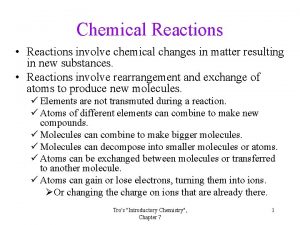
Nuclear Chemistry Chemical reactions involve changes with electrons




- Slides: 32

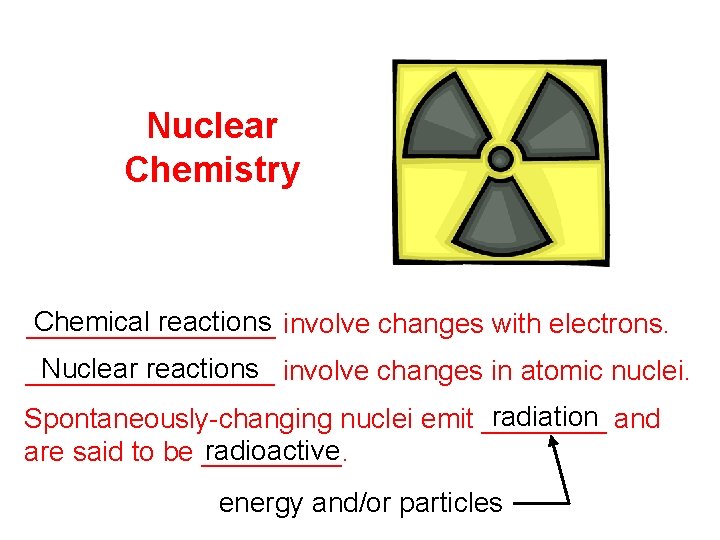
Nuclear Chemistry Chemical reactions involve changes with electrons. ________ Nuclear reactions involve changes in atomic nuclei. ________ radiation and Spontaneously-changing nuclei emit ____ radioactive are said to be _____. energy and/or particles

Radioactivity nucleons: protons (p+) and neutrons (n 0) mass number: (p+ + n 0) in a given atom isotopes: atoms having the same number of p+, but different numbers of n 0 radioisotopes -- radioactive ones are called ______ nuclide: a nucleus w/a specified number of p+ and n 0 radionuclides -- radioactive ones are called ______ atomic number: (Z); # of p+ these are unstable and emit radiation

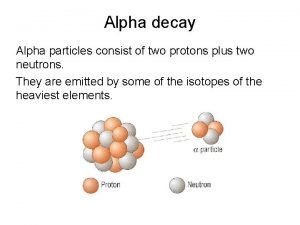
Radioactive Decay For nuclear equations, mass (top) and charge (bottom) must balance. alpha (a) decay: (go DOWN two #s on Table) 234 92 U 230 4 90 Th + 2 He 4 or 2 a a-particle (i. e. , a He nucleus): massive, slow-moving; stopped by skin

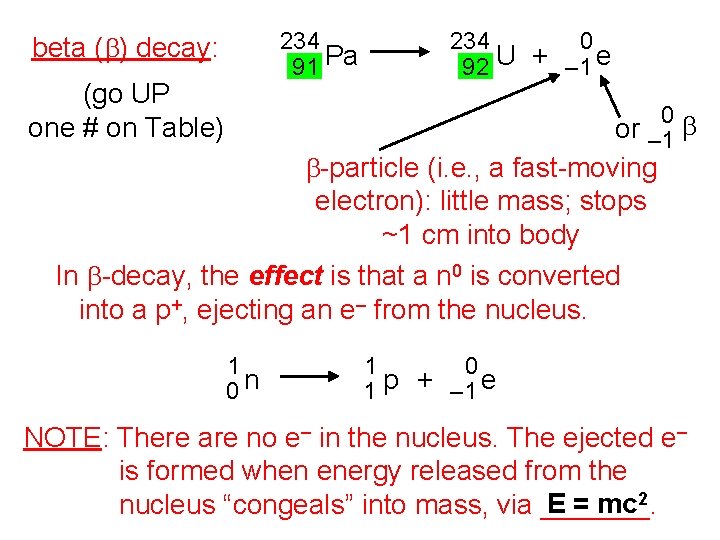
234 91 Pa beta (b) decay: (go UP one # on Table) 234 0 92 U + – 1 e 0 or – 1 b b-particle (i. e. , a fast-moving electron): little mass; stops ~1 cm into body In b-decay, the effect is that a n 0 is converted into a p+, ejecting an e– from the nucleus. 1 0 n 1 0 1 p + – 1 e NOTE: There are no e– in the nucleus. The ejected e– is formed when energy released from the E = mc 2 nucleus “congeals” into mass, via _______.

gamma radiation: consists of high-energy photons -- can penetrate to internal organs -- gamma ray: 0 g (or just g) 0 emitted when nucleons rearrange into a more stable configuration -- gamma radiation often accompanies other nuclear decays 234 92 U 230 4 0 90 Th + 2 He + 2 0 g

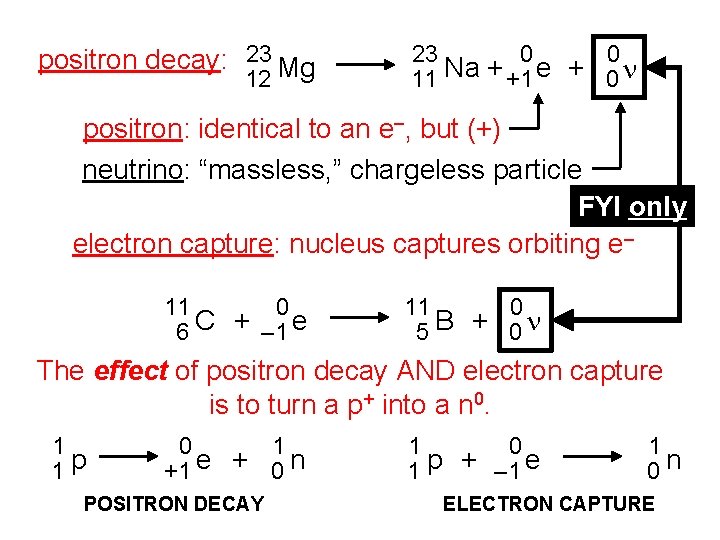
positron decay: 23 Mg 12 23 0 0 11 Na + +1 e + 0 n positron: identical to an e–, but (+) neutrino: “massless, ” chargeless particle FYI only electron capture: nucleus captures orbiting e– 11 0 6 C + – 1 e 11 0 5 B + 0 n The effect of positron decay AND electron capture is to turn a p+ into a n 0. 1 1 p 0 1 +1 e + 0 n POSITRON DECAY 1 0 1 p + – 1 e 1 0 n ELECTRON CAPTURE

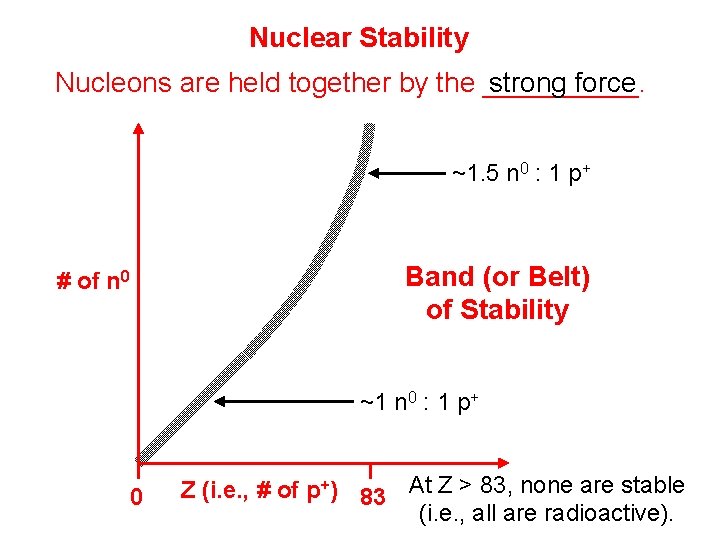
Nuclear Stability strong force Nucleons are held together by the _____. ~1. 5 n 0 : 1 p+ Band (or Belt) of Stability # of n 0 ~1 n 0 : 1 p+ 0 Z (i. e. , # of p+) 83 At Z > 83, none are stable (i. e. , all are radioactive).

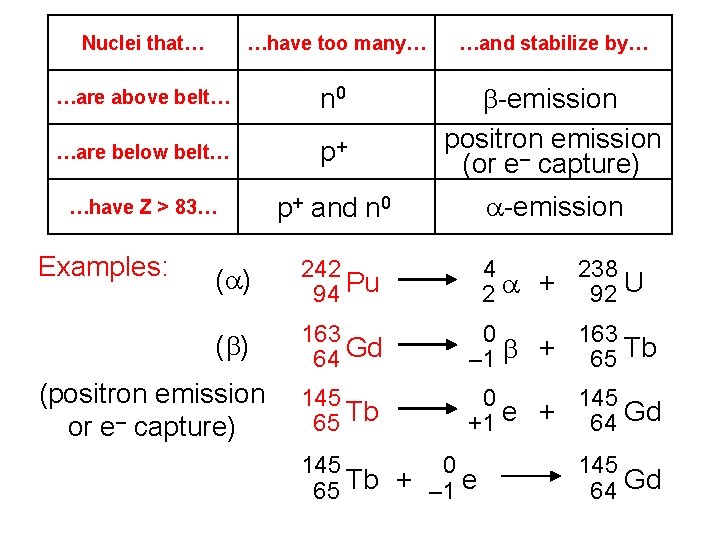
Nuclei that… …have too many… …and stabilize by… …are above belt… n 0 …are below belt… p+ b-emission positron emission (or e– capture) …have Z > 83… p+ and n 0 a-emission Examples: (a) 242 94 Pu 4 238 2 a + 92 U (b) 163 64 Gd 0 163 – 1 b + 65 Tb 145 65 Tb 0 145 +1 e + 64 Gd (positron emission or e– capture) 145 0 65 Tb + – 1 e 145 64 Gd

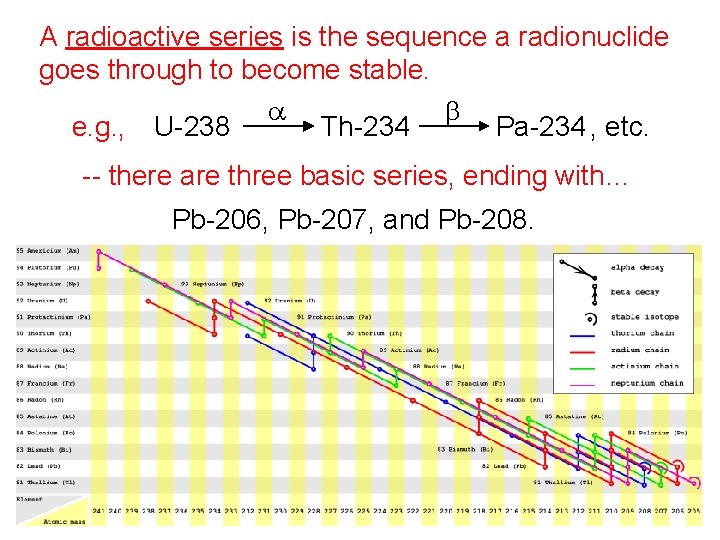
A radioactive series is the sequence a radionuclide goes through to become stable. a b e. g. , U-238 Th-234 Pa-234 , etc. -- there are three basic series, ending with… Pb-206, Pb-207, and Pb-208.

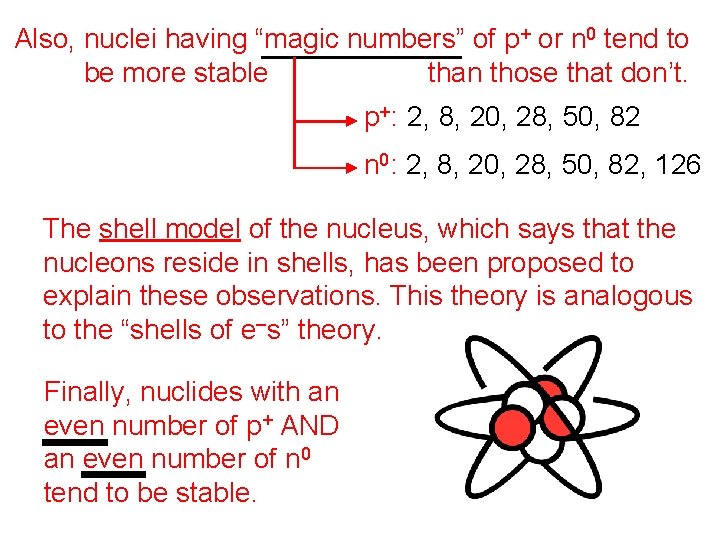
Also, nuclei having “magic numbers” of p+ or n 0 tend to be more stable than those that don’t. p+: 2, 8, 20, 28, 50, 82 n 0: 2, 8, 20, 28, 50, 82, 126 The shell model of the nucleus, which says that the nucleons reside in shells, has been proposed to explain these observations. This theory is analogous to the “shells of e–s” theory. Finally, nuclides with an even number of p+ AND an even number of n 0 tend to be stable.

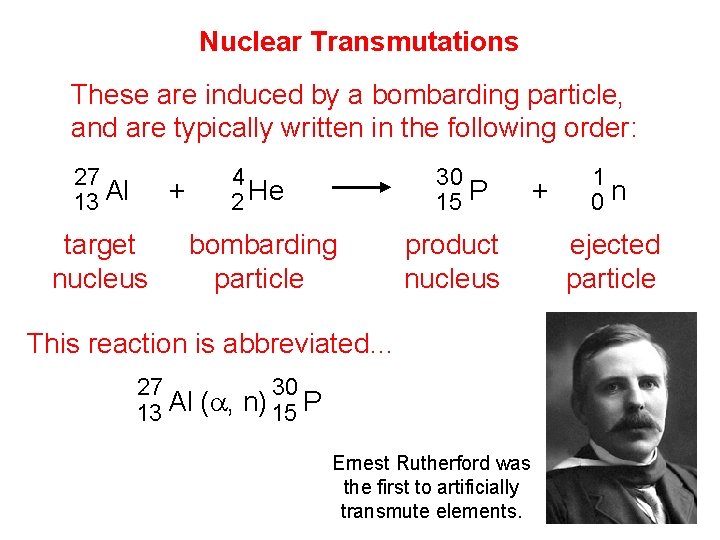
Nuclear Transmutations These are induced by a bombarding particle, and are typically written in the following order: 27 13 Al + target nucleus 4 2 He 30 15 P bombarding particle product nucleus This reaction is abbreviated… 27 30 13 Al (a, n) 15 P Ernest Rutherford was the first to artificially transmute elements. + 1 0 n ejected particle

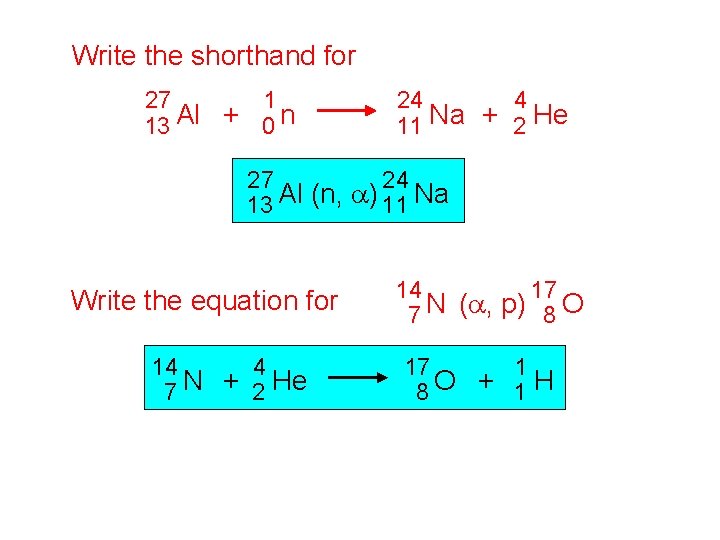
Write the shorthand for 27 1 Al + 13 0 n 24 4 Na + 11 2 He 27 24 13 Al (n, a) 11 Na Write the equation for 14 4 7 N + 2 He 14 17 7 N (a, p) 8 O 17 1 8 O + 1 H

Particle accelerators are used to accelerate charged particles (e. g. , a). We cannot accelerate neutrons, but we can use n 0 emitters to produce artificial isotopes. Often, b-emission Inside the particle accelerator at CERN (originally, Conseil Européen 0 follows n absorption. pour la Recherche Nucléaire, now Organisation Européenne pour la Recherche Nucléaire) For example: First… then… 238 92 U 239 92 U and then… 239 Np 93 1 + 0 n 239 92 U 239 0 93 Np + – 1 e 239 0 94 Pu + – 1 e

Rates of Radioactive Decay Each radioisotope has a unique rate of decay, its half-life, t 1/2, which is the time required for half of a sample of a radioisotope to decay into something stable. An isotope’s half-life is independent of… T, P, and its state of chemical combination. “Otzi” the Iceman lived circa 3300 B. C. , according to radiocarbon dating analyses.

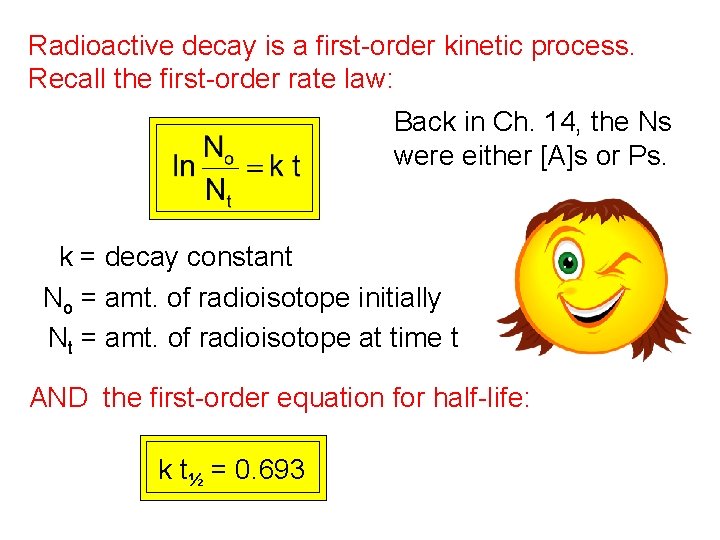
Radioactive decay is a first-order kinetic process. Recall the first-order rate law: Back in Ch. 14, the Ns were either [A]s or Ps. k = decay constant No = amt. of radioisotope initially Nt = amt. of radioisotope at time t AND the first-order equation for half-life: k t½ = 0. 693

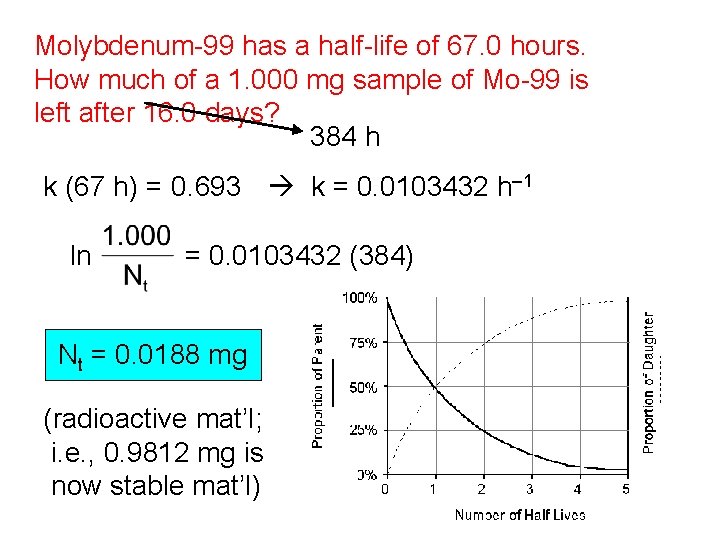
Molybdenum-99 has a half-life of 67. 0 hours. How much of a 1. 000 mg sample of Mo-99 is left after 16. 0 days? 384 h k (67 h) = 0. 693 k = 0. 0103432 h– 1 ln = 0. 0103432 (384) Nt = 0. 0188 mg (radioactive mat’l; i. e. , 0. 9812 mg is now stable mat’l)

Detection of Radioactivity photographic film (film badges): cheap, “ballpark quantitative” Geiger counter: ionization of gas produces a measurable electric current scintillation counter: radiation causes phosphors to glow; flashes counted electronically radiotracer: radioisotopes are monitored during chemical reactions -- all isotopes of an element behave the same… chemically

Nuclear Reactors -- fuel is… ~3% U-235 (natural U is ~0. 7% U-235) -- control rods of B or Cd… absorb n 0, slow down the reaction -- moderator: slows down n 0 to cause fission reactors in former USSR: graphite in the rest of the world: water WHY? low-molar-mass materials -- water is heated to steam, which spins electricalgenerating turbines control room at a nuclear power plant

Energy Changes in Nuclear Reactions Energy and mass are two sides of the same coin. c = 3. 00 x 108 m/s E = mc 2 m = mass, in kg E = energy, in J When a system loses/gains energy, it loses/gains mass. In chemical reactions, this mass change is nearly undetectable, so we speak of mass as being “conserved, ” when it really isn’t. The amount of “mass-and-energy-together, ” however, IS conserved. Mass changes in nuclear reactions are much larger than in chemical reactions, and are easily measured. All spontaneous nuclear reactions are exothermic.

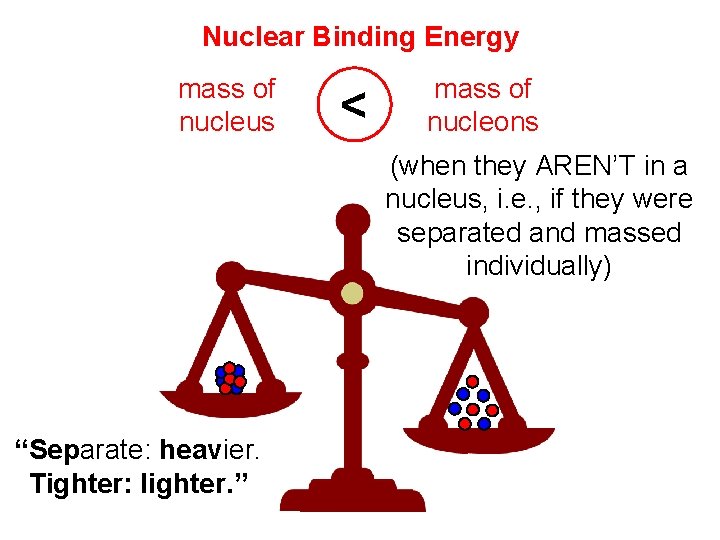
Nuclear Binding Energy mass of nucleus < mass of nucleons (when they AREN’T in a nucleus, i. e. , if they were separated and massed individually) “Separate: heavier. Tighter: lighter. ”

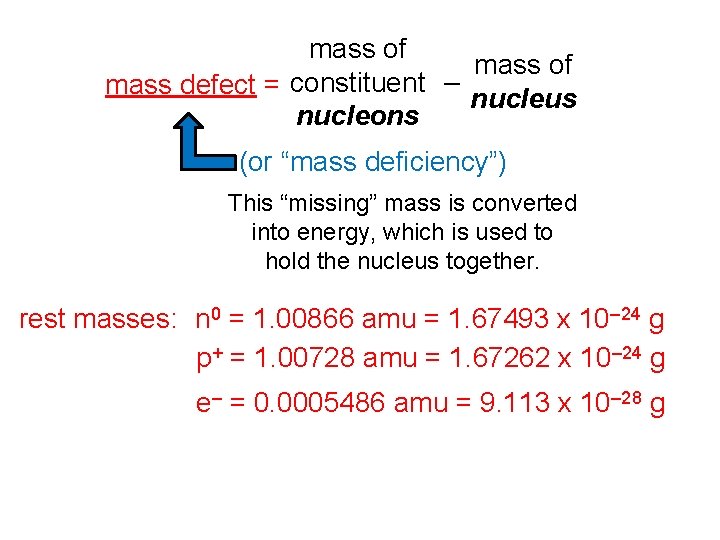
mass of mass defect = constituent – nucleus nucleons (or “mass deficiency”) This “missing” mass is converted into energy, which is used to hold the nucleus together. rest masses: n 0 = 1. 00866 amu = 1. 67493 x 10– 24 g p+ = 1. 00728 amu = 1. 67262 x 10– 24 g e– = 0. 0005486 amu = 9. 113 x 10– 28 g

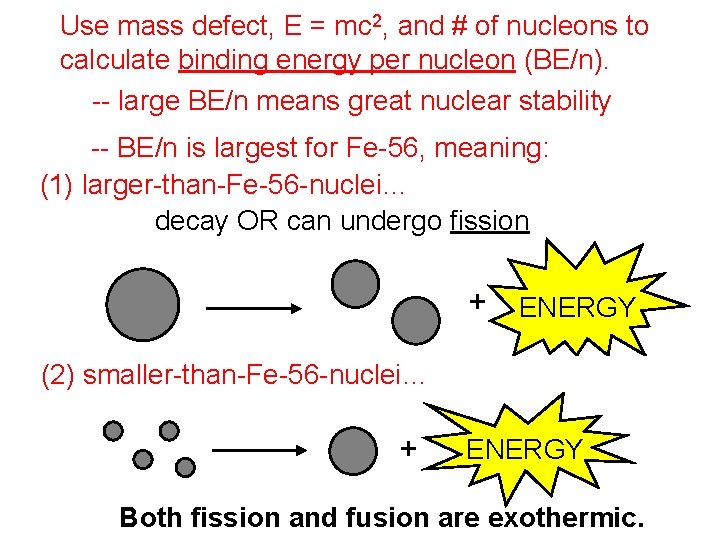
Use mass defect, E = mc 2, and # of nucleons to calculate binding energy per nucleon (BE/n). -- large BE/n means great nuclear stability -- BE/n is largest for Fe-56, meaning: (1) larger-than-Fe-56 -nuclei… decay OR can undergo fission + ENERGY (2) smaller-than-Fe-56 -nuclei… can undergo fusion + ENERGY Both fission and fusion are exothermic.

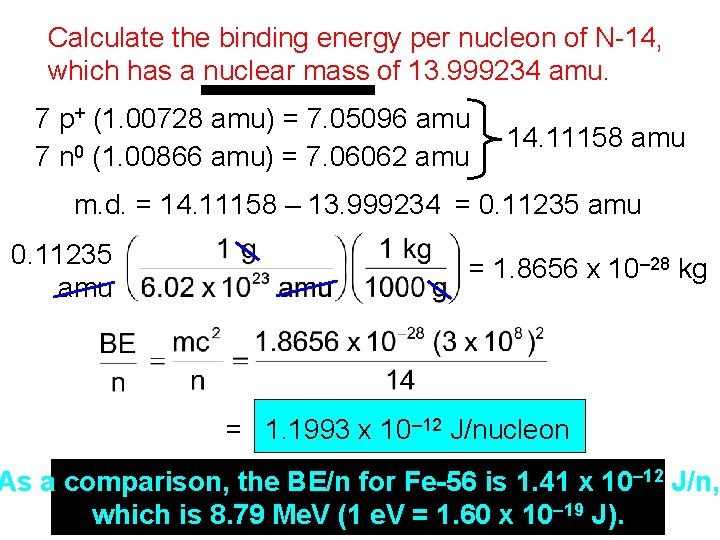
Calculate the binding energy per nucleon of N-14, which has a nuclear mass of 13. 999234 amu. 7 p+ (1. 00728 amu) = 7. 05096 amu 7 n 0 (1. 00866 amu) = 7. 06062 amu 14. 11158 amu m. d. = 14. 11158 – 13. 999234 = 0. 11235 amu = 1. 8656 x 10– 28 kg = 1. 1993 x 10– 12 J/nucleon As a comparison, the BE/n for Fe-56 is 1. 41 x 10– 12 J/n, which is 8. 79 Me. V (1 e. V = 1. 60 x 10– 19 J).

Nuclear Fission requires… slow-moving neutrons. distance too big; strong force weakens; +/+ repulsion takes over slow fast nn 00 released n 0; free to split more nuclei Important fissionable nuclei: U-233, U-235, Pu-239 chain reaction: one nuclear reaction leads to one or more others

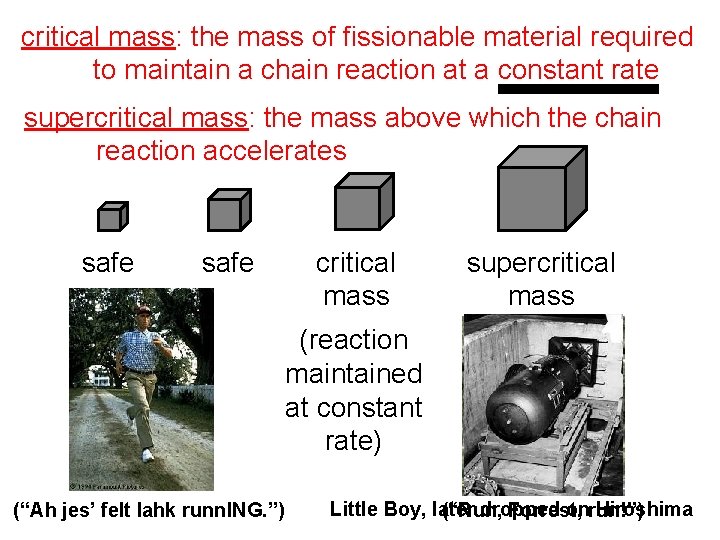
critical mass: the mass of fissionable material required to maintain a chain reaction at a constant rate supercritical mass: the mass above which the chain reaction accelerates safe critical mass supercritical mass (reaction maintained at constant rate) (“Ah jes’ felt lahk runn. ING. ”) Little Boy, later dropped onrun!”) Hiroshima (“Run, Forrest,

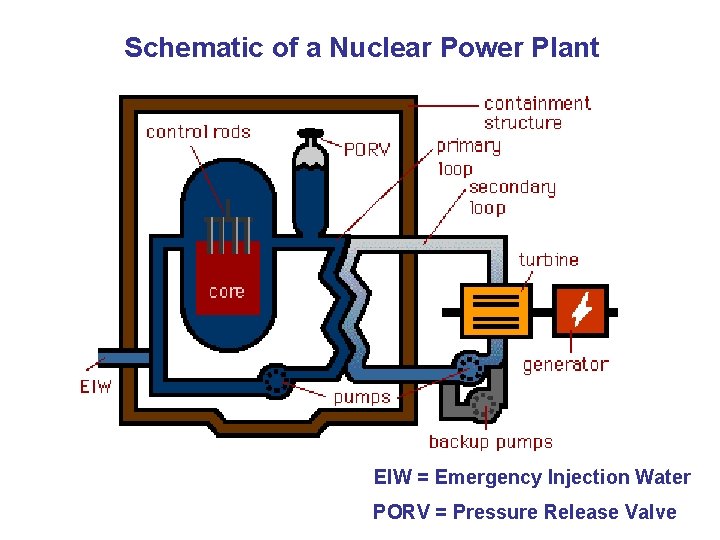
Schematic of a Nuclear Power Plant EIW = Emergency Injection Water PORV = Pressure Release Valve

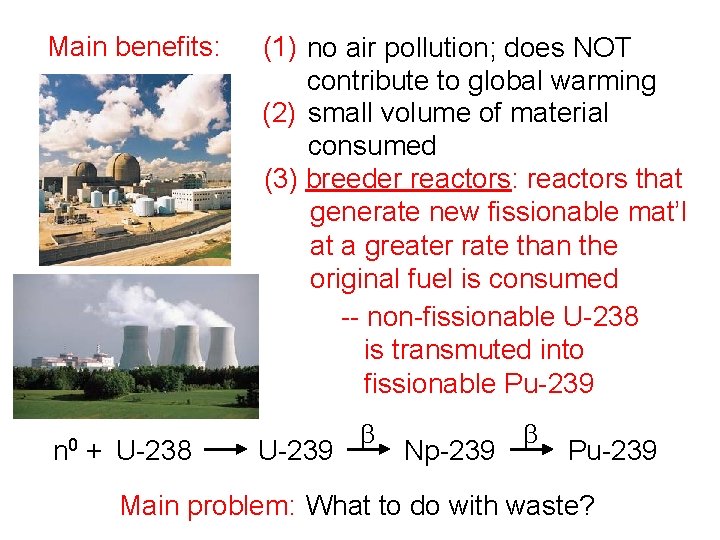
Main benefits: n 0 + U-238 (1) no air pollution; does NOT contribute to global warming (2) small volume of material consumed (3) breeder reactors: reactors that generate new fissionable mat’l at a greater rate than the original fuel is consumed -- non-fissionable U-238 is transmuted into fissionable Pu-239 U-239 b Np-239 b Pu-239 Main problem: What to do with waste?

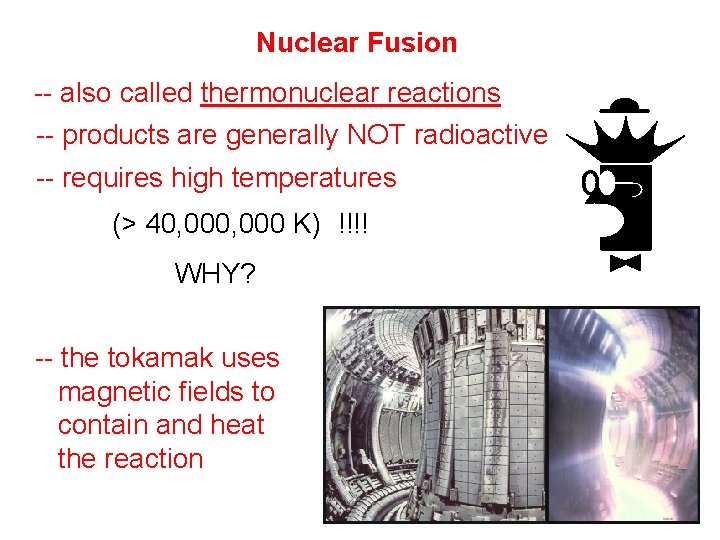
Nuclear Fusion -- also called thermonuclear reactions -- products are generally NOT radioactive -- requires high temperatures (> 40, 000 K) !!!! WHY? -- the tokamak uses magnetic fields to contain and heat the reaction

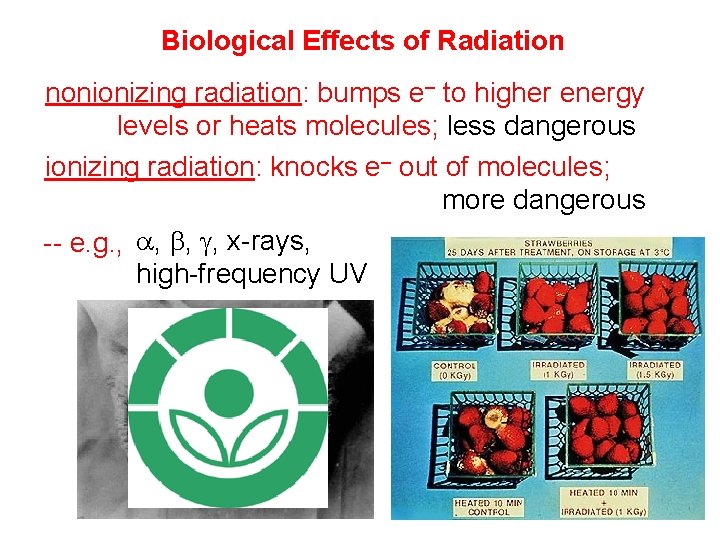
Biological Effects of Radiation nonionizing radiation: bumps e– to higher energy levels or heats molecules; less dangerous ionizing radiation: knocks e– out of molecules; more dangerous -- e. g. , a, b, g, x-rays, high-frequency UV

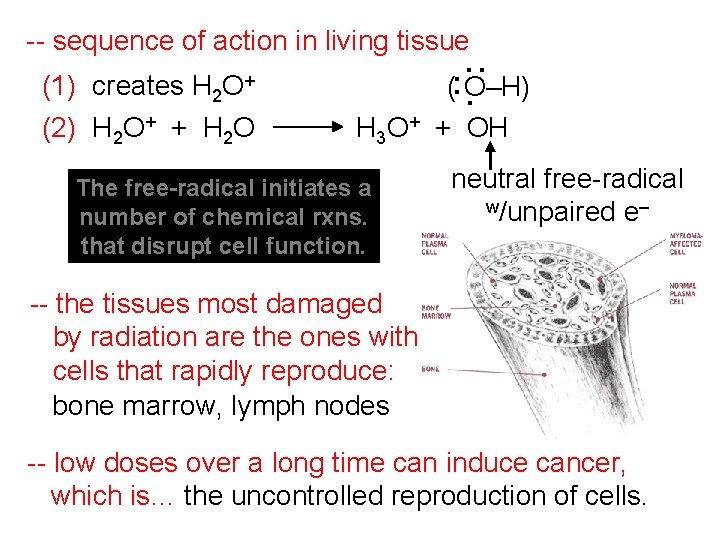
(1) creates H 2 O+ (2) H 2 O+ + H 2 O . : -- sequence of action in living tissue (: O–H) H 3 O+ + OH The free-radical initiates a number of chemical rxns. that disrupt cell function. neutral free-radical w/unpaired e– -- the tissues most damaged by radiation are the ones with cells that rapidly reproduce: bone marrow, lymph nodes -- low doses over a long time can induce cancer, which is… the uncontrolled reproduction of cells.

Units for Radiation Doses 1 bequerel (Bq) = 1 disintegration/sec 1 Curie (Ci) = 3. 7 x 1010 disintegrations/sec 1 gray (Gy) = absorbing 1 J/kg of tissue 1 rad = absorbing 1 x 10– 2 J/kg of tissue “radiation absorbed dose” Since the various types of radiation damage tissue with various degrees of efficiency, each type has its own relative biological effectiveness (RBE). photon or b RBE = 1 n 0 RBE = 10 a RBE = 20 “roentgen equivalent – man” rem = RBE x rad

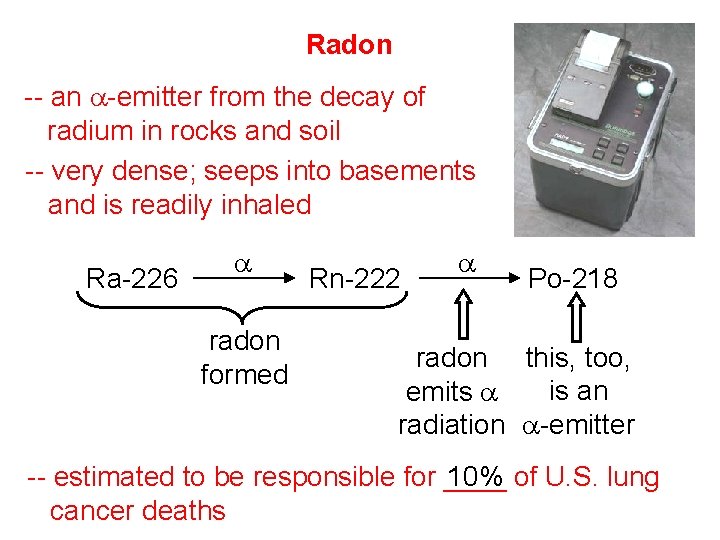
Radon -- an a-emitter from the decay of radium in rocks and soil -- very dense; seeps into basements and is readily inhaled Ra-226 a radon formed Rn-222 a Po-218 radon this, too, is an emits a radiation a-emitter -- estimated to be responsible for ____ 10% of U. S. lung cancer deaths
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 1 chemical changes
Section 1 chemical changes Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Types of reactions
Types of reactions What are the chemical change
What are the chemical change Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 Section 1 atoms elements and compounds
Section 1 atoms elements and compounds Chapter 6 section 1 atoms elements and compounds answer key
Chapter 6 section 1 atoms elements and compounds answer key Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Pixl knowit gcse chemistry chemical changes
Pixl knowit gcse chemistry chemical changes Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Nuclear decays and reactions section 2
Nuclear decays and reactions section 2 Two types of nuclear reactions
Two types of nuclear reactions Balancing nuclear reactions
Balancing nuclear reactions Balancing nuclear reactions
Balancing nuclear reactions Gamma decay nuclear equation
Gamma decay nuclear equation Flerov laboratory of nuclear reactions
Flerov laboratory of nuclear reactions Decay of carbon 14
Decay of carbon 14 Two types of nuclear reactions
Two types of nuclear reactions Nuclear bombardment equation
Nuclear bombardment equation Nuclear reactions are at
Nuclear reactions are at Key terms radioactivity and nuclear reactions
Key terms radioactivity and nuclear reactions Example of redox reaction
Example of redox reaction Lithium halflife
Lithium halflife Changes in latitudes, changes in attitudes meaning
Changes in latitudes, changes in attitudes meaning Chernobyl nuclear disaster webquest
Chernobyl nuclear disaster webquest Nuclear chemistry
Nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry Effective nuclear charge trend
Effective nuclear charge trend Chapter 24: nuclear chemistry answer key
Chapter 24: nuclear chemistry answer key