Nuclear Chemistry OBJECTIVE Describe and distinguish the nuclear

Nuclear Chemistry OBJECTIVE: Describe and distinguish the nuclear processes of fission, fusion, and radioactive decay. TASK: What do you think the GIFs at right are showing? Only one nation has ever deployed a nuclear weapon in war. Which nation is it?

How Good Are You at the Notes Thing? (JE): You will be taking your own notes today, in your science journal (which I will be collecting TOMORROW). Your job today will be to take the most useful set of notes you can. Remember: there are 2 reasons to take notes: 1. To process new information as it is presented, by summarizing, clarifying, and identifying important concepts. 2. To make a document you can refer back to, as a type of memory.

1. Radioactive Decay An isotope is radioactive if it spontaneously gives off particles and changes into something else. Example: carbon-14 Radioactive decay often leads to another unstable isotope, which then decays again, producing a “radioactive decay chain” Example: Neptunium-237 (right)
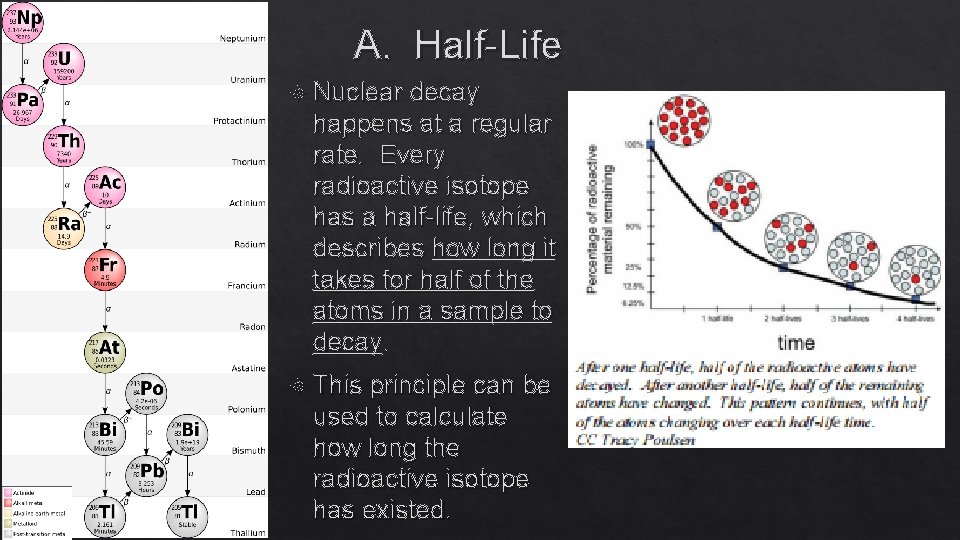
A. Half-Life Nuclear decay happens at a regular rate. Every radioactive isotope has a half-life, which describes how long it takes for half of the atoms in a sample to decay. This principle can be used to calculate how long the radioactive isotope has existed.
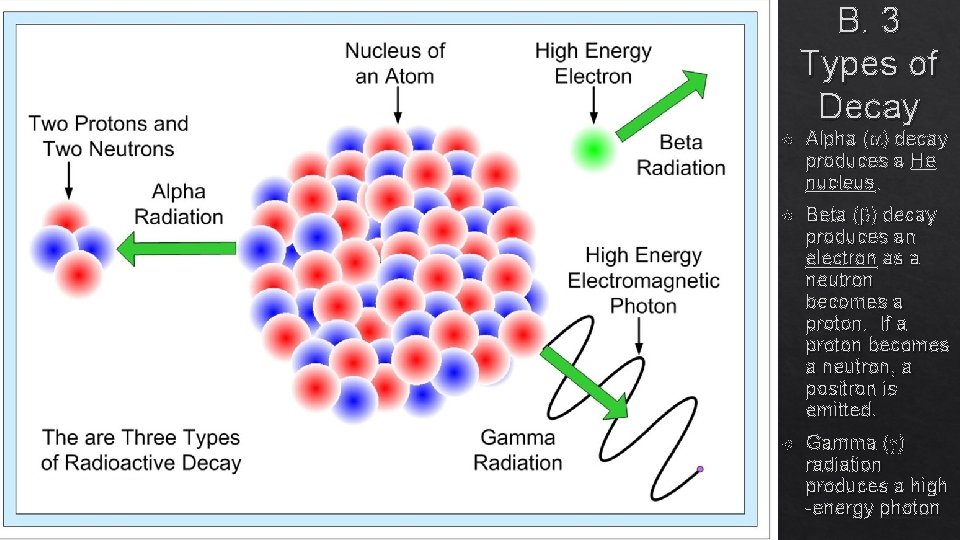
B. 3 Types of Decay Alpha ( ) decay produces a He nucleus. Beta ( ) decay produces an electron as a neutron becomes a proton. If a proton becomes a neutron, a positron is emitted. Gamma ( ) radiation produces a high -energy photon
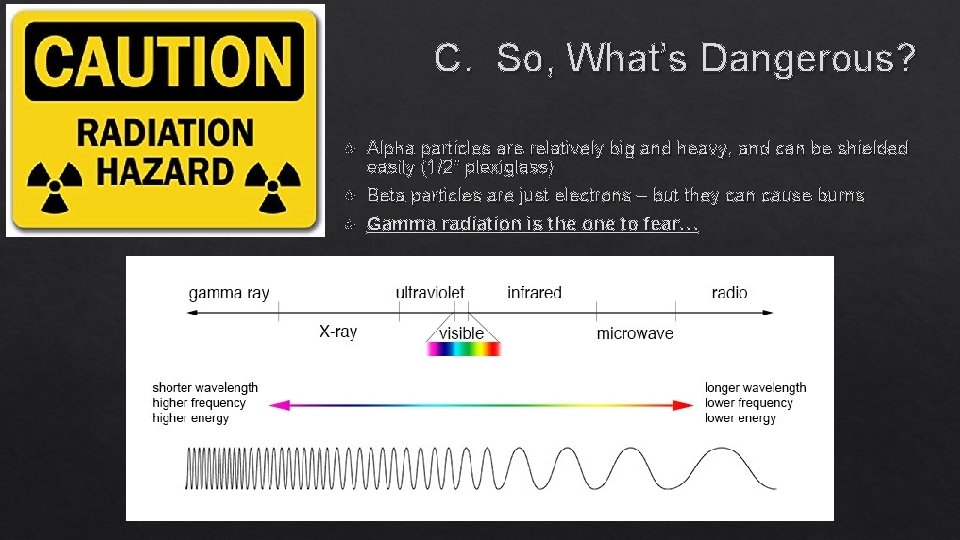
C. So, What’s Dangerous? Alpha particles are relatively big and heavy, and can be shielded easily (1/2” plexiglass) Beta particles are just electrons – but they can cause burns Gamma radiation is the one to fear…
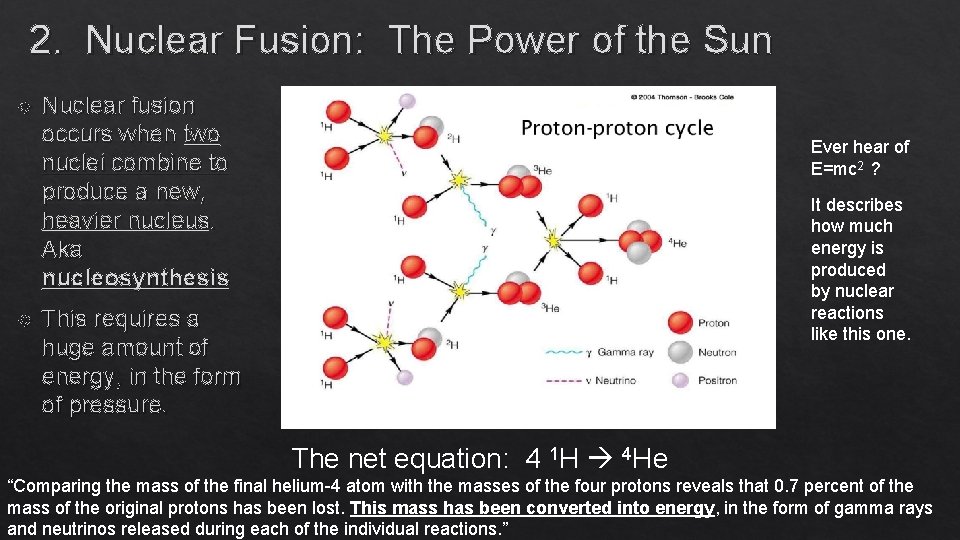
2. Nuclear Fusion: The Power of the Sun Nuclear fusion occurs when two nuclei combine to produce a new, heavier nucleus. Aka nucleosynthesis Ever hear of E=mc 2 ? It describes how much energy is produced by nuclear reactions like this one. This requires a huge amount of energy, in the form of pressure. The net equation: 4 1 H 4 He “Comparing the mass of the final helium-4 atom with the masses of the four protons reveals that 0. 7 percent of the mass of the original protons has been lost. This mass has been converted into energy, in the form of gamma rays and neutrinos released during each of the individual reactions. ”

Bigger Stars Do More Than Make Helium Our Sun produces energy by fusing hydrogen nuclei to make helium nuclei, releasing lots of energy in the process. This is called nucleosynthesis. Other stars (and especially supernovae) can preform other fusion reactions that make all of the different elements found in the entire universe. Hydrogen fusion requires temperatures of about 5, 000 K Heavier elements require higher temperatures (example: carbon fusion begins around 1, 000, 000 K) This is how we know our Sun is a 2 nd generation star. Most elements heavier than He are produced in stars 10 x as large as the I make some heavy elements!

Are You a Good Note-Taker? Do you have an outline that looks something like: 1. Decay A. Half life B. 3 Types of Decay C. What’s Dangerous? Level 1: Congratulations! You know what we were talking about in class today. 2. Fusion Do you have definitions for radioactive decay, half life, fusion, and nucleosynthesis? Did you include any relevant details including alpha, beta, and gamma radiation? Positrons? The Sun? Level 2: Good Job! You were able to pick out the key words and main ideas that are new and important from the presentation. Level 3: Excellent! These details might not show up on an assessment, but they help you get the whole story, and have a more complete understanding.

Nuclear Chemistry, Day 2 Objective: Describe and distinguish the nuclear processes of fission, fusion, and radioactive decay. (repeat of yesterday) Use the image to answer: A. Which type of decay changes the atomic number (Z), but not the mass number? B. Which type of decay changes the mass number (A) of an isotope? C. Which type of decay changes neither the mass number (A) or atomic number (Z)?
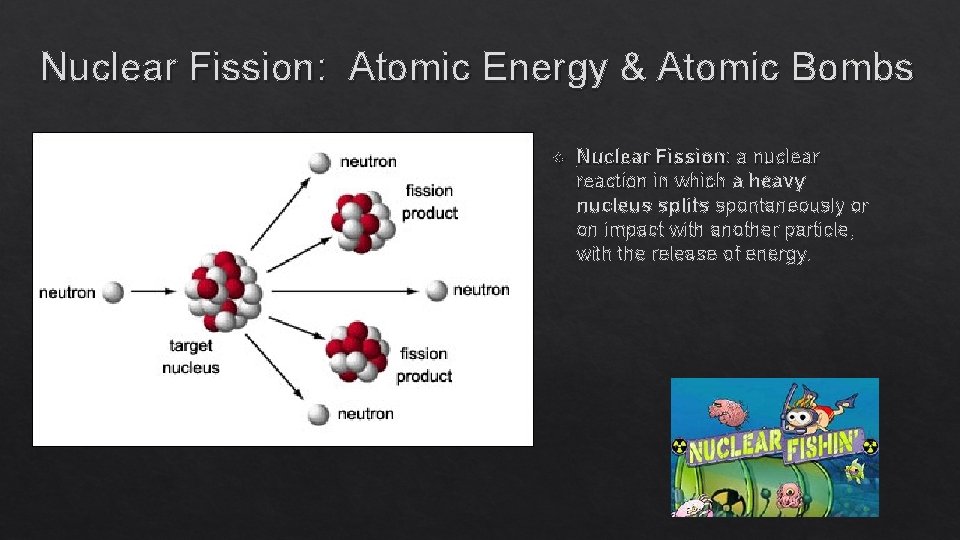
Nuclear Fission: Atomic Energy & Atomic Bombs Nuclear Fission: a nuclear reaction in which a heavy nucleus splits spontaneously or on impact with another particle, with the release of energy.
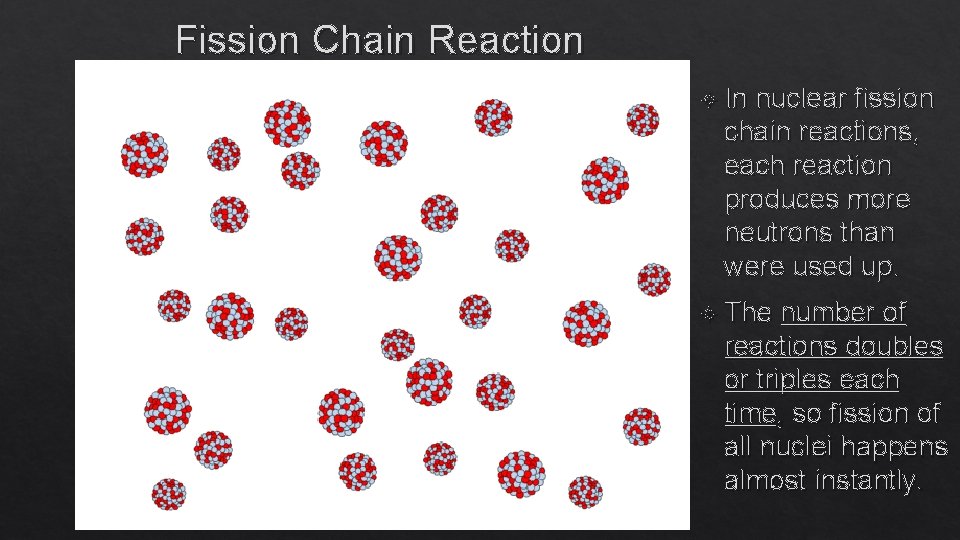
Fission Chain Reaction In nuclear fission chain reactions, each reaction produces more neutrons than were used up. The number of reactions doubles or triples each time, so fission of all nuclei happens almost instantly.

What We’ve Seen So Far… Radioactive Unstable, radioactive isotopes spontaneously change and emit particles. Alpha and Beta decay result in new isotopes Decay happens at a regular rate, measured as a half-life, which can be used to determine how long an isotope has been decaying. Nuclear Decay: radioactive isotopes emit particles Fusion: nuclei fuse together Stars produce energy by fusing nuclei together at extreme temperatures and pressures. Our Sun, being rather small, can only fuse hydrogen into helium. Other, larger stars can fuse heavier elements Nuclear Fission: a heavy nucleus splits

Fission Application 1: Nuclear Energy The controlled fission of Uranium-235 produces heat. The heat is used to boil water, and the resulting steam turns a turbine. The spinning of the turbine makes electricity.

Nuclear Energy Risks and Benefits Risk 1: Meltdown Risk 2: Waste After the uranium fuel rods are used, they are still highly radioactive, and need to be stored carefully (see risk #1!) Benefit 1: Green Energy (? ) If the reactor gets too hot (e. g. because the water leaks out) the fission can become uncontrolled and lead to a reactor core MELTDOWN. Nuclear power does not directly produce greenhouse gasses, like CO 2. Benefit 2: Stable Long-term Power Nuclear reactors can provide electricity for a LONG time without refueling Submarines

Fission Application #2: Nuclear Weapons Fission chain reactions release massive amounts of energy almost instantly. There’s a lot of complex engineering, and combinations of fission and fusion (i. e. “thermonuclear”) are most common.

Hiroshima and Nagasaki: Nuclear Shadows “Nuclear Shadow” ~1 mile from blast center, Hiroshima In 1945, the US military dropped atomic bombs on the Japanese cities of Hiroshima and Nagasaki, effectively ending WWII in the Pacific. They are the only times atomic weapons have been used… so
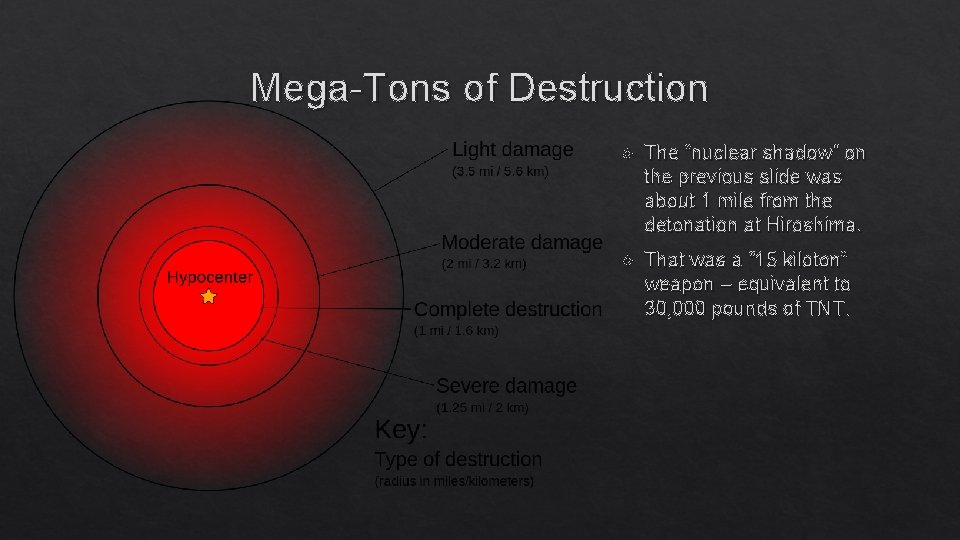
Mega-Tons of Destruction The “nuclear shadow” on the previous slide was about 1 mile from the detonation at Hiroshima. That was a “ 15 kiloton” weapon – equivalent to 30, 000 pounds of TNT.
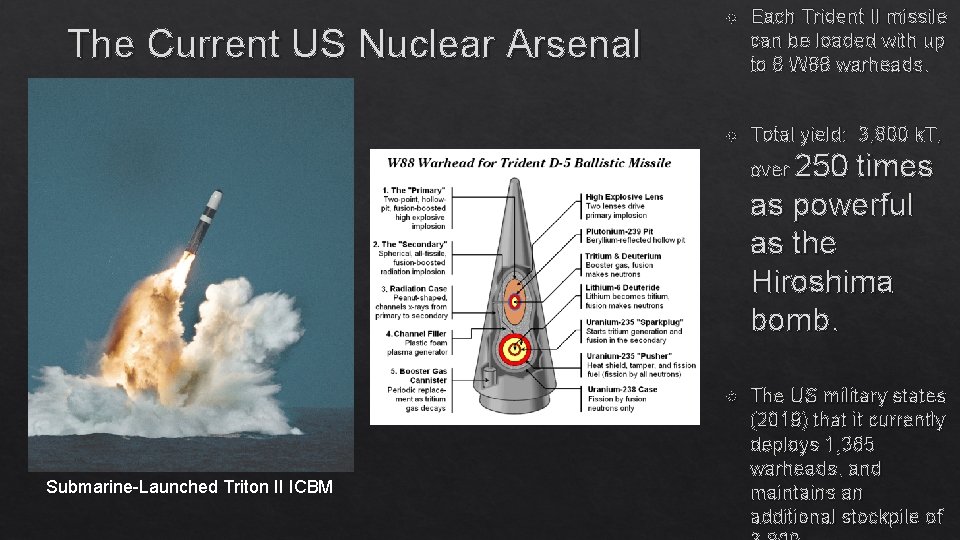
The Current US Nuclear Arsenal Each Trident II missile can be loaded with up to 8 W 88 warheads. Total yield: 3, 800 k. T, over 250 times as powerful as the Hiroshima bomb. Submarine-Launched Triton II ICBM The US military states (2019) that it currently deploys 1, 365 warheads, and maintains an additional stockpile of


Recap Nuclear Fission Chain Reaction Nuclear Power: Controlled How fission does it work? Risks and benefits? Nuclear Fission Weapons: Fusion (“thermonuclear”) Unimaginable destructive capability
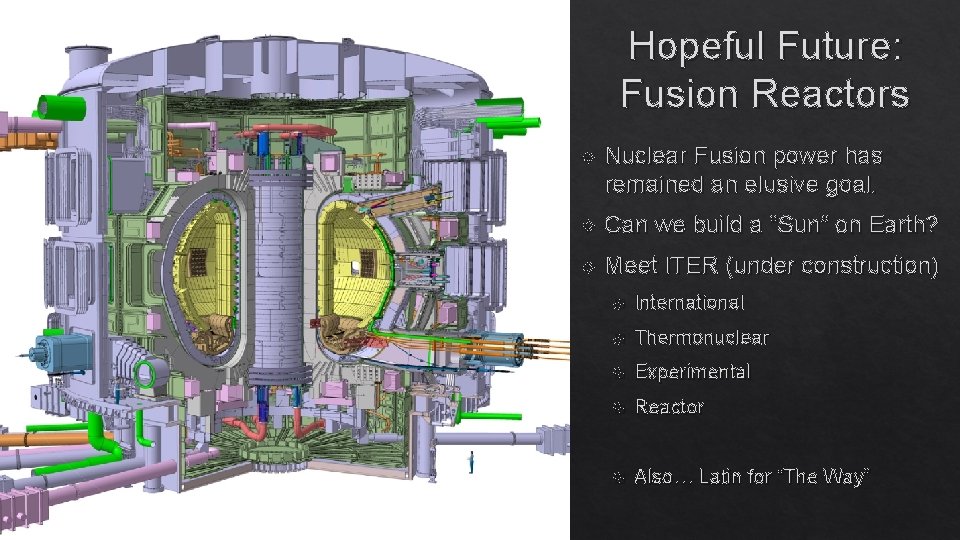
Hopeful Future: Fusion Reactors Nuclear Fusion power has remained an elusive goal. Can we build a “Sun” on Earth? Meet ITER (under construction) International Thermonuclear Experimental Reactor Also… Latin for “The Way” ITER: A Fusion Reactor
- Slides: 22