NUCLEAR CHEMISTRY Radioactivity discovered by Henri Becquerel fluorescence
NUCLEAR CHEMISTRY Radioactivity - discovered by Henri Becquerel (fluorescence) - Marie Curie (search for substances- Po and Ra that spontaneously emitted radiation) - property of certain unstable nuclides to spontaneously emit radiation in order to form a more stable species.

Terms to remember Nuclide – any atom having a nucleus of mass number (A), atomic number (Z) and number of neutrons (n) - frequently used in place of the term nucleus Isotopes – elements with different atomic mass (A) but same atomic number.

Terms to remember Radioactive isotopes / Radionuclides – isotopes having an UNSTABLE nuclei Nucleons – particles that make up the nucleus (protons & neutrons) * Thus, mass number is the total mass of nucleons in the nucleus.
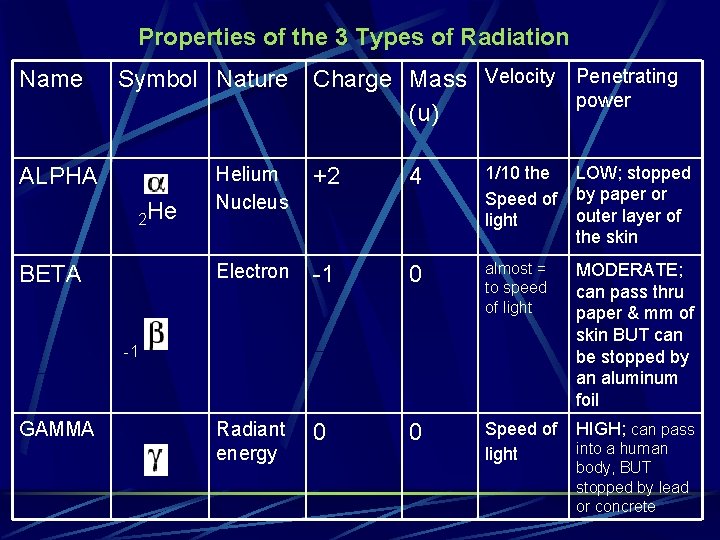
Properties of the 3 Types of Radiation Name Symbol Nature ALPHA 2 He BETA Charge Mass Velocity Penetrating power (u) Helium Nucleus +2 4 1/10 the Speed of light LOW; stopped by paper or outer layer of the skin Electron -1 0 almost = to speed of light MODERATE; can pass thru paper & mm of skin BUT can be stopped by an aluminum foil Radiant energy 0 0 Speed of light HIGH; can pass -1 GAMMA into a human body, BUT stopped by lead or concrete
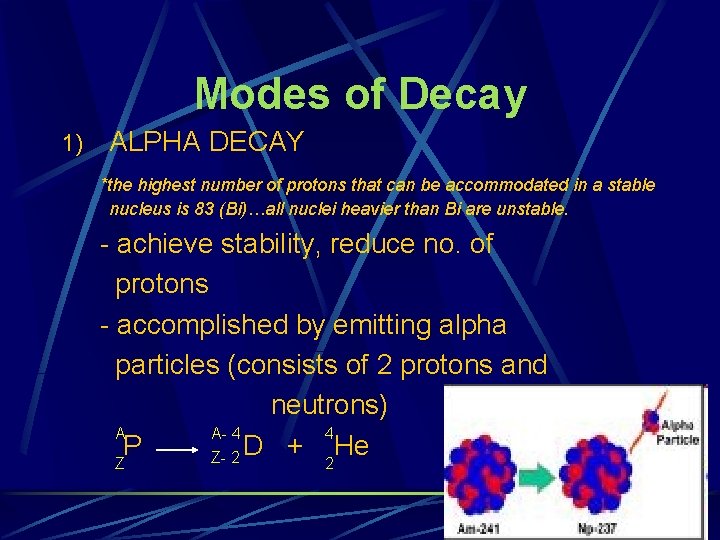
Modes of Decay 1) ALPHA DECAY *the highest number of protons that can be accommodated in a stable nucleus is 83 (Bi)…all nuclei heavier than Bi are unstable. - achieve stability, reduce no. of protons - accomplished by emitting alpha particles (consists of 2 protons and neutrons) A A- 4 4 P D + He Z- 2 Z 2

Modes of Decay 2) Beta Decay - involves the ejection of the beta particle from the nucleus. - beta particle results from the conversion of a neutron into a proton. n 1 0 1 -1 p +

Modes of Decay 3) Gamma Decay - gamma rays are high energy radiation similar to x-rays. - has no mass & electrical charge. - A and Z number remain the same. - Gamma emitters (cancer therapy & radioactive tracer nuclides)
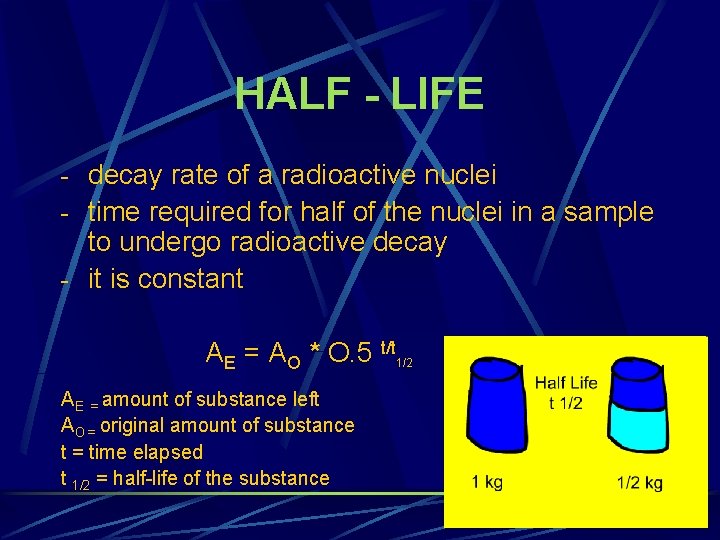
HALF - LIFE - decay rate of a radioactive nuclei - time required for half of the nuclei in a sample to undergo radioactive decay - it is constant AE = AO * O. 5 t/t 1/2 AE = amount of substance left AO = original amount of substance t = time elapsed t 1/2 = half-life of the substance
- Slides: 8