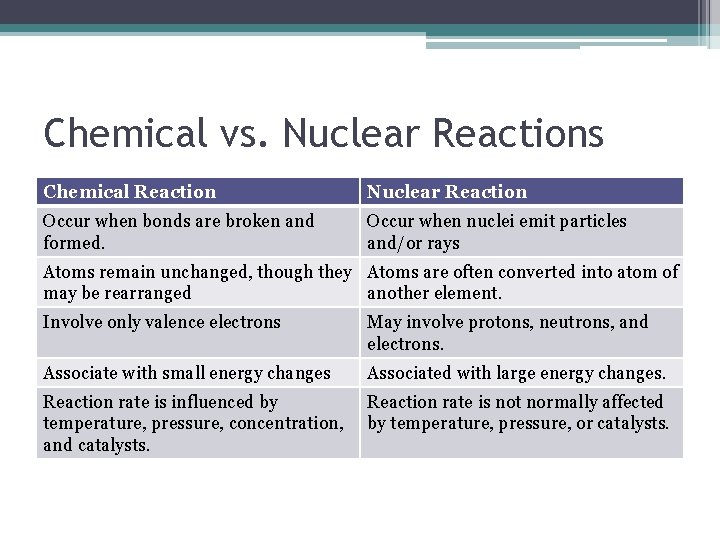
Nuclear Chemistry Chemical vs Nuclear Reactions Chemical Reaction
























- Slides: 45

Nuclear Chemistry

Chemical vs. Nuclear Reactions Chemical Reaction Nuclear Reaction Occur when bonds are broken and formed. Occur when nuclei emit particles and/or rays Atoms remain unchanged, though they Atoms are often converted into atom of may be rearranged another element. Involve only valence electrons May involve protons, neutrons, and electrons. Associate with small energy changes Associated with large energy changes. Reaction rate is influenced by temperature, pressure, concentration, and catalysts. Reaction rate is not normally affected by temperature, pressure, or catalysts.

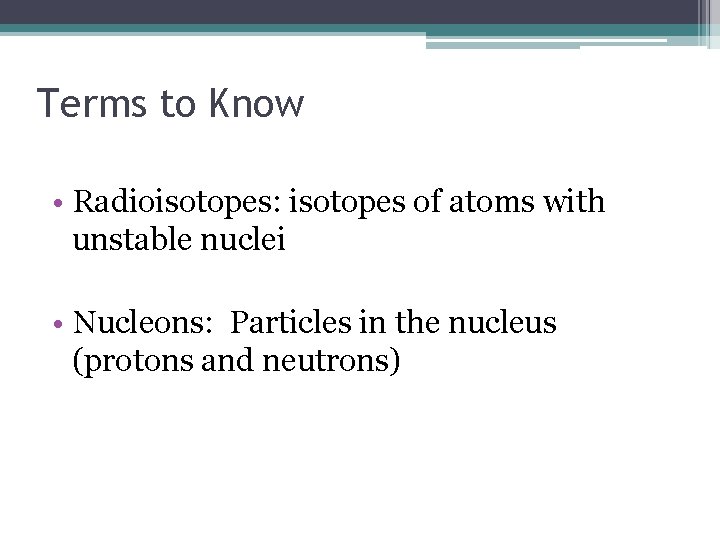
Terms to Know • Radioisotopes: isotopes of atoms with unstable nuclei • Nucleons: Particles in the nucleus (protons and neutrons)

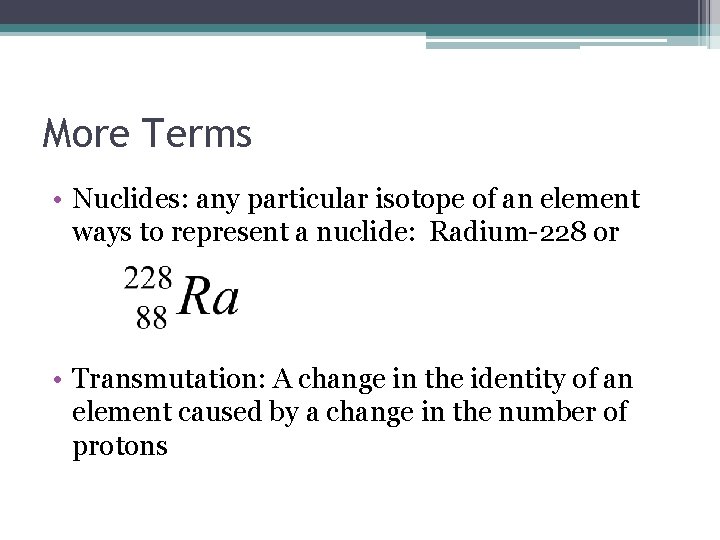
More Terms • Nuclides: any particular isotope of an element ways to represent a nuclide: Radium-228 or • Transmutation: A change in the identity of an element caused by a change in the number of protons

Why do nuclear reactions occur? To make a nucleus more stable! Example:

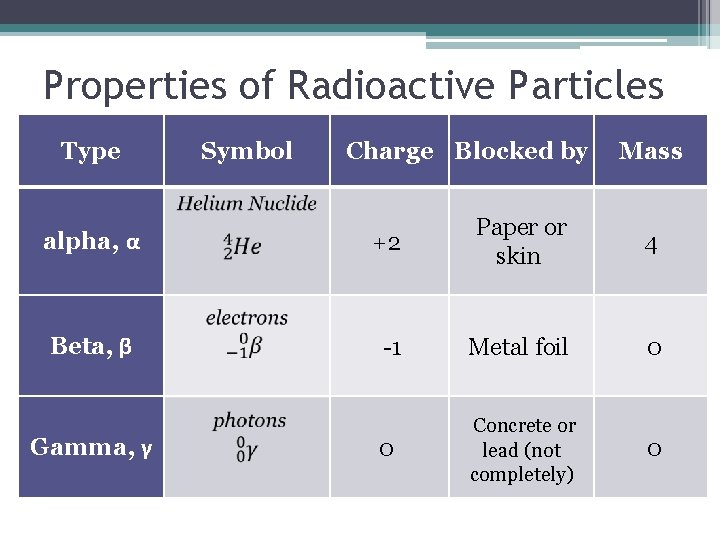
Properties of Radioactive Particles Type Symbol Charge Blocked by Mass alpha, α +2 Paper or skin Beta, β -1 Metal foil 0 0 Concrete or lead (not completely) 0 Gamma, γ 4

Energy of the Particles • Alpha has the least energy. • Gamma has the most energy.

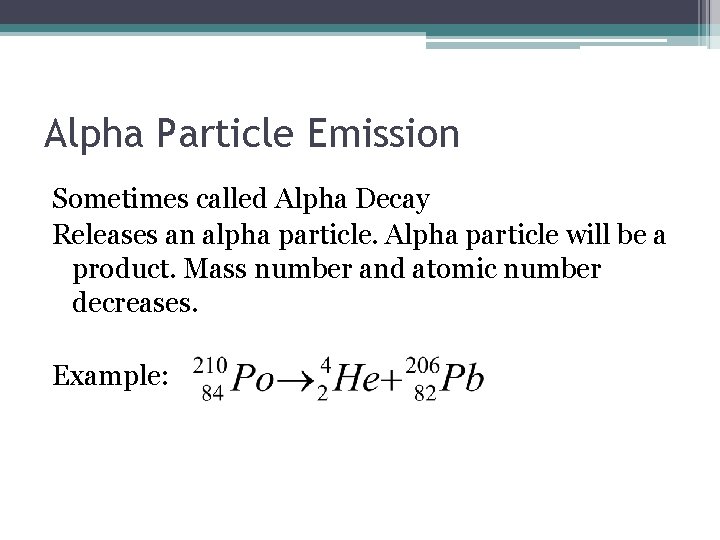
Alpha Particle Emission Sometimes called Alpha Decay Releases an alpha particle. Alpha particle will be a product. Mass number and atomic number decreases. Example:

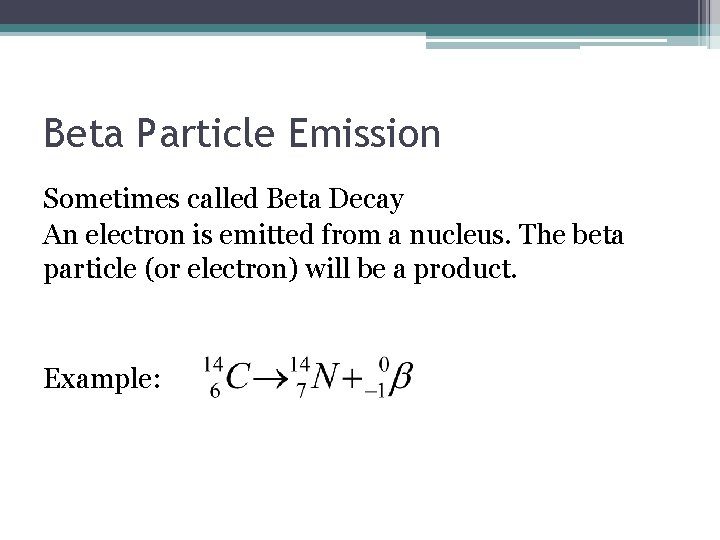
Beta Particle Emission Sometimes called Beta Decay An electron is emitted from a nucleus. The beta particle (or electron) will be a product. Example:

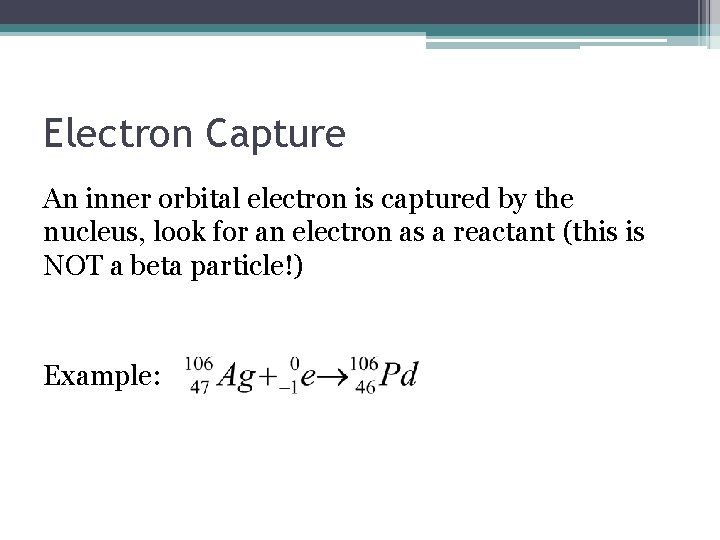
Electron Capture An inner orbital electron is captured by the nucleus, look for an electron as a reactant (this is NOT a beta particle!) Example:

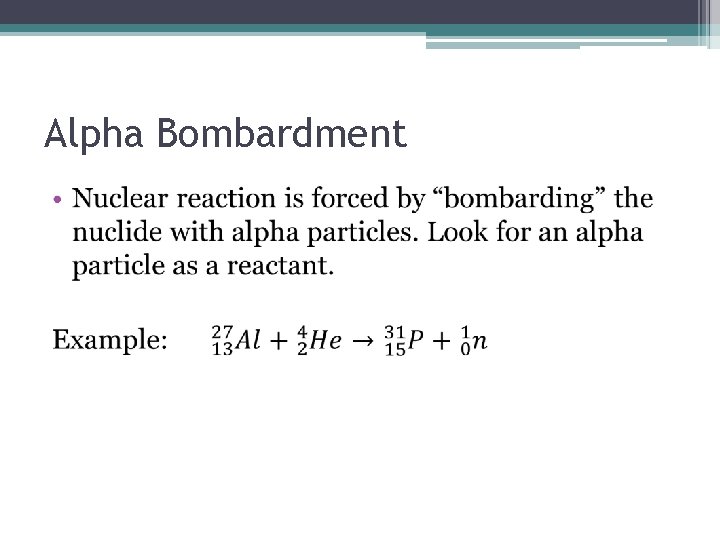
Alpha Bombardment •

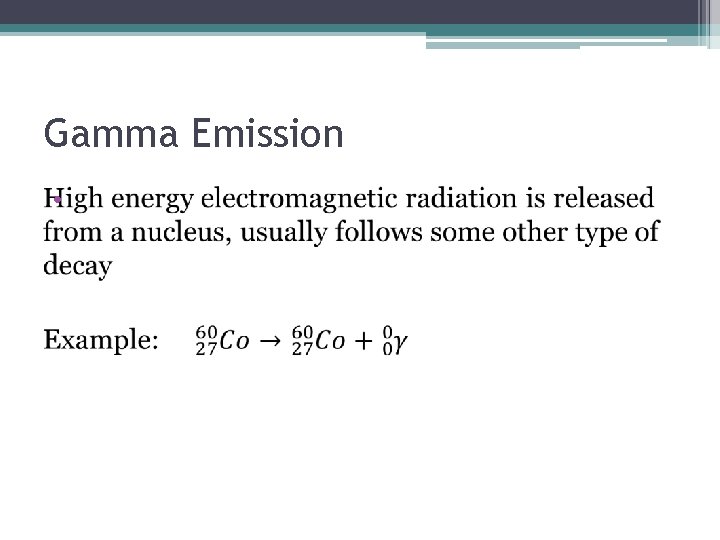
Gamma Emission •

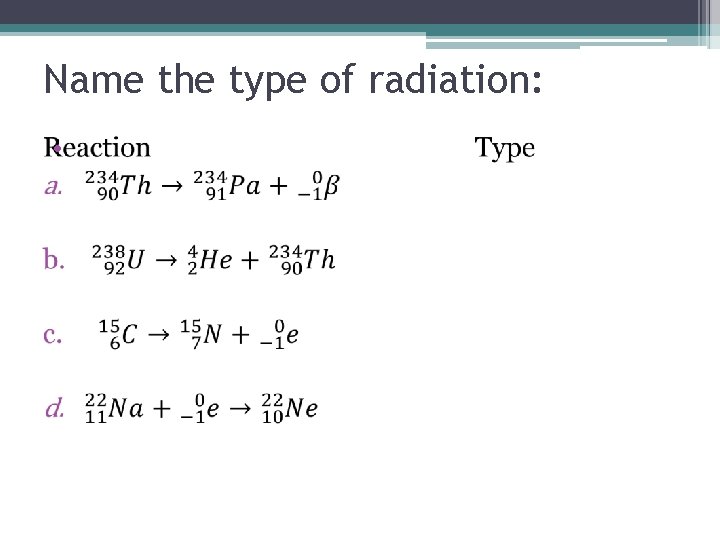
Name the type of radiation: •

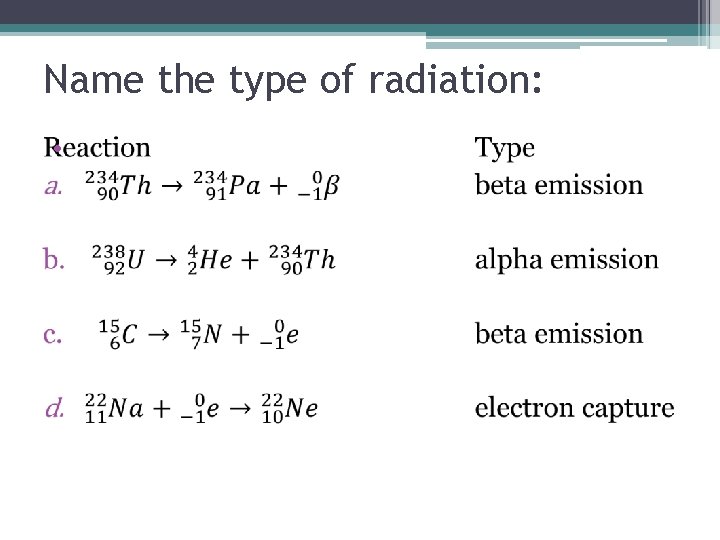
Name the type of radiation: •

Complete the following nuclear reactions: •

Try this! • Write a balanced nuclear equation for the alpha decay of Thorium-230.

Why is nuclear radiation dangerous? It ionizes atoms and molecules, which causes them to become charged. It damages living tissue and mutates DNA. Causing cancer, genetic defects, etc.

Units of Radiation: Roentgen (R), rad, rem, Detection methods for radiation Film badge, Geiger-Muller counter, Scintillation Counter

Roentgen • The roentgen (R) unit refers to the amount of ionization present in the air. • This unit is used to express gamma ray intensity in the air from radioactive materials in the earth and in the atmosphere.

REM • Roentgen Equivalent Man • Unit that measures the effects of ionizing radiation on humans.

Applications of nuclear radiation

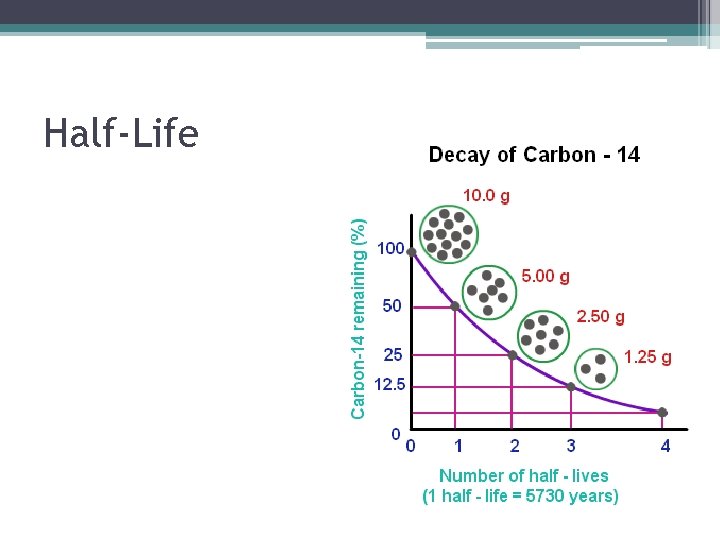
RADIOACTIVE DATING Approximates age of an object based on the presence of a radioactive nuclide with a known half-life ex: Carbon-14, t 1/2 = 5715 years

Nuclear medicine • Radioactive tracers (Barium) used to find things like a blocked kidney • Radiation therapy for cancer (Cobalt-60) • CAT (computed tomography) scans shows cross-sectional views of the body • PET (positron emission tomography) scans images the cellular function of the human body demonstrates biological function of the body • Sterilization of medical instruments

Nuclear agriculture • Tracers in fertilizer to monitor effectiveness • Prolong shelf life of foods (zapping with gamma rays from cobalt-60) (strawberries are often irradiated to maintain shelf life)

Radon • A radioactive gas that naturally occurs in the atmosphere. It is often found in basements and many homes undergo radon testing to decrease the level that inhabitants are exposed to.

Smoke detectors Use Americium-241, ionizes air when the small electric current is blocked the alarm sounds (smoke has stopped the signal)

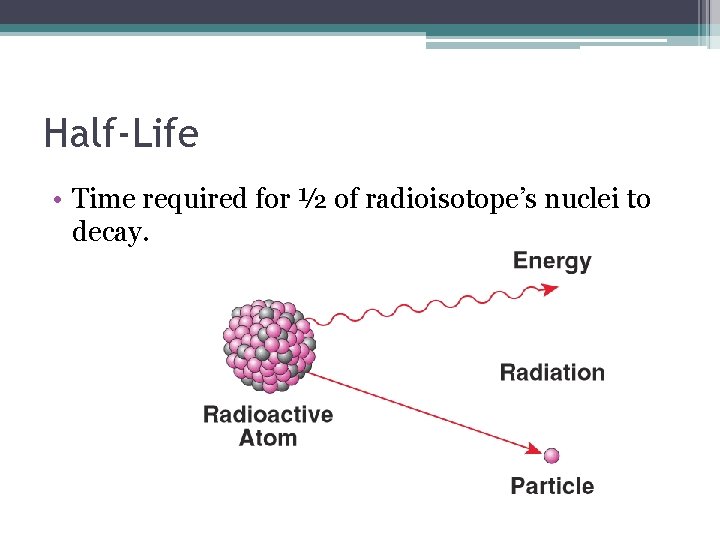
Half-Life • Time required for ½ of radioisotope’s nuclei to decay.

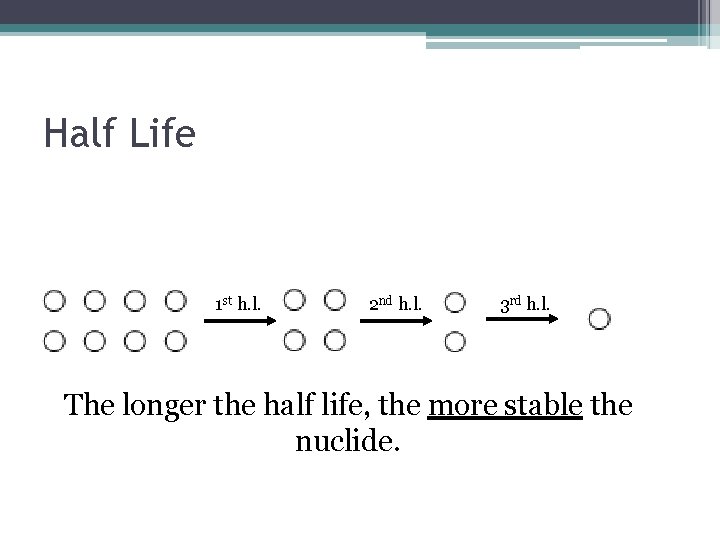
Half Life 1 st h. l. 2 nd h. l. 3 rd h. l. The longer the half life, the more stable the nuclide.

Half-Life

Solve the following problems 1. The half life of polonium-210 is 138. 4 days. How many milligrams of polonium-210 remain after 415. 2 days, if you start with 2. 0 mg of the isotope?

2. Assuming a half-life of 1599 years, how many years will be needed for the decay of 15/16 of a given amount of radium-226?

3. The half-life of polonium-218 is 3. 0 minutes. If you start with 16 mg, how long will it be before only 1. 0 mg remains?

4. If it takes 6. 5 hours for 20. g to decay to 2. 5 g, what is the half-life?

Determine your Radiation Exposure • Calculate your annual exposure to nuclear radiation

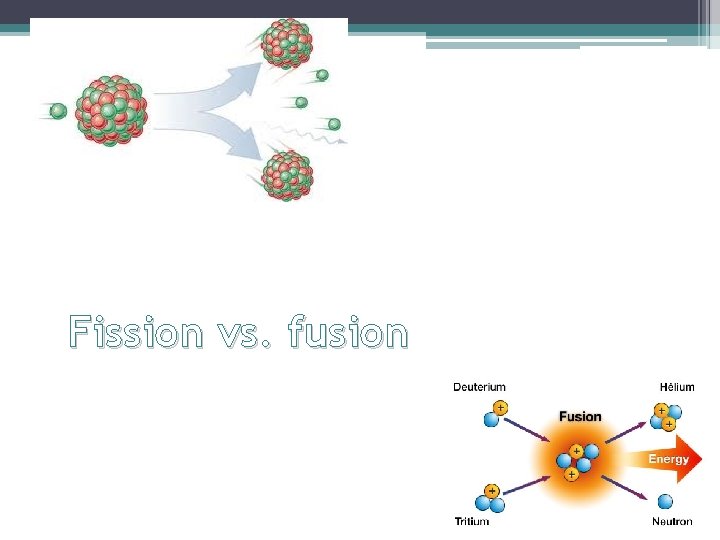
Fission vs. fusion

Definitions • Fission: splitting of a heavy nucleus into more stable nuclei of intermediate mass • Fusion: light nuclei combine to form heavier, more stable nuclei • Chain reaction: reaction in which the starting material is also one of the products. Ex: a decay series An uncontrolled chain reaction results in an atomic bomb. A controlled chain reaction can be used to produce nuclear power. • Nuclear power plant: device used to control a fission chain reaction to produce energy or radioactive nuclei

Fission • Fission is the splitting of a large atom into two or more smaller atoms. • It does not take a lot of energy for fission to occur, but fission releases a lot of energy, almost a million times more than any chemical reaction. However, it releases less energy than fusion. • Reaction:

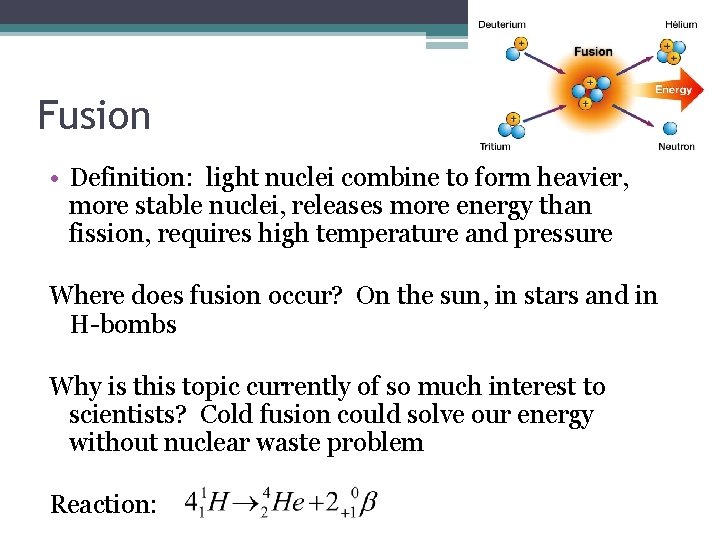
Fusion • Definition: light nuclei combine to form heavier, more stable nuclei, releases more energy than fission, requires high temperature and pressure Where does fusion occur? On the sun, in stars and in H-bombs Why is this topic currently of so much interest to scientists? Cold fusion could solve our energy without nuclear waste problem Reaction:

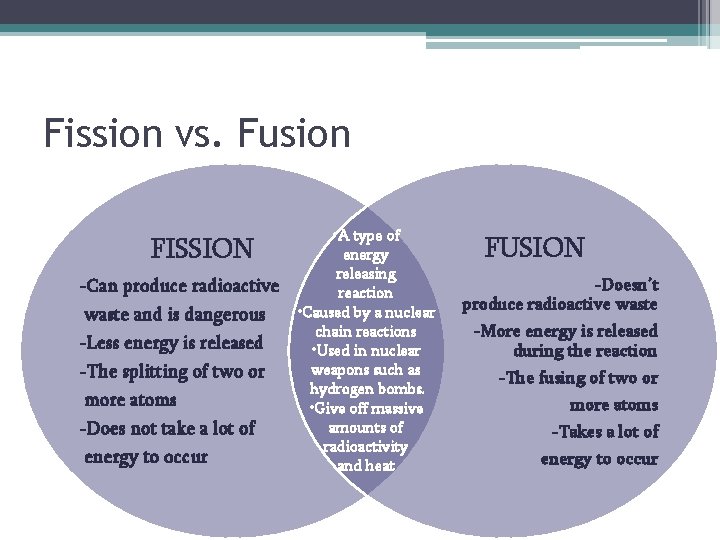
Fission vs. Fusion FISSION -Can produce radioactive waste and is dangerous -Less energy is released -The splitting of two or more atoms -Does not take a lot of energy to occur • A type of energy releasing reaction • Caused by a nuclear chain reactions • Used in nuclear weapons such as hydrogen bombs. • Give off massive amounts of radioactivity and heat FUSION -Doesn’t produce radioactive waste -More energy is released during the reaction -The fusing of two or more atoms -Takes a lot of energy to occur

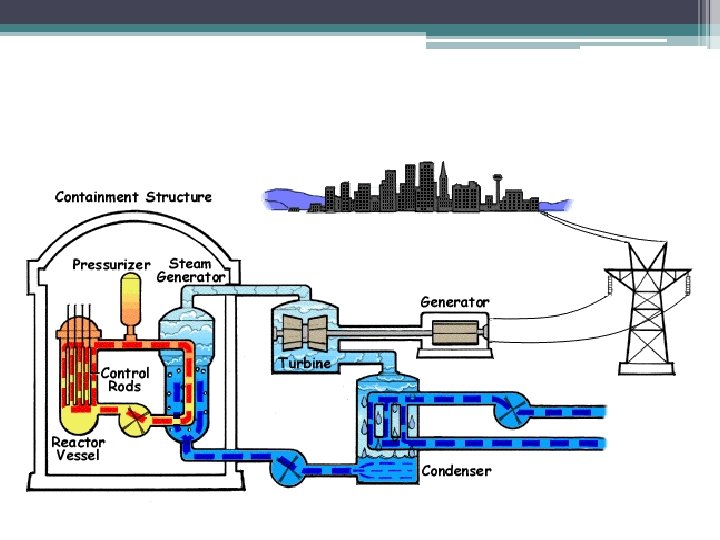
Parts of a nuclear reactor • Fuel: Usually uranium-235 or plutonium-239, used to convert fission to heat • Control Rods: absorbs neutrons to control the chain reaction; made of boron, cadmium, gadolinium • Moderator: increase efficiency of reaction by slowing down fast moving neutrons • Coolant: heated by fission drives turbine; made of water, liquid sodium, helium


Description of a nuclear power plant • Nuclear fuel, such as uranium-235, goes through fission to produce a lot of heat and energy. • This energy heats up the coolant (water), causing the water to boil. • The boiling water goes through the turbine causing it to rotate which generates electricity.

Review the particles Particle blocked by concrete Helium nucleus The same as an electron Particle blocked by paper/skin Particle with no mass or charge Heaviest particle Particle with the greatest charge

Review the particles Particle blocked by concrete Gamma Helium nucleus Alpha The same as an electron Beta Particle blocked by paper/skin Alpha Particle with no mass or charge Gamma Heaviest particle Alpha Particle with the greatest charge Alpha (+2)

Quiz Topics • • Balancing and Identifying Nuclear Reactions Half-Life calculation Alpha, beta, gamma radiation (facts about each) Fusion vs. Fission