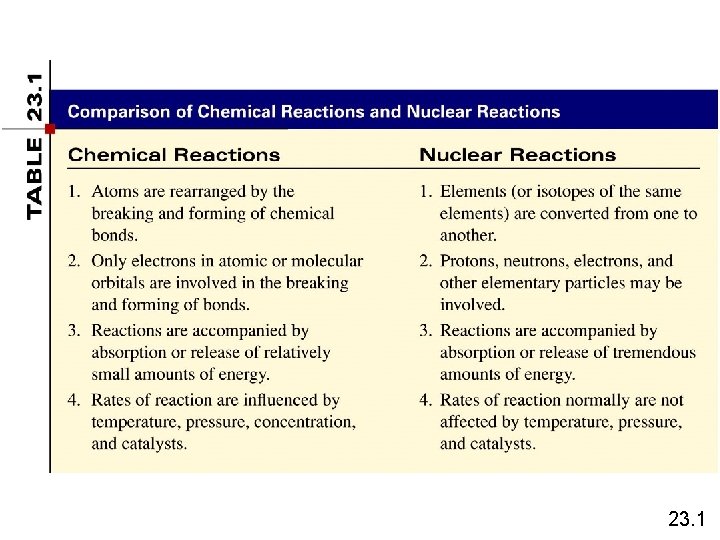
Nuclear Reactions vs. Normal Chemical Changes • Nuclear reactions involve the nucleus • The nucleus opens, and protons and neutrons are rearranged • The opening of the nucleus releases a tremendous amount of energy that holds the nucleus together – called binding energy • “Normal” Chemical Reactions involve electrons, not protons and neutrons

23. 1

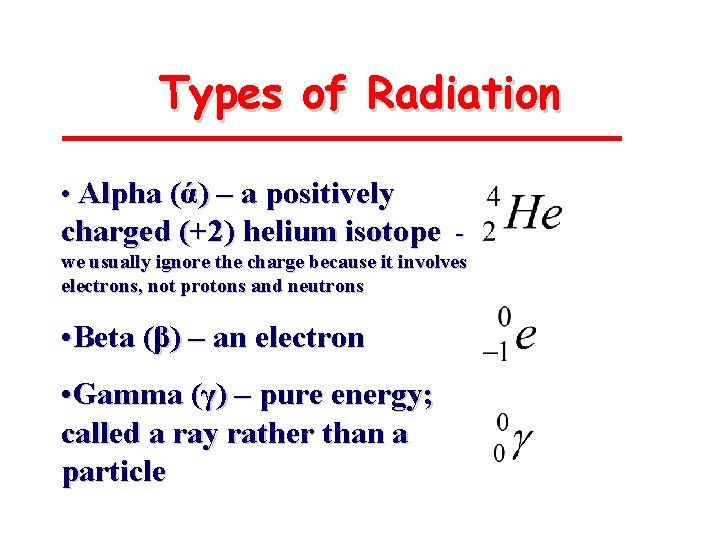
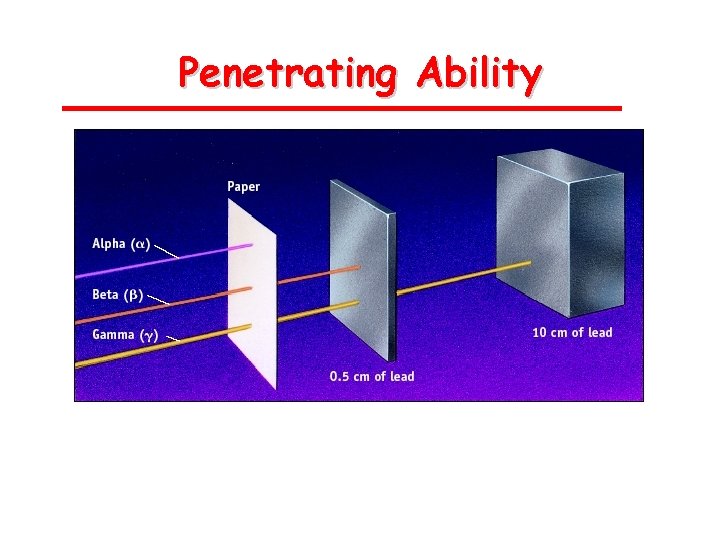
Types of Radiation • Alpha (ά) – a positively charged (+2) helium isotope we usually ignore the charge because it involves electrons, not protons and neutrons • Beta (β) – an electron • Gamma (γ) – pure energy; called a ray rather than a particle

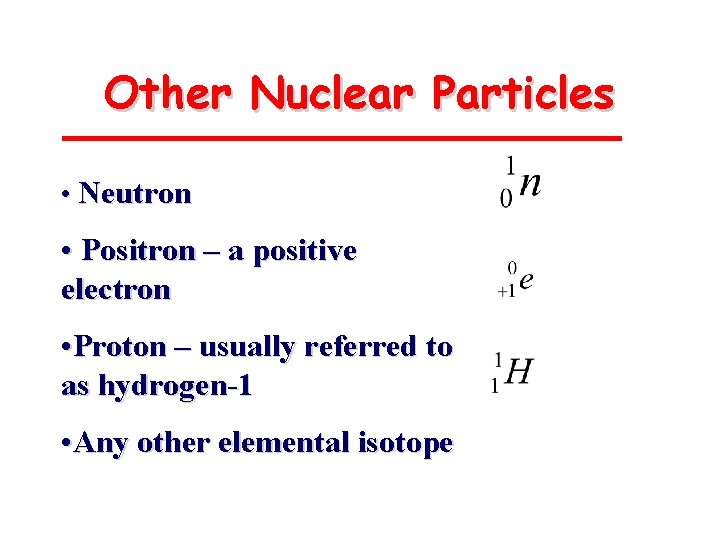
Other Nuclear Particles • Neutron • Positron – a positive electron • Proton – usually referred to as hydrogen-1 • Any other elemental isotope

Penetrating Ability

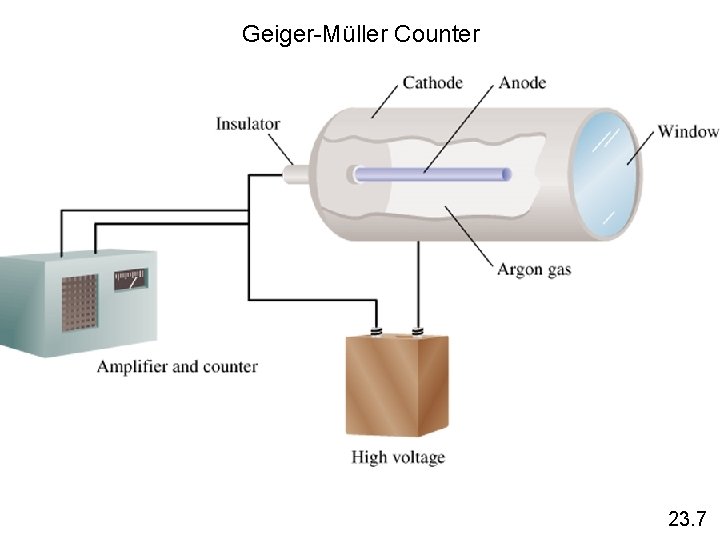
Geiger-Müller Counter 23. 7

Geiger Counter • Used to detect radioactive substances

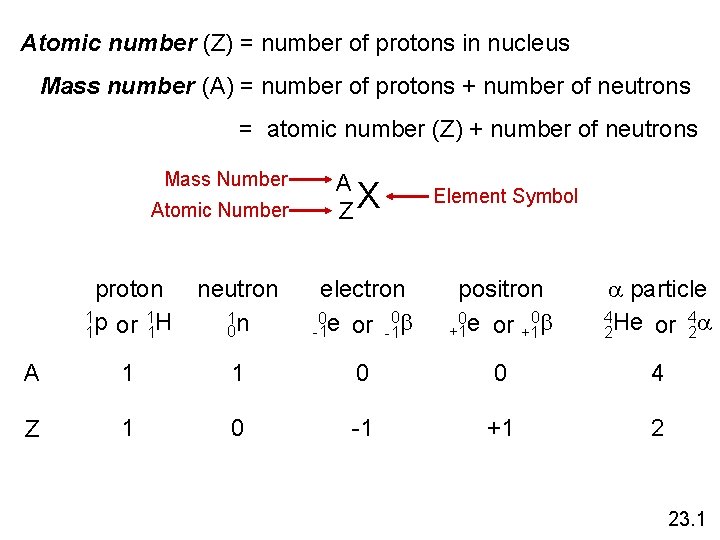
Atomic number (Z) = number of protons in nucleus Mass number (A) = number of protons + number of neutrons = atomic number (Z) + number of neutrons Mass Number Atomic Number A ZX Element Symbol proton 1 p 1 H or 1 1 neutron 1 n 0 electron 0 b 0 e or -1 -1 positron 0 b 0 e or +1 +1 a particle 4 He 4 a or 2 2 A 1 1 0 0 4 Z 1 0 -1 +1 2 23. 1

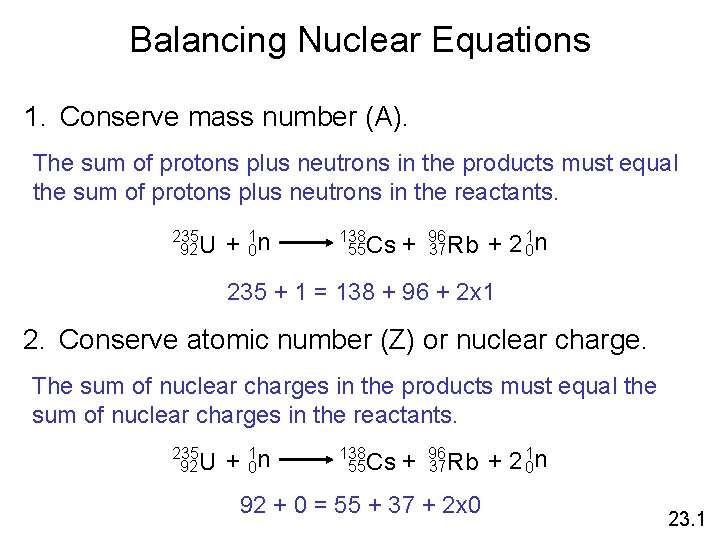
Balancing Nuclear Equations 1. Conserve mass number (A). The sum of protons plus neutrons in the products must equal the sum of protons plus neutrons in the reactants. 235 92 U + 10 n 138 55 Cs + 96 37 Rb + 2 10 n 235 + 1 = 138 + 96 + 2 x 1 2. Conserve atomic number (Z) or nuclear charge. The sum of nuclear charges in the products must equal the sum of nuclear charges in the reactants. 235 92 U + 10 n 138 55 Cs + 96 37 Rb 92 + 0 = 55 + 37 + 2 x 0 + 2 10 n 23. 1

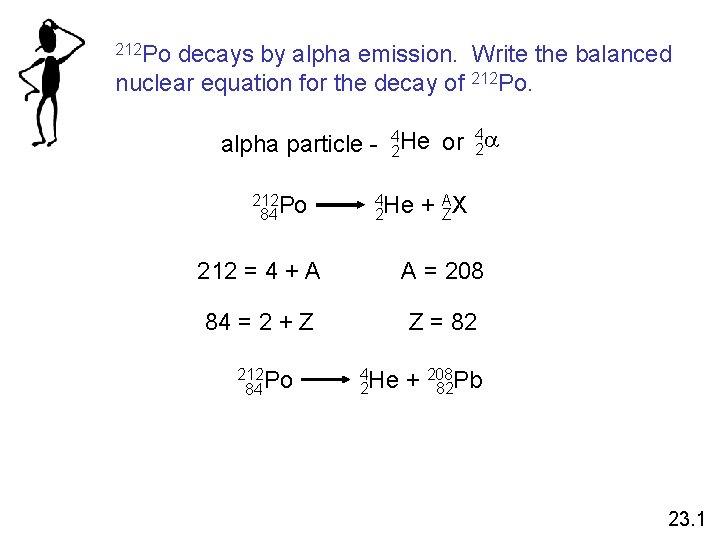
212 Po decays by alpha emission. Write the balanced nuclear equation for the decay of 212 Po. 4 alpha particle - 42 He or 2 a 212 Po 84 4 He 2 + AZX 212 = 4 + A A = 208 84 = 2 + Z Z = 82 212 Po 84 4 He 2 + 208 82 Pb 23. 1

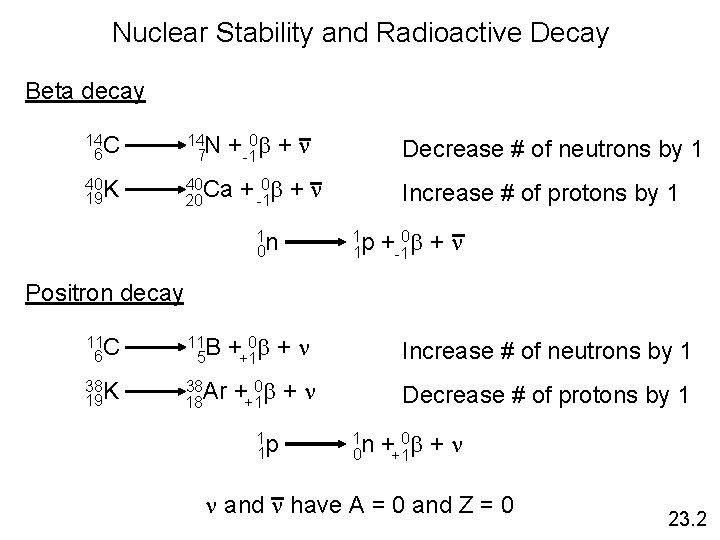
Nuclear Stability and Radioactive Decay Beta decay +-10 b + n 14 C 6 14 N 7 40 K 19 40 Ca 20 Decrease # of neutrons by 1 + -10 b + n 1 n 0 Increase # of protons by 1 1 p 1 +-10 b + n Positron decay 11 C 6 11 B 5 38 19 K 38 Ar 18 ++10 b + n Increase # of neutrons by 1 ++10 b + n Decrease # of protons by 1 1 p 1 1 n 0 ++10 b + n n and n have A = 0 and Z = 0 23. 2

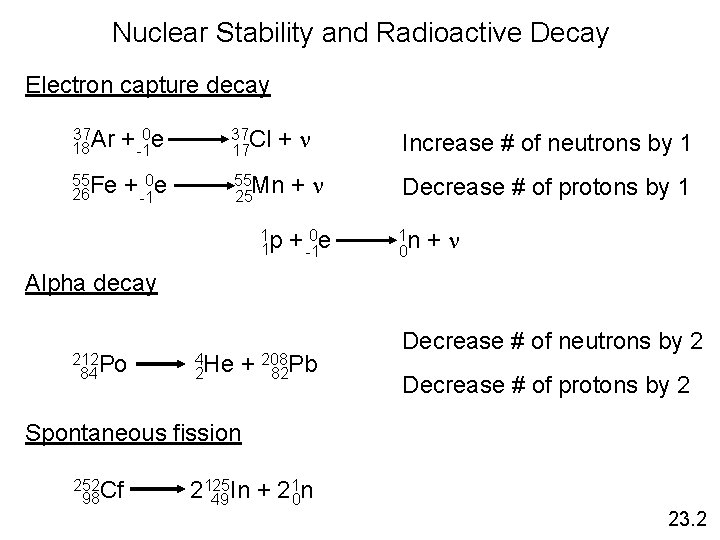
Nuclear Stability and Radioactive Decay Electron capture decay +n 37 Ar 18 + -10 e 37 Cl 17 55 Fe 26 + -10 e 55 Mn 25 1 p 1 Increase # of neutrons by 1 +n Decrease # of protons by 1 + -10 e 1 n 0 +n Alpha decay 212 Po 84 4 He 2 + 208 82 Pb Decrease # of neutrons by 2 Decrease # of protons by 2 Spontaneous fission 252 Cf 98 1 n 2125 In + 2 49 0 23. 2

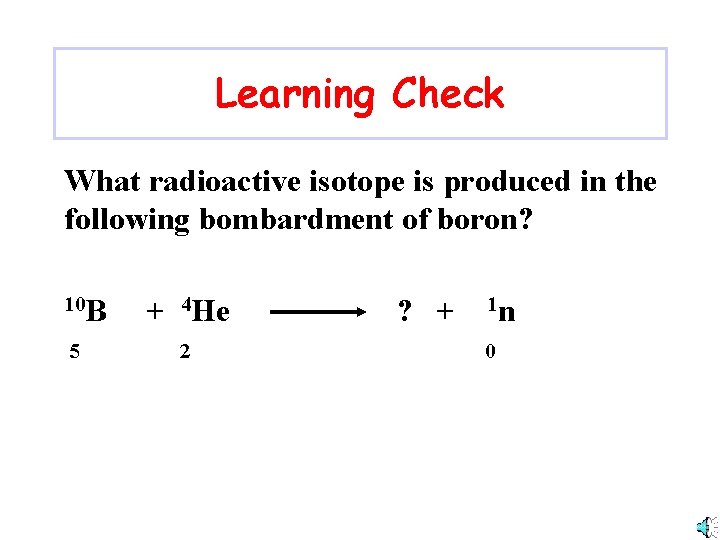
Learning Check What radioactive isotope is produced in the following bombardment of boron? 10 B 5 + 4 He 2 ? + 1 n 0

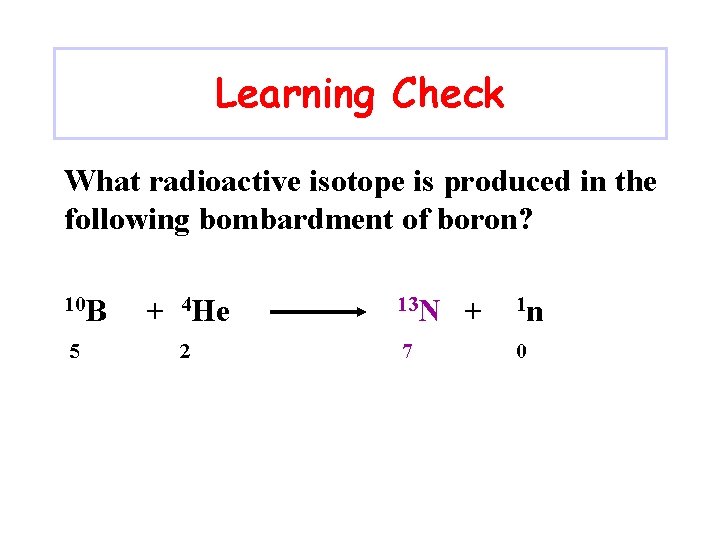
Learning Check What radioactive isotope is produced in the following bombardment of boron? 10 B 5 + 4 He 2 13 N 7 + 1 n 0

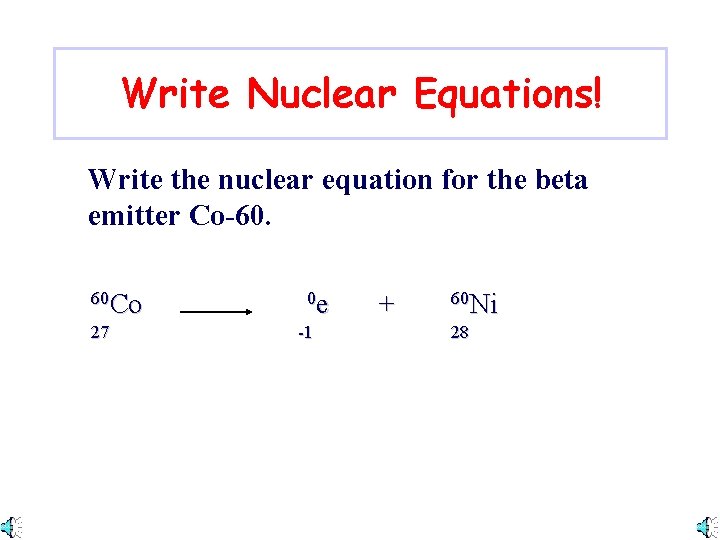
Write Nuclear Equations! Write the nuclear equation for the beta emitter Co-60. 60 Co 27 0 e -1 + 60 Ni 28

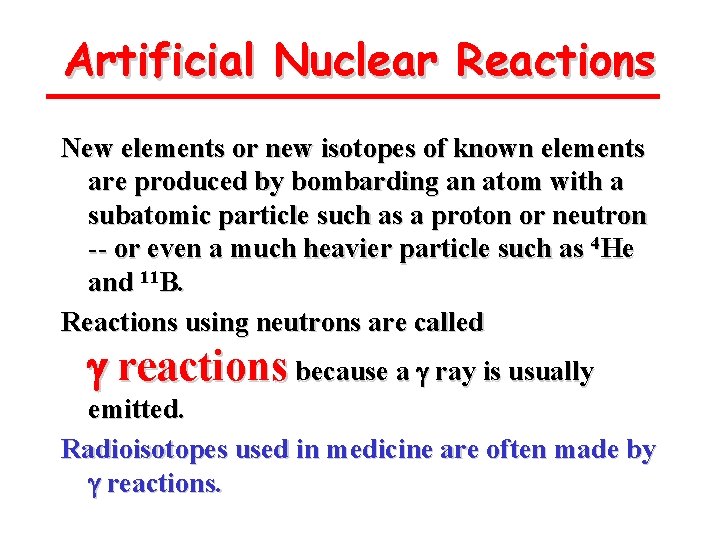
Artificial Nuclear Reactions New elements or new isotopes of known elements are produced by bombarding an atom with a subatomic particle such as a proton or neutron -- or even a much heavier particle such as 4 He and 11 B. Reactions using neutrons are called g reactions because a g ray is usually emitted. Radioisotopes used in medicine are often made by g reactions.

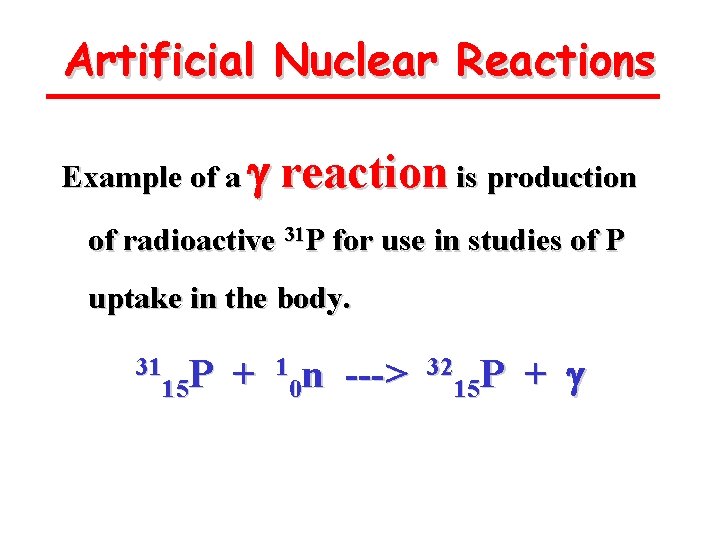
Artificial Nuclear Reactions Example of a g reaction is production of radioactive 31 P for use in studies of P uptake in the body. 31 P 15 + 1 n 0 ---> 32 P 15 + g

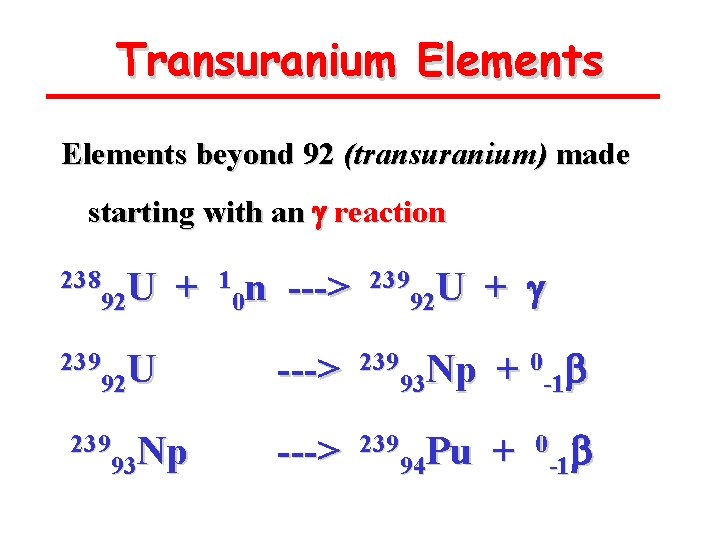
Transuranium Elements beyond 92 (transuranium) made starting with an g reaction 238 U 92 + 239 U 92 239 Np 93 1 n 0 ---> 239 U 92 + g ---> 239 Np 93 + 0 -1 b ---> 239 Pu 94 + 0 b -1

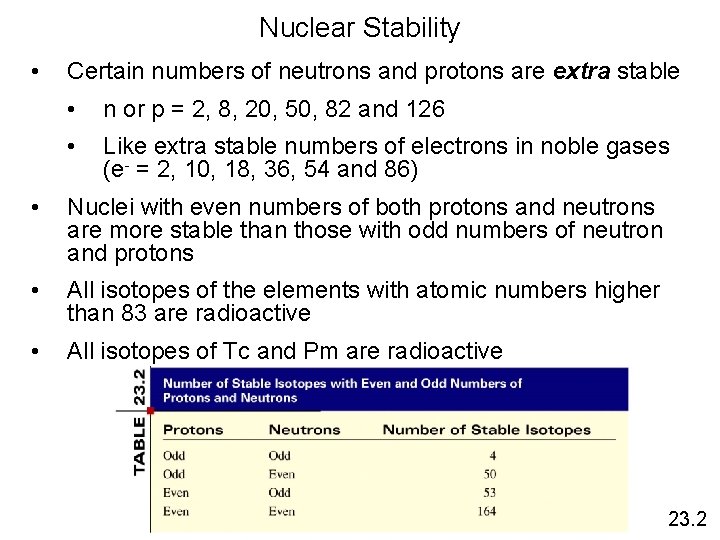
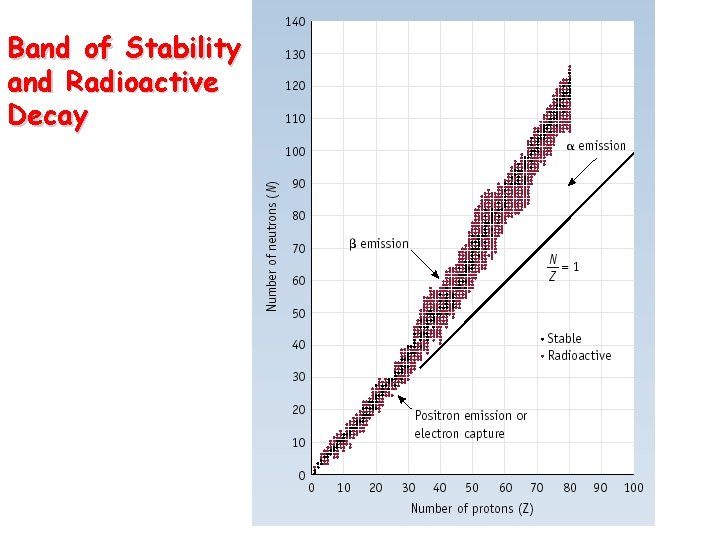
Nuclear Stability • Certain numbers of neutrons and protons are extra stable • n or p = 2, 8, 20, 50, 82 and 126 • Like extra stable numbers of electrons in noble gases (e- = 2, 10, 18, 36, 54 and 86) • Nuclei with even numbers of both protons and neutrons are more stable than those with odd numbers of neutron and protons • All isotopes of the elements with atomic numbers higher than 83 are radioactive • All isotopes of Tc and Pm are radioactive 23. 2

Band of Stability and Radioactive Decay

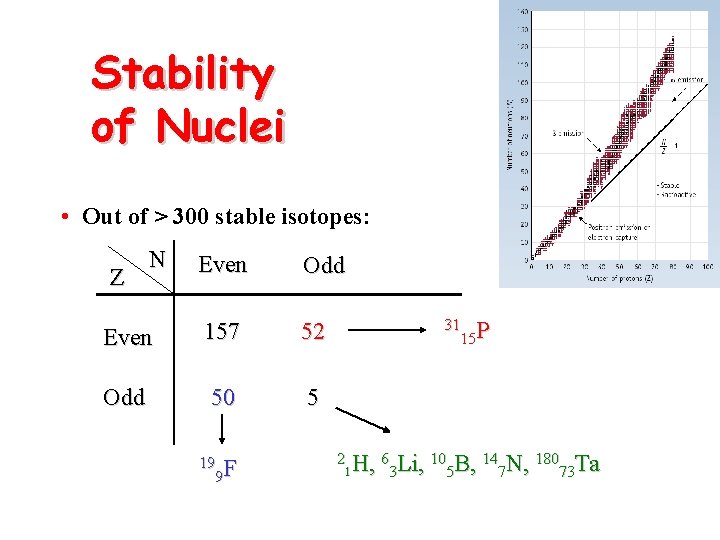
Stability of Nuclei • Out of > 300 stable isotopes: N Even Odd Even 157 52 Odd 50 5 Z 19 F 9 31 P 15 2 H, 63 Li, 105 B, 147 N, 18073 Ta 1

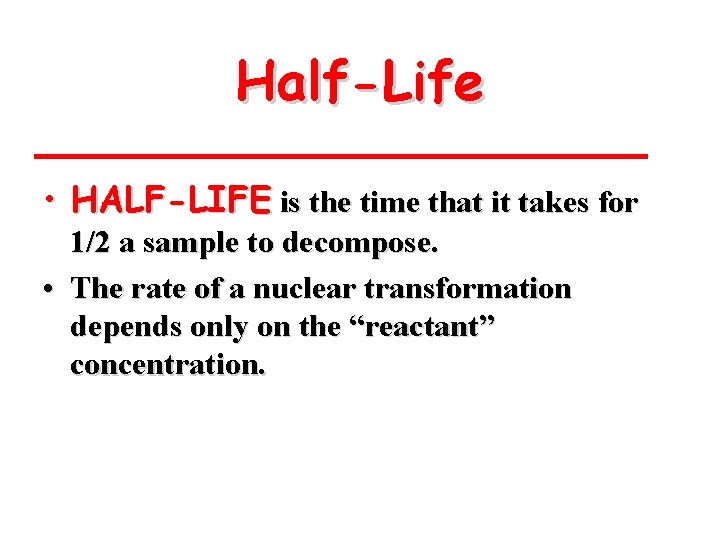
Half-Life • HALF-LIFE is the time that it takes for 1/2 a sample to decompose. • The rate of a nuclear transformation depends only on the “reactant” concentration.

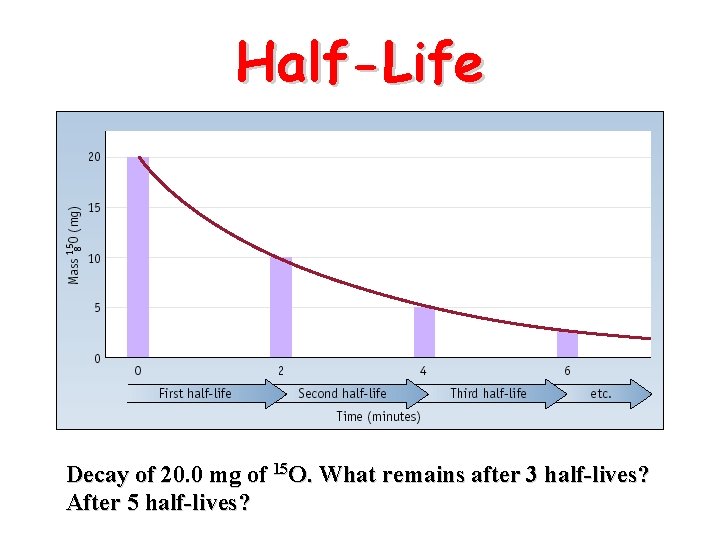
Half-Life Decay of 20. 0 mg of 15 O. What remains after 3 half-lives? After 5 half-lives?

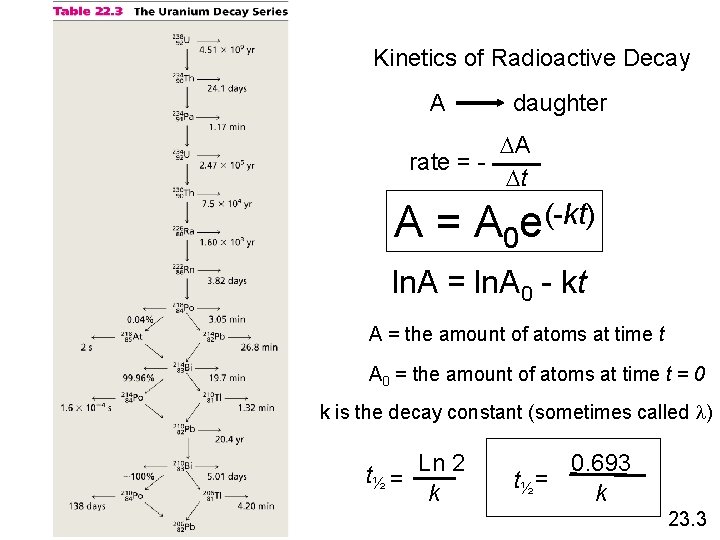
Kinetics of Radioactive Decay For each duration (half-life), one half of the substance decomposes. For example: Ra-234 has a half-life of 3. 6 days If you start with 50 grams of Ra-234 After 3. 6 days > 25 grams After 7. 2 days > 12. 5 grams After 10. 8 days > 6. 25 grams

Kinetics of Radioactive Decay A daughter DA rate = Dt A = A 0 (-kt) e ln. A = ln. A 0 - kt A = the amount of atoms at time t A 0 = the amount of atoms at time t = 0 k is the decay constant (sometimes called l) t½ = Ln 2 k 0. 693 t½ = k 23. 3
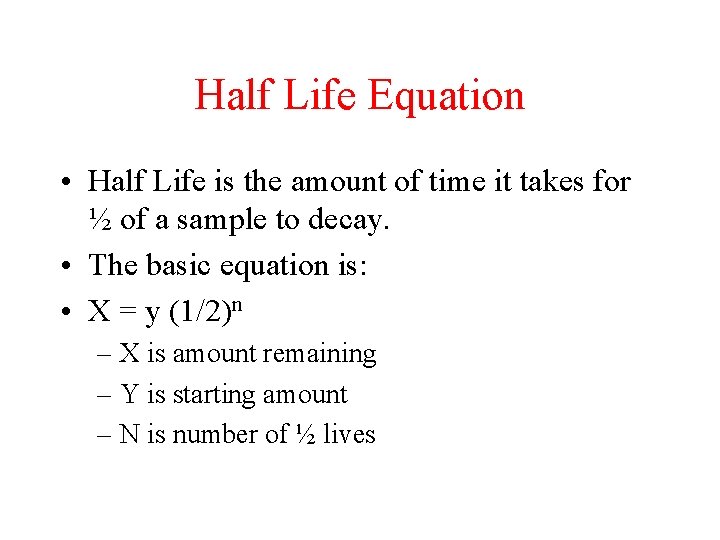
Half Life Equation • Half Life is the amount of time it takes for ½ of a sample to decay. • The basic equation is: • X = y (1/2)n – X is amount remaining – Y is starting amount – N is number of ½ lives

Sample question • How much U-238 is left over if a 100 gram sample undergoes 8 life lives? • X = 100(1/2)8 = 0. 391 grams

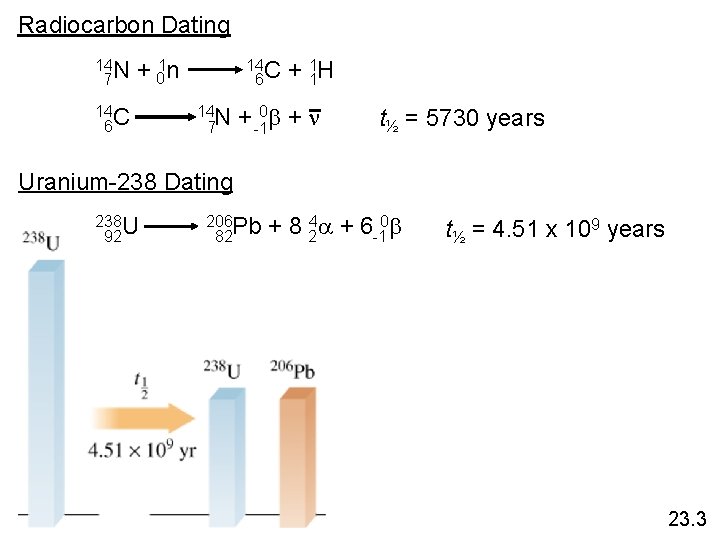
Radiocarbon Dating 14 N 7 + 01 n 14 C 6 14 N 7 + 11 H + -10 b + n t½ = 5730 years Uranium-238 Dating 238 U 92 206 Pb 82 + 8 24 a + 6 -10 b t½ = 4. 51 x 109 years 23. 3

Learning Check! The half life of I-123 is 13 hr. How much of a 64 mg sample of I-123 is left after 31 hours?

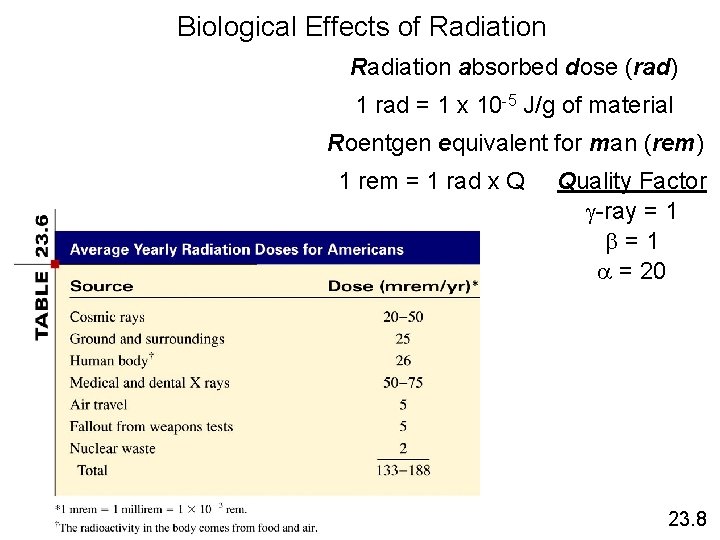
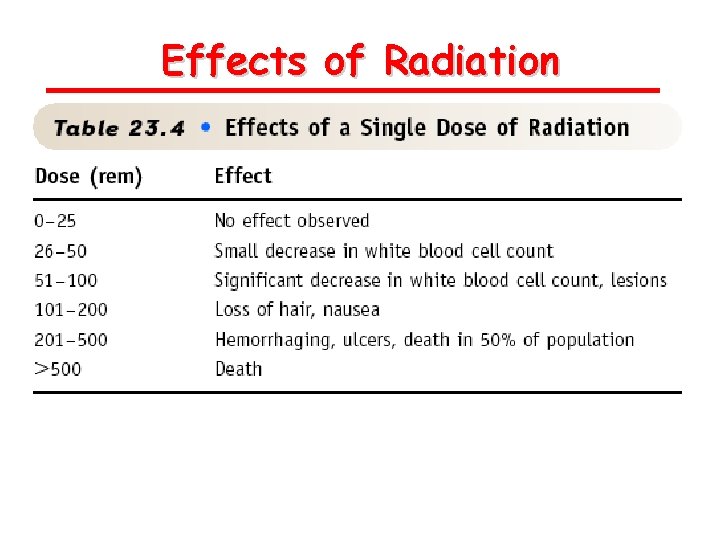
Biological Effects of Radiation absorbed dose (rad) 1 rad = 1 x 10 -5 J/g of material Roentgen equivalent for man (rem) 1 rem = 1 rad x Q Quality Factor g-ray = 1 b=1 a = 20 23. 8

Effects of Radiation

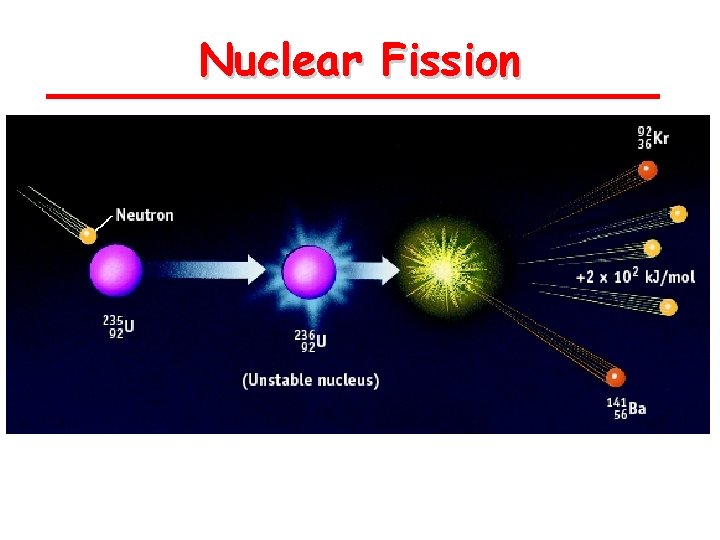
Nuclear Fission is the splitting of atoms These are usually very large, so that they are not as stable Fission chain has three general steps: 1. Initiation. Reaction of a single atom starts the chain (e. g. , 235 U + neutron) 2. Propagation. 236 U fission releases neutrons that initiate other fissions 3. Termination.

Nuclear Fission

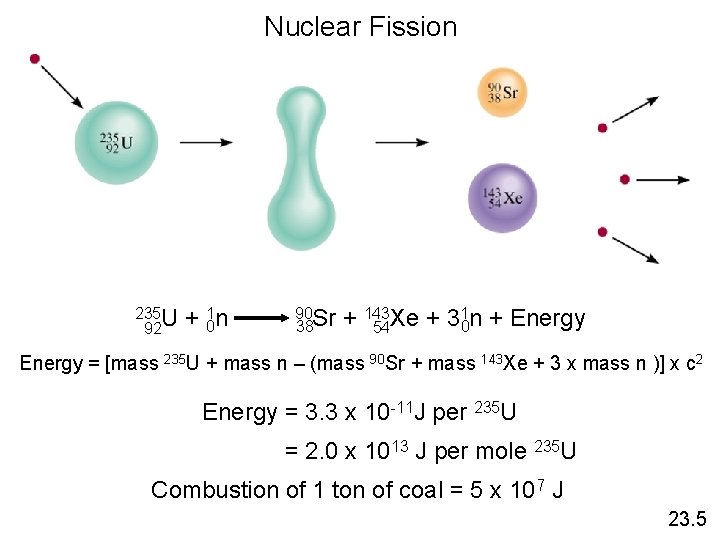
Nuclear Fission 235 U 92 + 01 n 90 Sr 38 1 n + Energy + 143 Xe + 3 0 54 Energy = [mass 235 U + mass n – (mass 90 Sr + mass 143 Xe + 3 x mass n )] x c 2 Energy = 3. 3 x 10 -11 J per 235 U = 2. 0 x 1013 J per mole 235 U Combustion of 1 ton of coal = 5 x 107 J 23. 5

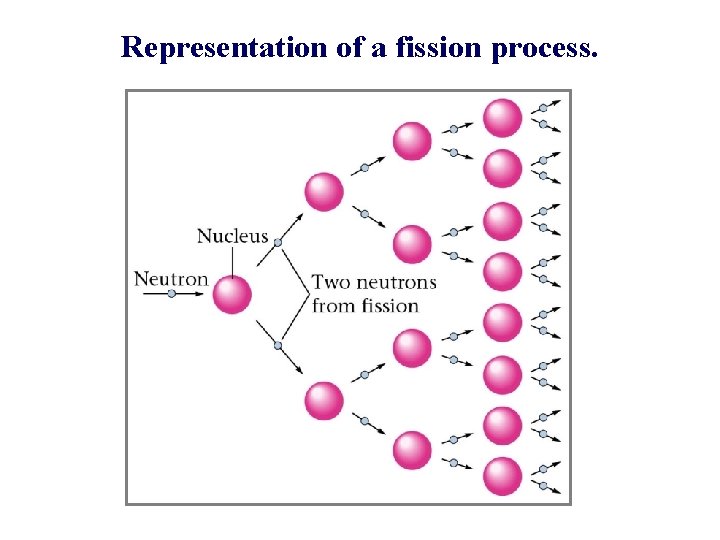
Representation of a fission process.

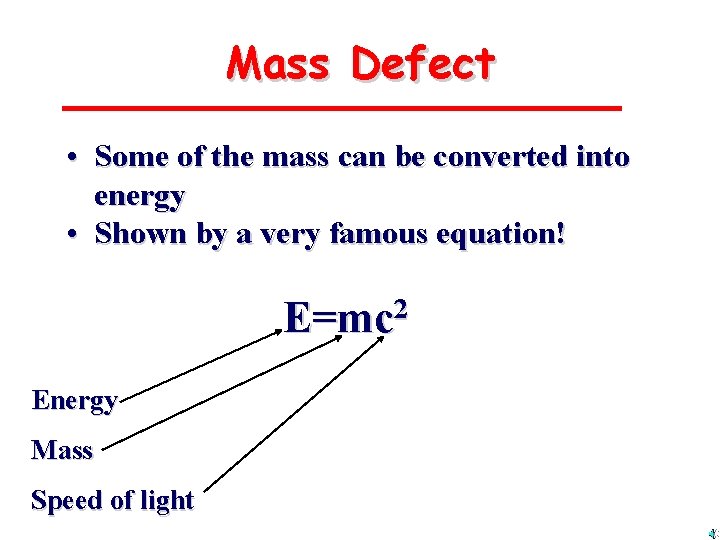
Mass Defect • Some of the mass can be converted into energy • Shown by a very famous equation! E=mc 2 Energy Mass Speed of light

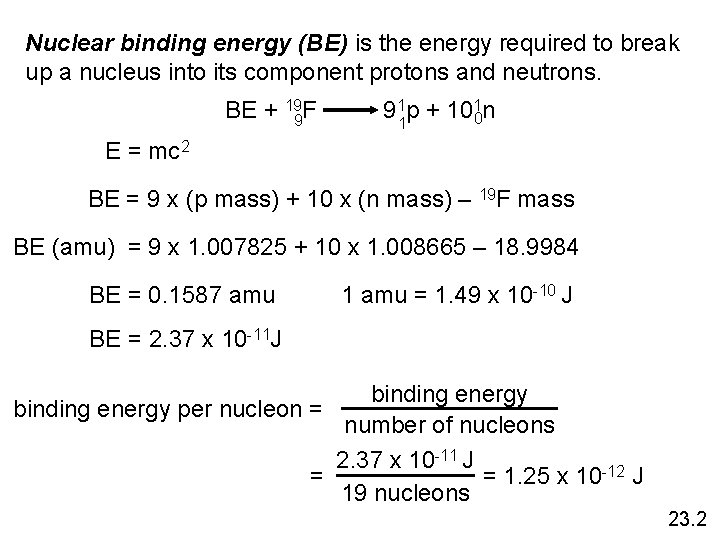
Nuclear binding energy (BE) is the energy required to break up a nucleus into its component protons and neutrons. BE + 199 F 911 p + 1010 n E = mc 2 BE = 9 x (p mass) + 10 x (n mass) – 19 F mass BE (amu) = 9 x 1. 007825 + 10 x 1. 008665 – 18. 9984 BE = 0. 1587 amu 1 amu = 1. 49 x 10 -10 J BE = 2. 37 x 10 -11 J binding energy per nucleon = number of nucleons 2. 37 x 10 -11 J = 1. 25 x 10 -12 J = 19 nucleons 23. 2

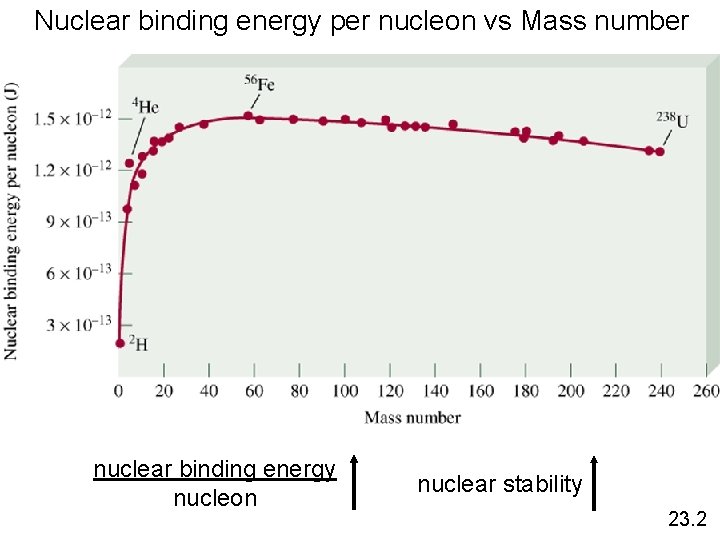
Nuclear binding energy per nucleon vs Mass number nuclear binding energy nucleon nuclear stability 23. 2

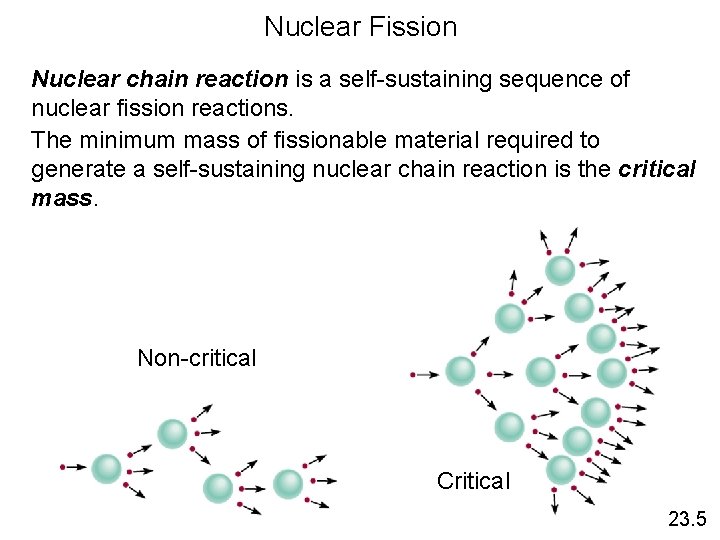
Nuclear Fission Nuclear chain reaction is a self-sustaining sequence of nuclear fission reactions. The minimum mass of fissionable material required to generate a self-sustaining nuclear chain reaction is the critical mass. Non-critical Critical 23. 5

Nuclear Fission & POWER • Currently about 103 nuclear power plants in the U. S. and about 435 worldwide. • 17% of the world’s energy comes from nuclear.

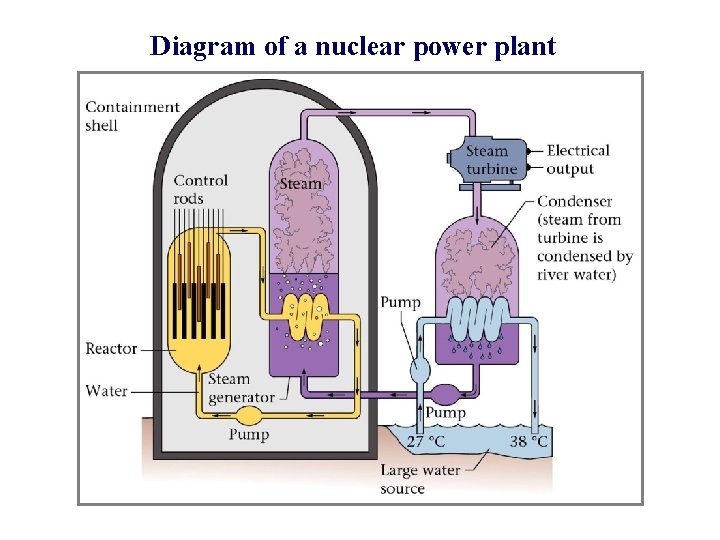
Diagram of a nuclear power plant

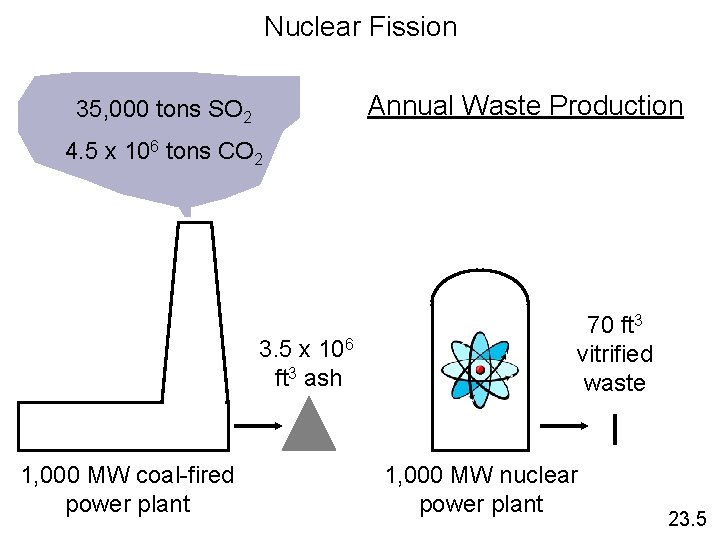
Nuclear Fission Annual Waste Production 35, 000 tons SO 2 4. 5 x 106 tons CO 2 3. 5 x 106 ft 3 ash 1, 000 MW coal-fired power plant 70 ft 3 vitrified waste 1, 000 MW nuclear power plant 23. 5

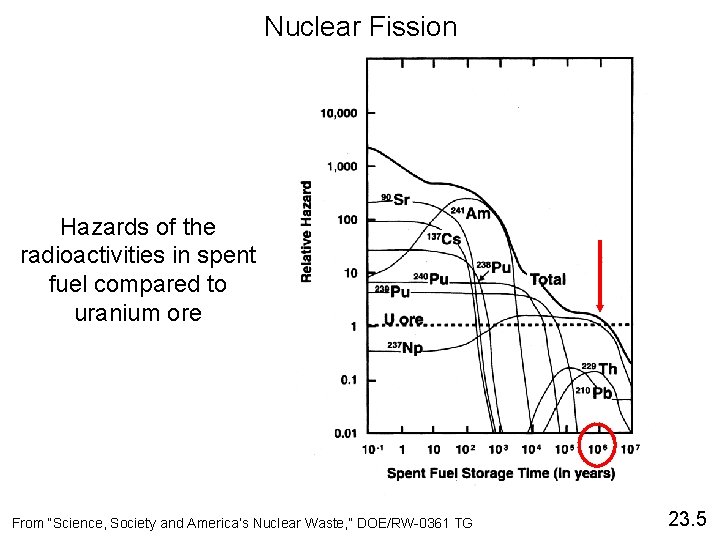
Nuclear Fission Hazards of the radioactivities in spent fuel compared to uranium ore From “Science, Society and America’s Nuclear Waste, ” DOE/RW-0361 TG 23. 5

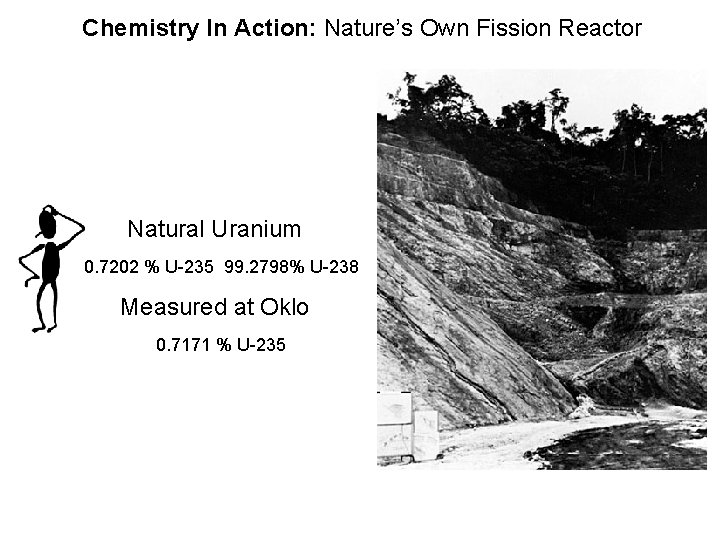
Chemistry In Action: Nature’s Own Fission Reactor Natural Uranium 0. 7202 % U-235 99. 2798% U-238 Measured at Oklo 0. 7171 % U-235

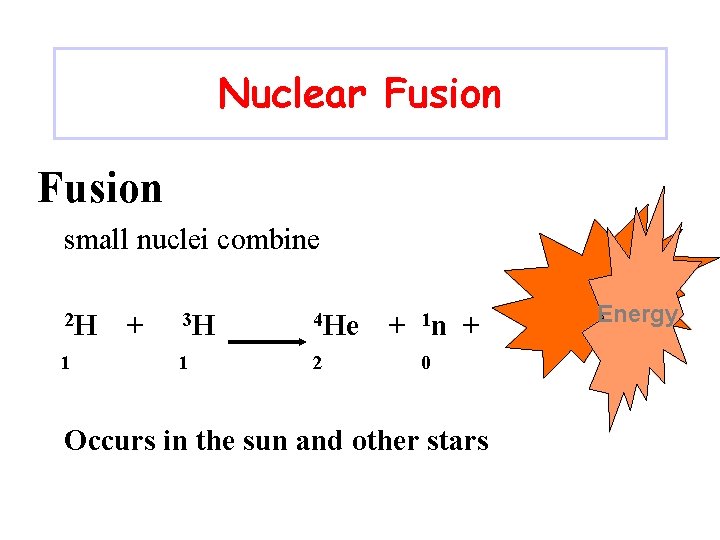
Nuclear Fusion small nuclei combine 2 H 1 + 3 H 4 He 1 2 + 1 n + 0 Occurs in the sun and other stars Energy

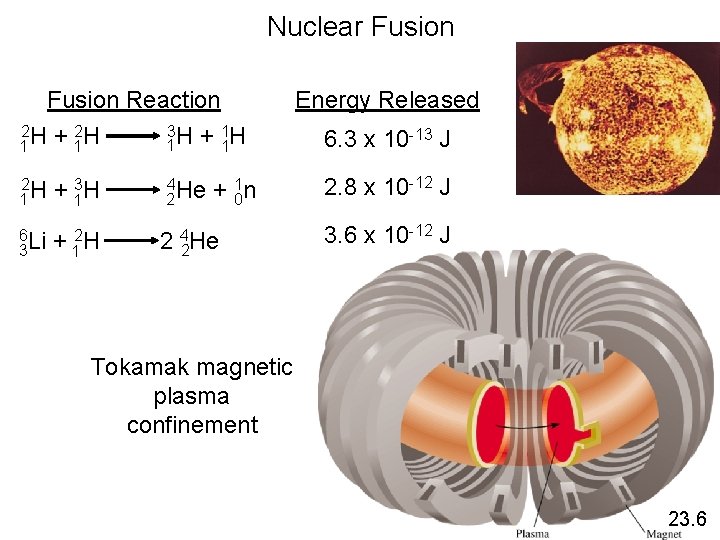
Nuclear Fusion Reaction 2 2 3 1 1 H + 1 H 2 H 1 + 13 H 6 Li 3 + 12 H 4 He 2 2 + 01 n 4 He 2 Energy Released 6. 3 x 10 -13 J 2. 8 x 10 -12 J 3. 6 x 10 -12 J Tokamak magnetic plasma confinement 23. 6

Nuclear Fusion • Excessive heat can not be contained • Attempts at “cold” fusion have FAILED. • “Hot” fusion is difficult to contain

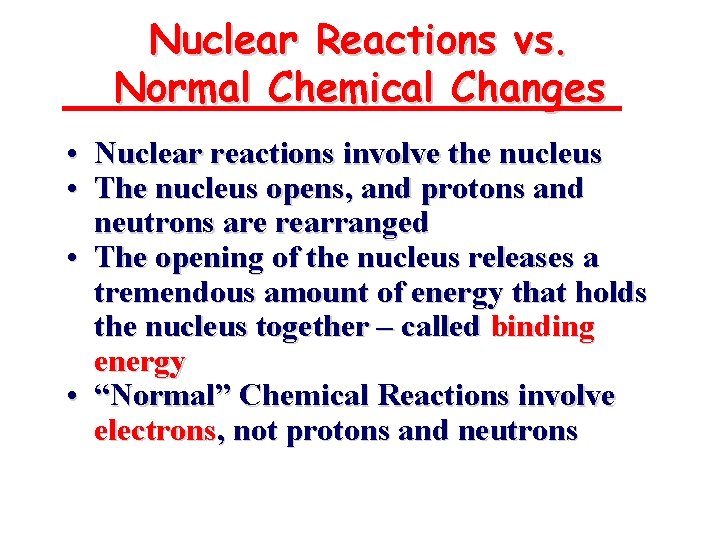
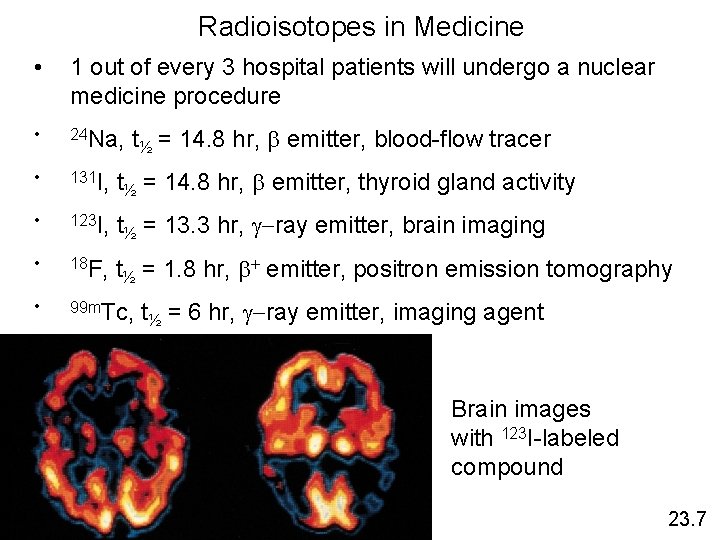
Radioisotopes in Medicine • 1 out of every 3 hospital patients will undergo a nuclear medicine procedure • 24 Na, • 131 I, t½ = 14. 8 hr, b emitter, thyroid gland activity • 123 I, t½ = 13. 3 hr, g-ray emitter, brain imaging • 18 F, t½ = 1. 8 hr, b+ emitter, positron emission tomography • 99 m. Tc, t½ = 14. 8 hr, b emitter, blood-flow tracer t½ = 6 hr, g-ray emitter, imaging agent Brain images with 123 I-labeled compound 23. 7

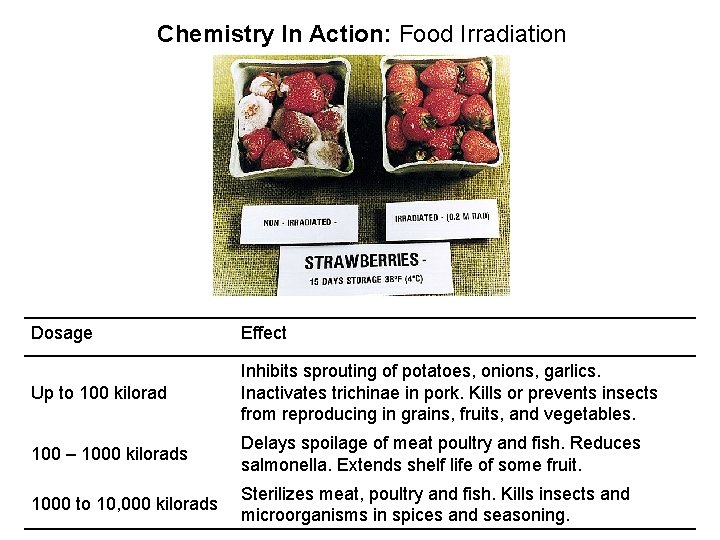
Chemistry In Action: Food Irradiation Dosage Effect Up to 100 kilorad Inhibits sprouting of potatoes, onions, garlics. Inactivates trichinae in pork. Kills or prevents insects from reproducing in grains, fruits, and vegetables. 100 – 1000 kilorads Delays spoilage of meat poultry and fish. Reduces salmonella. Extends shelf life of some fruit. 1000 to 10, 000 kilorads Sterilizes meat, poultry and fish. Kills insects and microorganisms in spices and seasoning.