Nuclear Changes Objectives Students will Use the symbols

Nuclear Changes Objectives: Students will: • Use the symbols for alpha, beta, and gamma radiation, and compare the penetrating ability. • Write and analyze nuclear equations to balance and solve for unknowns.

Radioactivity

Radioactive Atoms �There about 2000 different isotopes that are known. �Of this number, only 279 are stable-they do not change over time. �All other isotopes are called radioisotopes, because the nucleus will decay (change) over time and release radiation-energy or particles. �This change is spontaneous-occurs without action/intervention. �Humans have learned to capitalize/manipulate nuclear changes and have developed many tools incorporating radioisotopes.

Discovery �Antoine Henri Becquerel- Accidental observation �fogging on photographic film plates in the presence of Uranium. �Paul & Marie Curie were able to show that it was the radioactive rays emitted by the Uranium atoms that caused the fogging. �We still use film badges to detect radiation.
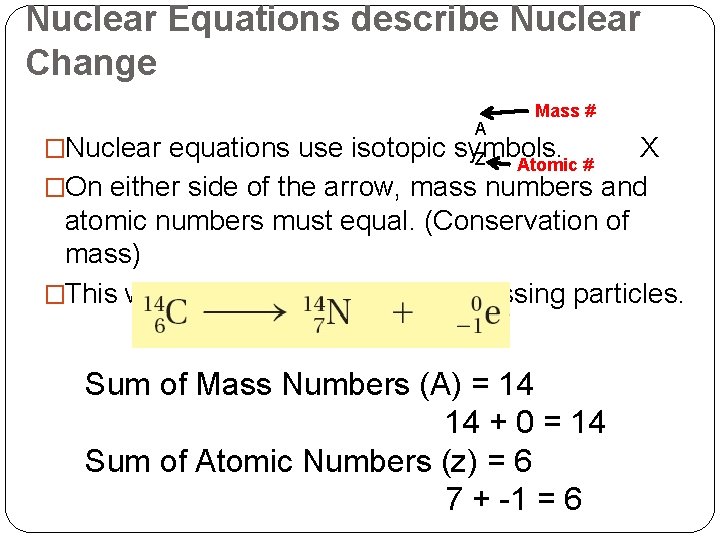
Nuclear Equations describe Nuclear Change A Mass # �Nuclear equations use isotopic symbols. Z X �On either side of the arrow, mass numbers and atomic numbers must equal. (Conservation of mass) �This will help you determine any missing particles. Atomic # Sum of Mass Numbers (A) = 14 14 + 0 = 14 Sum of Atomic Numbers (z) = 6 7 + -1 = 6

Types of Radioactive Decay: Alpha �Alpha (α) Particles, which is a Helium nucleus, are commonly emitted by heavy nuclides, and can be written in two ways: �Alpha particles have a charge of +2. �Ex: Parent Nuclide Radiation + Daughter Nuclide �Write the equation for when Thorium-230 undergoes alpha decay.

Types of Decay: Beta �A beta (β) particle is a negatively charged electron that formed when a neutron splits into a proton and an electron, and the electron is ejected from the nucleus. �It can be written two ways: �Remember, the mass of an electron is so small that it gets assigned a mass number of 0, so the net effect of β-particle production is to change a neutron to a proton: �Write the equation for Actinium-227 decay, a beta particle producer.

Types of Radiation: Gamma �A gamma (γ) ray is a high energy photon (packet) of electromagnetic radiation with no mass and no charge. �It can be written two ways: �Visible light is also composed of photons of electromagnetic radiation, but those photons contain significantly less energy than gamma photons.
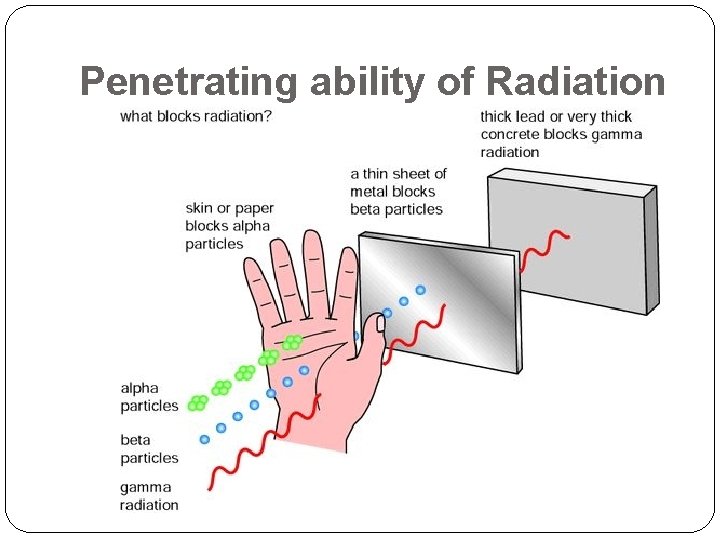
Penetrating ability of Radiation

Other useful nuclear particles/info �Proton: �Neutron: �If you see a number in front of a particle, it is a coefficient, which means multiple of that particle. �Distribute the coefficient to A and Z to solve.

Other useful information �Emission vs. Capture �Emission(Emit/Emitted): Indicates the particle is being released, and Right should be on the ________ side of the arrow in the equation. �Capture/Bombardment: Indicates the particle is being absorbed, and should Left be on the ________ side of the arrow in the equation.

Why Radioactive? �Since over 85% of isotopes are radioactive, it is helpful to identify some similarities. �All atoms over atomic # 83 are radioactive �There are simply too many protons/neutrons to be held together by the nuclear force, which holds particles together at extremely short distances. �Will undergo decay, sometimes many cycles, until they get to a smaller atom. �Ex: Uranium-238 eventually decays to Pb-206

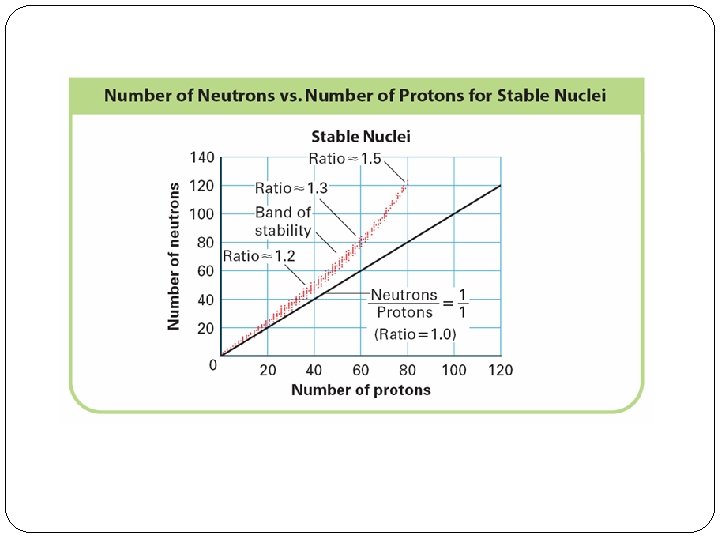
Why Radioactive? �The radioactivity of smaller atoms can be predicted based on the neutron to proton ratio in the nucleus. �Atomic Numbers 1 -20, a 1: 1 ratio is stable. �Atomic Numbers 21 -83, the band of stability approaches 1. 5: 1 �This is because the function of the neutron is to buffer the repulsion of the positive protons.

Nuclear Transformutations & Applications

History of Nuclear Transmutations: �The first nuclear transmutation, or change of one element into another, was observed in 1911 when Lord Rutherford bombarded Nitrogen-14 with an alpha particle to produce Oxygen-17 and a proton. �Write the equation for this transmutation. �The bombarding particle here is an alpha particle which is positively charged. �So that the alpha particle isn’t repelled by the nucleus, the bombarding particle must move at
Particle Accelerators allow the particles to achieve high speeds �Through neutron, proton, and alpha particle bombardment, scientist have been able to extend the periodic table. �Prior to 1940, the heaviest element known was Uranium. �In 1940, Neptunium-239 was produced by neutron bombardment of Uranium-238. �Since then, elements #93 & beyond have been synthesized. These are called the transuranium elements.

Alpha, Beta, and Gamma are ionizing radiation (so are other Electromagnetic waves) �Ionizing radiation carries enough energy to knock electrons off of atoms when they travel through matter, creating positive ions and lose electrons. �The familiar instrument for detecting radiation is a Geiger Counter. �Contains Argon Gas �Connected to a speaker which clicks every time a high speed particle knocks an electron off an Argon atom. �A scintillation counter uses a substance that gives off light when struck by a high-energy particle, and the detector senses the flashes of

Geiger Counter Scintillation Counter
Scintillation Counters are used to detect decay events… • An important characteristic of a radioisotope is its half-life, or the time required for half the original sample to decay. • For example, if a certain radioactive sample contains 1000 nuclei at a given time, and 500 nuclei 7. 5 days later, then the half-life of that isotope is 7. 5 days. • There is a broad spectrum of half-life times from milliseconds to millions or billions of years. • The length of the half-life, and the type of

Sample Problem #1 �Silicon-31 has a half-life of approximately 2. 5 hours. If we begin with a sample containing 1000. mg of Si 31, what is the amount remaining after 10 hours?
Sample Problem #2 �Carbon-14 emits beta radiation and decays with a half-life of 5730 years. Assume you begin with 2. 00 x 10 -12 g of C-14. �Write the equation for the beta decay of Carbon 14 �How long is three half lives? �How many grams are left at the end of 22920 years?
Sample Problem #3 �A 0. 456 -mg sample of Hydrogen-3 was collected. After 36. 78 years, 0. 114 -mg remains. �What is the half life of Hydrogen-3?
- Slides: 23