Nuclear Chemistry Chemical Reactivity Normal Chemical Behavior Example

Nuclear Chemistry

Chemical Reactivity • • “Normal” Chemical Behavior • Example: 2 H 2 + O 2 2 H 2 O • “Atoms that go in must come out. ” • Nuclei of atoms remain the same. Nuclear Chemistry • • Resembles Alchemy “Turning Lead into Gold!” Nucleus changes as reactions occur Radioactivity

Nuclear Stability • Most stable nuclei have a 1: 1 neutron: proton ratio • As number of protons increase, stability decreases • All isotopes with an atomic number > 83 are radioactive. • Elements below 83 may have some isotopes that are radioactive, but not all of them will be. Ex: 14 C • Half-life gives an indication of stability of nucleus – see Table N.
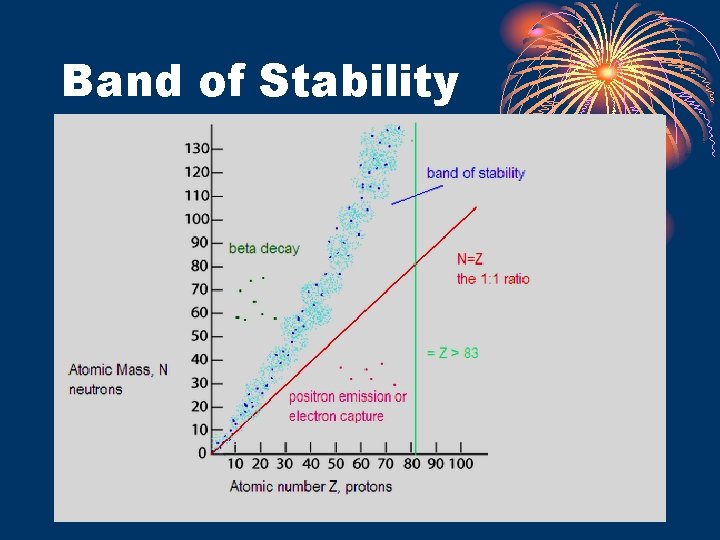
Band of Stability
Radioactivity • Radioactive elements decay spontaneously. • Nothing will stop the decay. • Decay = Transmutation • change of one element into another • change in atomic number • Not necessarily bad!
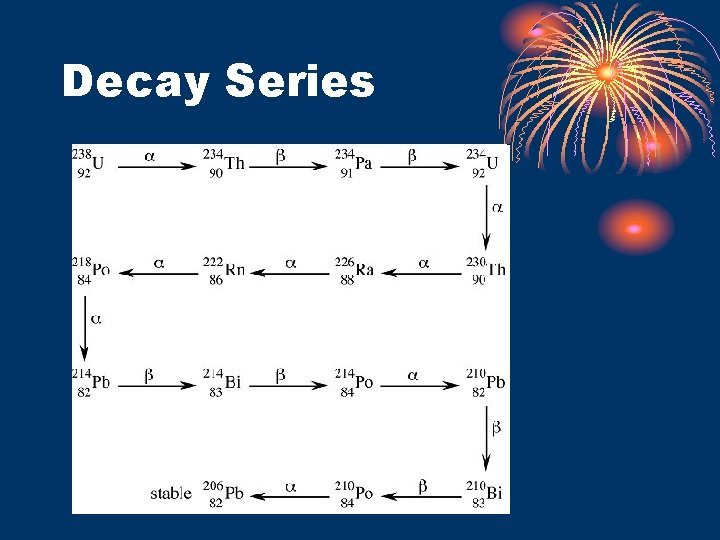
Decay Series
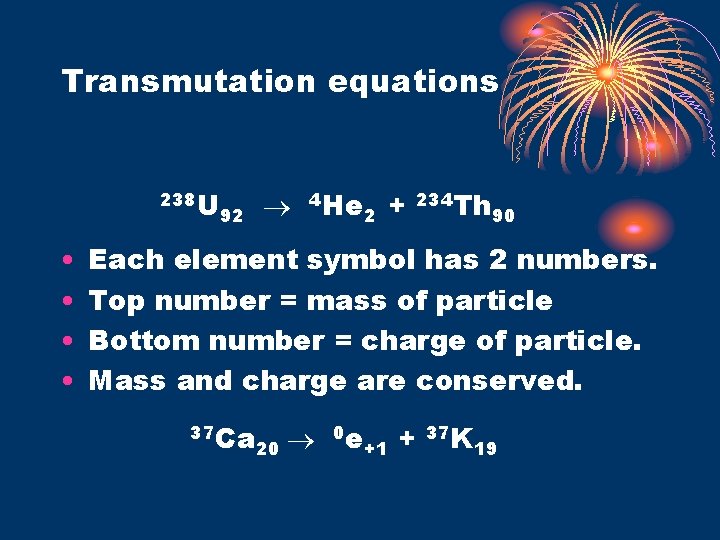
Transmutation equations 238 U • • 92 4 He 2 + 234 Th 90 Each element symbol has 2 numbers. Top number = mass of particle Bottom number = charge of particle. Mass and charge are conserved. 37 Ca 20 0 e +1 + 37 K 19
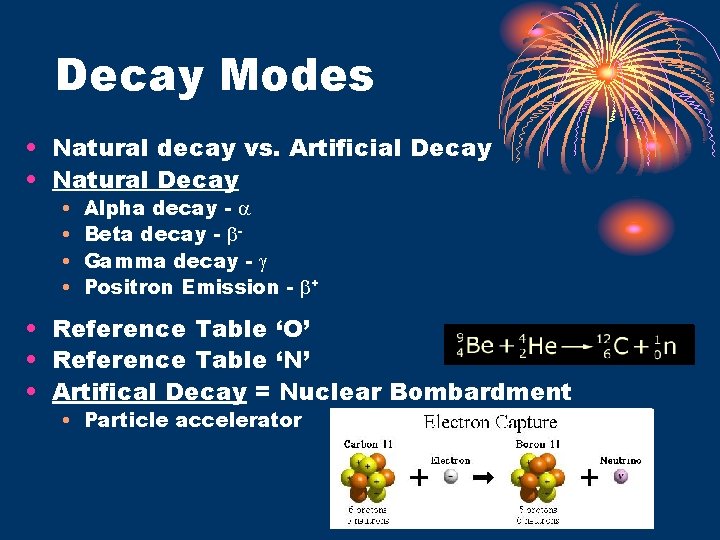
Decay Modes • Natural decay vs. Artificial Decay • Natural Decay • • Alpha decay - Beta decay - Gamma decay - Positron Emission - + • Reference Table ‘O’ • Reference Table ‘N’ • Artifical Decay = Nuclear Bombardment • Particle accelerator

Alpha decay • Alpha particle • • • nucleus of He atom : 4 He 2 or 4 2 • +2 charge – symbol on Ref. Table “O” Attracted to negative charges Slow moving – travels only approx. 6 in. Stopped by piece of paper Alpha Transmutation • natural • atomic number decreases by 2 • mass number deceases by 4 • Ex. 238 U 92 4 He 2 + 234 Th 90
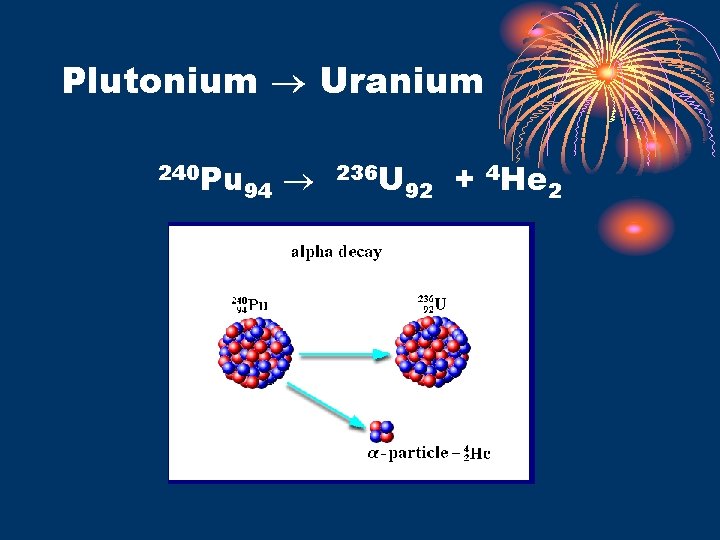
Plutonium Uranium 240 Pu 94 236 U 92 + 4 He 2

Beta Decay • Beta Particle • Beta = an electron = 0 e-1 • -1 charge, 0 mass • attracted to positive charges • WHAT? An electron in the nucleus? ? ? • Produced by “decomposition” of neutron into a proton and an electron: 1 n 0 1 p 1 + 0 e-1 • Faster moving than alpha (smaller), travels farther, stopped by Al foil or thick cardboard • Beta Transmutation • natural • atomic number increases by 1 • mass number remains the same • Ex. 239 U 92 0 e-1 + 239 Np 93
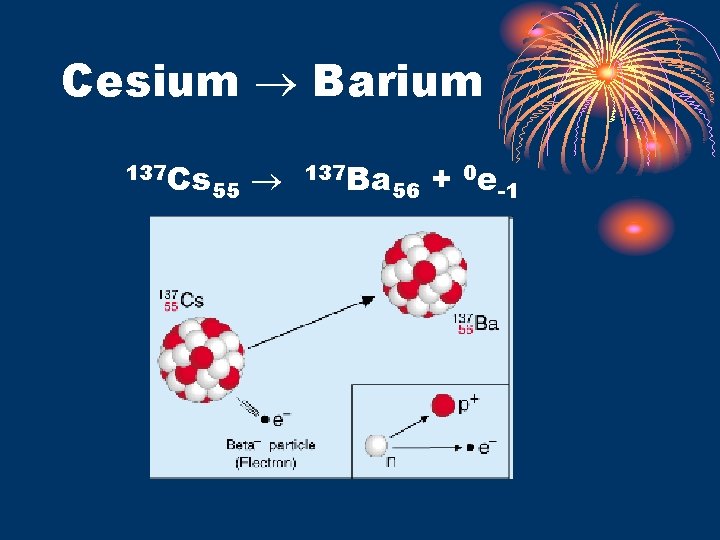
Cesium Barium 137 Cs 55 137 Ba 56 + 0 e-1

Gamma Rays • Energy only • no mass, charge, volume • like X-ray energy except more powerful – attracted to nothing • Travels though thick material • can be stopped by thick metal, Pb, concrete • Travels at speed of light • Symbol – Ref. Table “O”
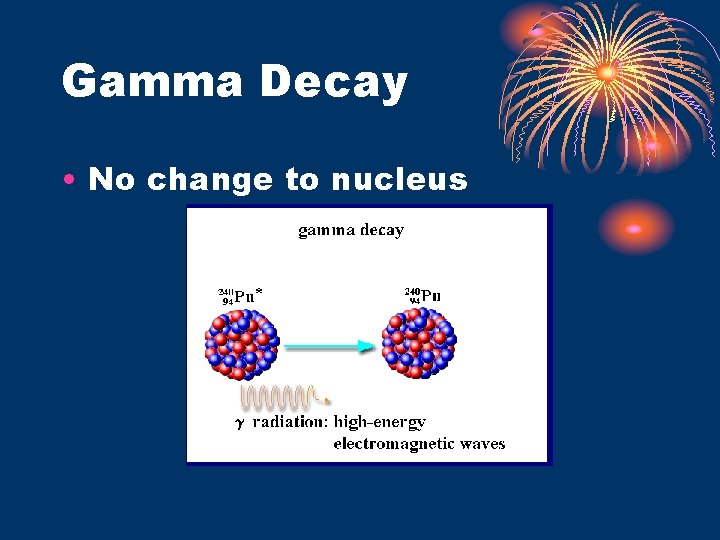
Gamma Decay • No change to nucleus
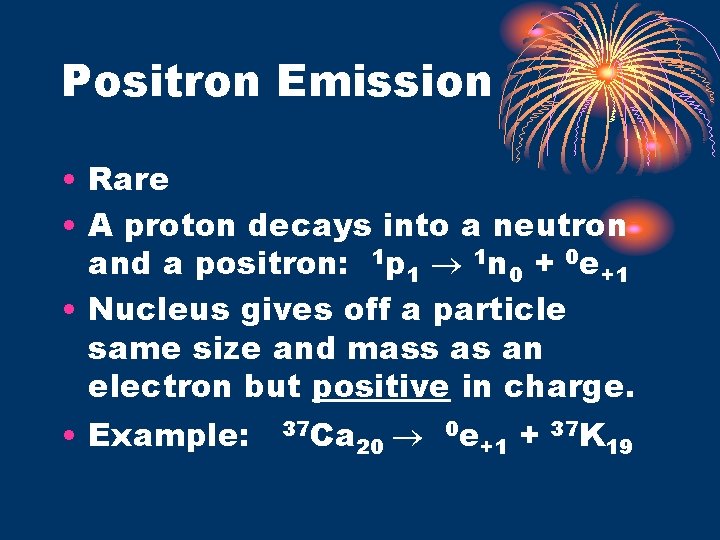
Positron Emission • Rare • A proton decays into a neutron and a positron: 1 p 1 1 n 0 + 0 e+1 • Nucleus gives off a particle same size and mass as an electron but positive in charge. • Example: 37 Ca 20 0 e +1 + 37 K 19
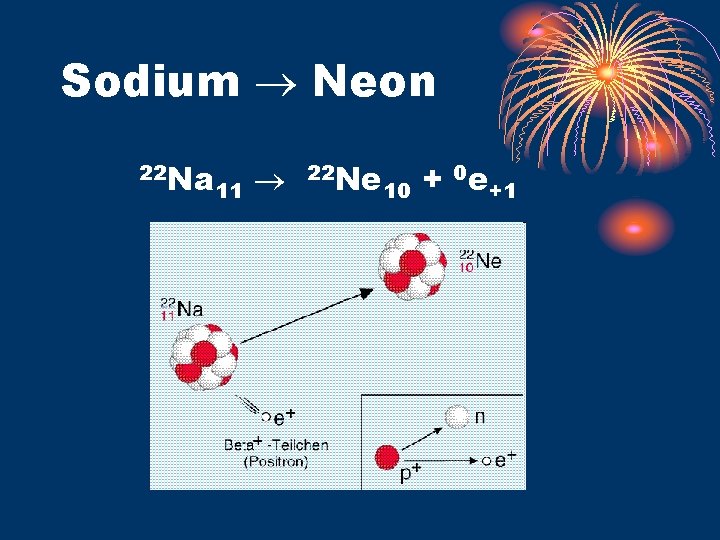
Sodium Neon 22 Na 11 22 Ne 10 + 0 e+1
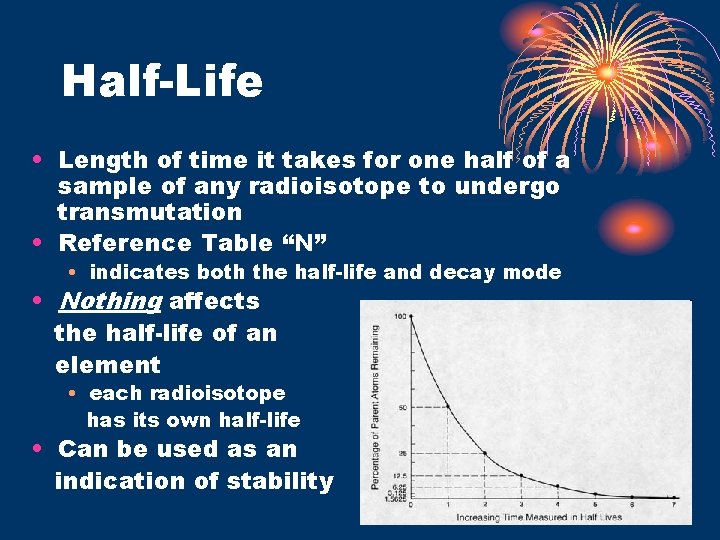
Half-Life • Length of time it takes for one half of a sample of any radioisotope to undergo transmutation • Reference Table “N” • indicates both the half-life and decay mode • Nothing affects the half-life of an element • each radioisotope has its own half-life • Can be used as an indication of stability

Nuclear Fission • In a fission reaction, an atom absorbs a neutron and splits into two or more smaller atoms and energy 235 U 92 + 1 n 0 141 Ba 56 + 92 Kr 36 + 3 1 n 0 + energy • The reaction is an uncontrolled chain reaction if it occurs as an atomic bomb • The energy produced can be explained using Einstein’s equation: E=mc 2 (next slide) • Other elements capable of fission include and 233 U. 239 Pu
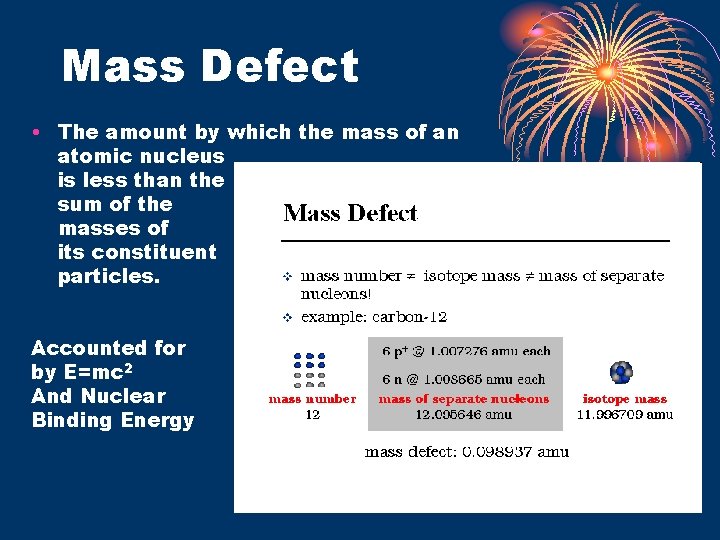
Mass Defect • The amount by which the mass of an atomic nucleus is less than the sum of the masses of its constituent particles. Accounted for by E=mc 2 And Nuclear Binding Energy
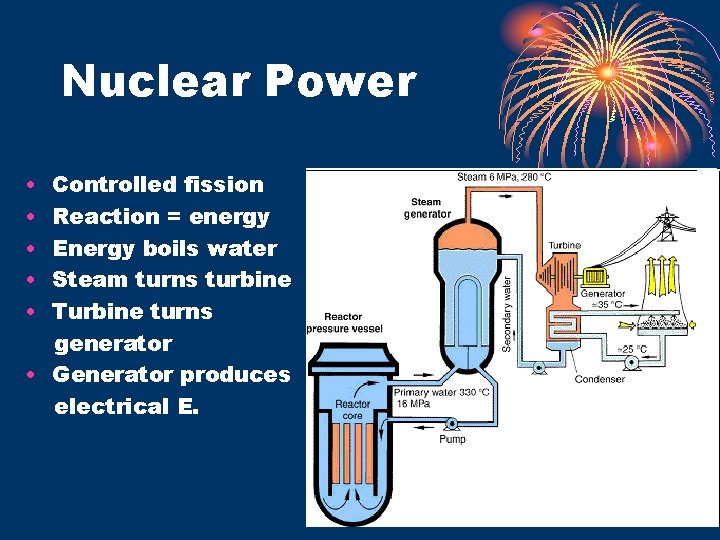
Nuclear Power • • • Controlled fission Reaction = energy Energy boils water Steam turns turbine Turbine turns generator • Generator produces electrical E.

Other details… • Plant Operation: • Fuel • U 235 or Pu 239 • Moderator – slows neutrons • water, heavy water, graphite • Control rods – absorb neutrons • boron &/or cadmium) • used to stop or start reaction • Coolant – control temperature • water, heavy water, liquid sodium • Shielding – contain radiation • reinforced concrete, steel, water

Nuclear Power • Benefits: • large quantities of energy from small masses; • no air pollution; • low operation cost • Negatives: • • residual radioactive waste; high initial cost; potential thermal pollution; release of radiation from accidents Chernobyl, Three Mile Island, Japan) (ex.

Fusion • Two nuclei unite • under tremendous heat and pressure • forms a heavier nucleus • the difference in attraction needed for new atom is released as energy • 2 H • • + 2 H 1 4 He 2 + energy More powerful than fission Ex. - the sun, hydrogen bomb Uncontrollable with present technology Ultimate source of energy 1

Uses of Radioisotopes • Tracers – ex: C-14 • follow chemical or biological reactions • Medical – ex: Tc-99, I-131, Ra, Co-60 • detection/treatment of diseases • Food storage – • destroy bacteria, yeast, mold • permits storage w/o refrigeration for longer periods • Radioactive dating - • U-238 to Pb-206, C-14 • Industrial
- Slides: 24