NRGLU 002 Puneeth Iyengar MD Ph D NRG










- Slides: 24

NRG-LU 002 Puneeth Iyengar, MD, Ph. D NRG Semiannual Meeting, Thoracic Committee July 17, 2020 @NRGOnc NRG Oncology

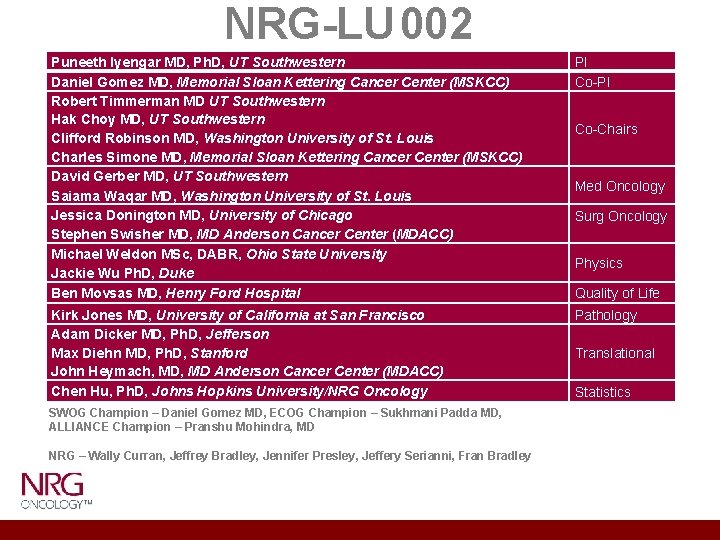
NRG-LU 002 Puneeth Iyengar MD, Ph. D, UT Southwestern Daniel Gomez MD, Memorial Sloan Kettering Cancer Center (MSKCC) Robert Timmerman MD UT Southwestern Hak Choy MD, UT Southwestern Clifford Robinson MD, Washington University of St. Louis Charles Simone MD, Memorial Sloan Kettering Cancer Center (MSKCC) David Gerber MD, UT Southwestern Saiama Waqar MD, Washington University of St. Louis Jessica Donington MD, University of Chicago Stephen Swisher MD, MD Anderson Cancer Center (MDACC) Michael Weldon MSc, DABR, Ohio State University Jackie Wu Ph. D, Duke Ben Movsas MD, Henry Ford Hospital PI Co-PI Kirk Jones MD, University of California at San Francisco Adam Dicker MD, Ph. D, Jefferson Max Diehn MD, Ph. D, Stanford John Heymach, MD Anderson Cancer Center (MDACC) Chen Hu, Ph. D, Johns Hopkins University/NRG Oncology Pathology SWOG Champion – Daniel Gomez MD, ECOG Champion – Sukhmani Padda MD, ALLIANCE Champion – Pranshu Mohindra, MD NRG – Wally Curran, Jeffrey Bradley, Jennifer Presley, Jeffery Serianni, Fran Bradley Co-Chairs Med Oncology Surg Oncology Physics Quality of Life Translational Statistics

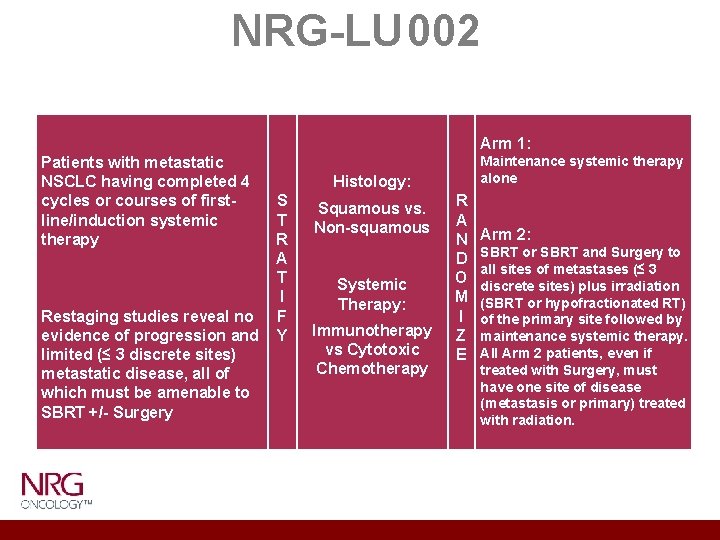
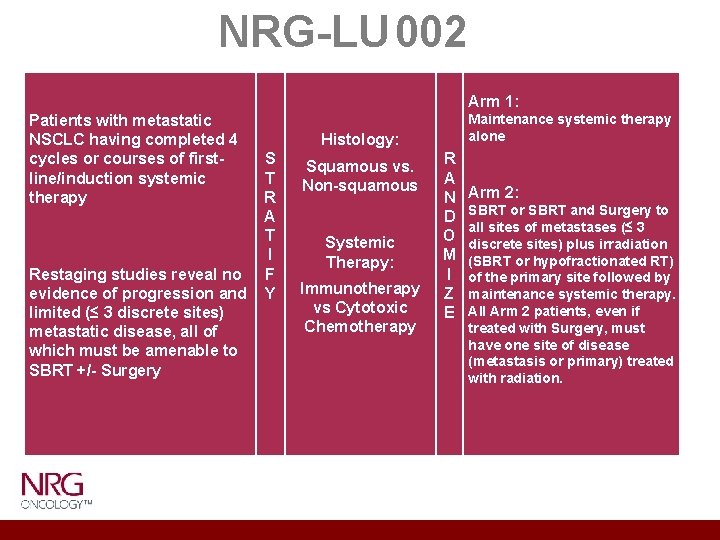
NRG-LU 002 Patients with metastatic NSCLC having completed 4 Histology: cycles or courses of first. S Squamous vs. line/induction systemic T Non-squamous therapy R A T Systemic I Therapy: Restaging studies reveal no F Immunotherapy evidence of progression and Y vs Cytotoxic limited (≤ 3 discrete sites) Chemotherapy metastatic disease, all of which must be amenable to SBRT +/- Surgery Arm 1: Maintenance systemic therapy alone R A N D O M I Z E Arm 2: SBRT or SBRT and Surgery to all sites of metastases (≤ 3 discrete sites) plus irradiation (SBRT or hypofractionated RT) of the primary site followed by maintenance systemic therapy. All Arm 2 patients, even if treated with Surgery, must have one site of disease (metastasis or primary) treated with radiation.

Limited Metastatic Disease ü Support for the benefits of aggressive local therapy in limited metastatic states was first derived from other primary cancers. ü Chemo/IO trials never describe limited versus diffuse disease distributions ü ¾ of patients with NSCLC respond to induction systemic therapy but often fail in original sites of gross disease as 1 st site of failure ü A significant majority of these sites could have been treated with SBRT (Rusthoven et al) / local therapy

Limited Metastatic Disease Data Suggest: 1. Metastases are not always widely disseminated 2. Metastases do not always progress in multiple sites 3. Patients with limited sites of metastases may not progress or progress only in sites of initial disease 4. Therefore there may be a role for local therapy in selected patients

Treatment in Stage IV NSCLC How do we currently treat stage IV NSCLC patients after 1 st line systemic therapy if they have had partial response/stable disease and limited sites of gross residual disease? Standard as of NRG-LU 002 activation: • Maintenance chemotherapy/immunotherapy • Observation with initiation of 2 nd line therapy at time of progression Possible new paradigm: • Local treatment (radiation and/or surgery) as part of 1 st line/ maintenance therapy

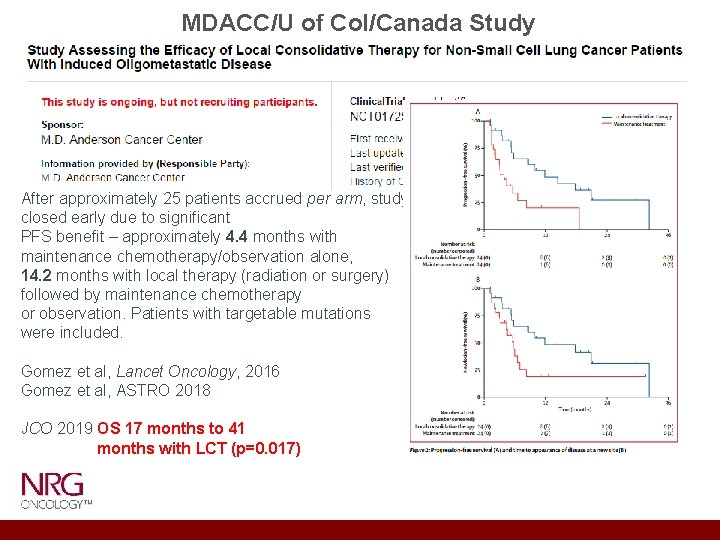
MDACC/U of Col/Canada Study After approximately 25 patients accrued per arm, study closed early due to significant PFS benefit – approximately 4. 4 months with maintenance chemotherapy/observation alone, 14. 2 months with local therapy (radiation or surgery) followed by maintenance chemotherapy or observation. Patients with targetable mutations were included. Gomez et al, Lancet Oncology, 2016 Gomez et al, ASTRO 2018 JCO 2019 OS 17 months to 41 months with LCT (p=0. 017)

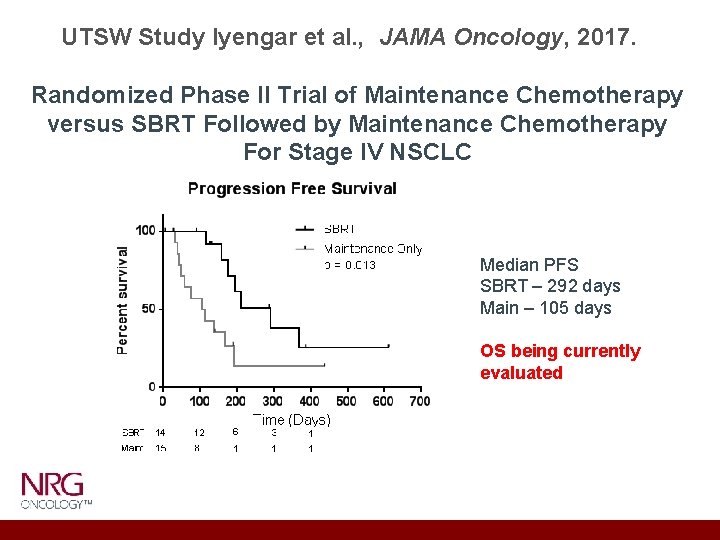
UTSW Study Iyengar et al. , JAMA Oncology, 2017. Randomized Phase II Trial of Maintenance Chemotherapy versus SBRT Followed by Maintenance Chemotherapy For Stage IV NSCLC Median PFS SBRT – 292 days Main – 105 days OS being currently evaluated

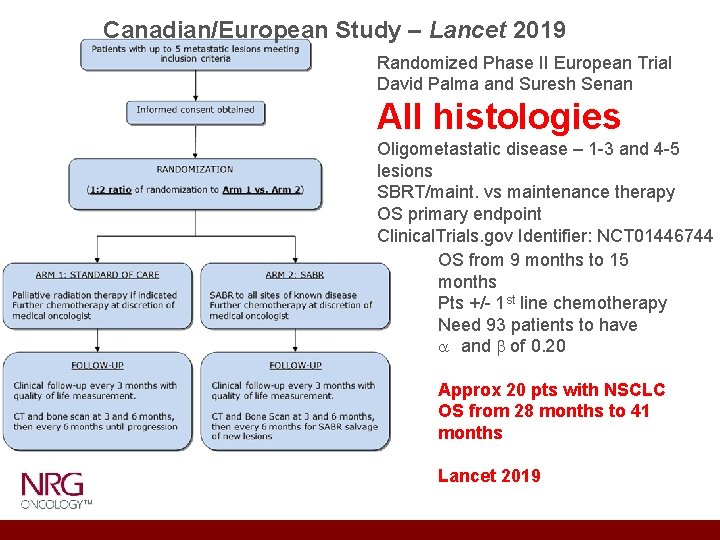
Canadian/European Study – Lancet 2019 Randomized Phase II European Trial David Palma and Suresh Senan All histologies Oligometastatic disease – 1 -3 and 4 -5 lesions SBRT/maint. vs maintenance therapy OS primary endpoint Clinical. Trials. gov Identifier: NCT 01446744 OS from 9 months to 15 months Pts +/- 1 st line chemotherapy Need 93 patients to have a and b of 0. 20 Approx 20 pts with NSCLC OS from 28 months to 41 months Lancet 2019

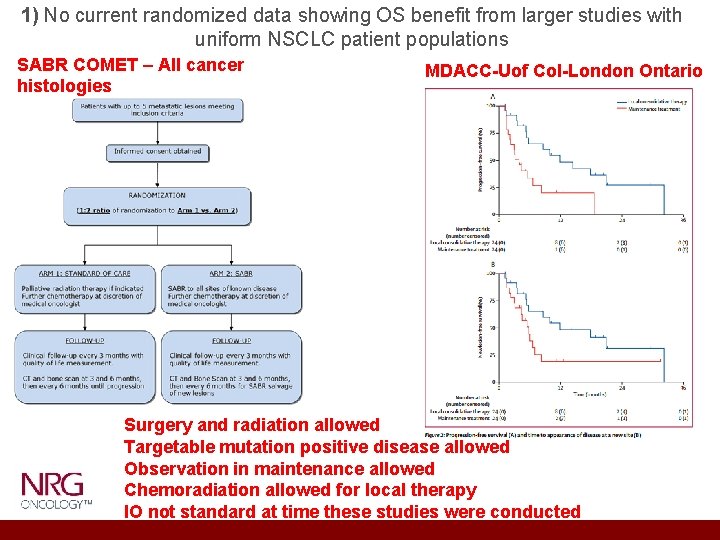
1) No current randomized data showing OS benefit from larger studies with uniform NSCLC patient populations SABR COMET – All cancer histologies MDACC-Uof Col-London Ontario Surgery and radiation allowed Targetable mutation positive disease allowed Observation in maintenance allowed Chemoradiation allowed for local therapy IO not standard at time these studies were conducted

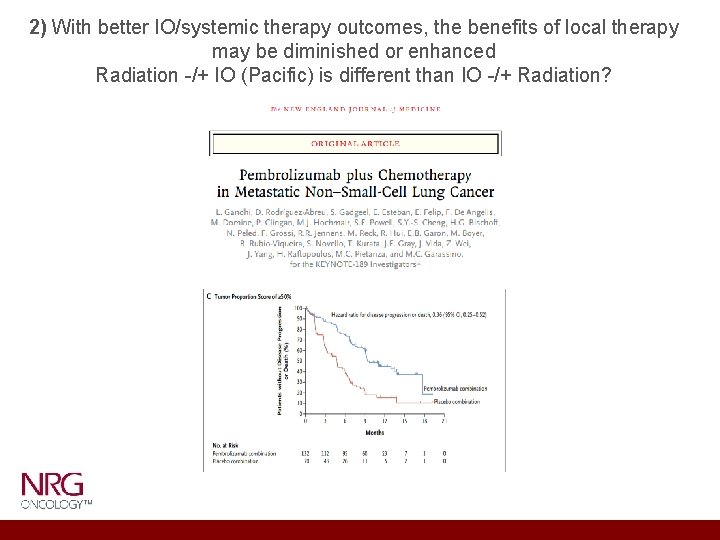
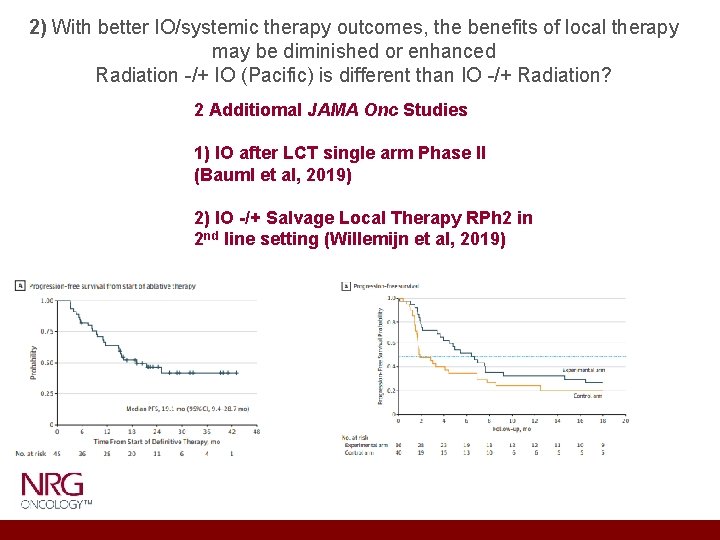
2) With better IO/systemic therapy outcomes, the benefits of local therapy may be diminished or enhanced Radiation -/+ IO (Pacific) is different than IO -/+ Radiation?

2) With better IO/systemic therapy outcomes, the benefits of local therapy may be diminished or enhanced Radiation -/+ IO (Pacific) is different than IO -/+ Radiation? 2 Additiomal JAMA Onc Studies 1) IO after LCT single arm Phase II (Bauml et al, 2019) 2) IO -/+ Salvage Local Therapy RPh 2 in 2 nd line setting (Willemijn et al, 2019)

NRG-LU 002 Patients with metastatic NSCLC having completed 4 Histology: cycles or courses of first. S Squamous vs. line/induction systemic T Non-squamous therapy R A T Systemic I Therapy: Restaging studies reveal no F Immunotherapy evidence of progression and Y vs Cytotoxic limited (≤ 3 discrete sites) Chemotherapy metastatic disease, all of which must be amenable to SBRT +/- Surgery Arm 1: Maintenance systemic therapy alone R A N D O M I Z E Arm 2: SBRT or SBRT and Surgery to all sites of metastases (≤ 3 discrete sites) plus irradiation (SBRT or hypofractionated RT) of the primary site followed by maintenance systemic therapy. All Arm 2 patients, even if treated with Surgery, must have one site of disease (metastasis or primary) treated with radiation.

NRG LU 002 PRIMARY OBJECTIVES • • Ph II: Evaluate impact on PFS of adding local consolidative therapy (LCT) to maintenance systemic therapy versus maintenance systemic therapy alone for patients with metastatic NSCLC → no evidence of progression/limited metastatic sites after first-line systemic therapy Ph III: Evaluate impact on OS of adding LCT to maintenance systemic therapy versus maintenance systemic therapy alone for patients with metastatic NSCLC → no evidence of progression/limited metastatic sites after first-line systemic therapy SECONDARY OBJECTIVES • • Evaluate effect on Quality of Life of adding LCT to systemic therapy in limited stage IV NSCLC Collect biospecimens → at registration, after local therapy, and at first recurrence

Eligibility Criteria NSCLC diagnosis → Metastatic presentation prior to registration - Synchronous or Metachronous Disease. Received 1 st line/induction systemic therapy (4 cycles/courses) → stable disease or partial response Permitted: • Prior systemic therapy as part of concurrent treatment approach for previously diagnosed stage III NSCLC, as adjuvant therapy for previously resected NSCLC or other previous cancers • Patients with brain metastases are eligible if these lesions previously have been treated and patient has no clinical or radiographic evidence of progression prior to enrollment Must have measurable disease at baseline and 3 or fewer discrete, extracranial metastatic disease sites that are technically amenable to LCT. As long as any disease is remaining after induction that is treatable with radiation, primary and/or metastasis, the patient is eligible for study.

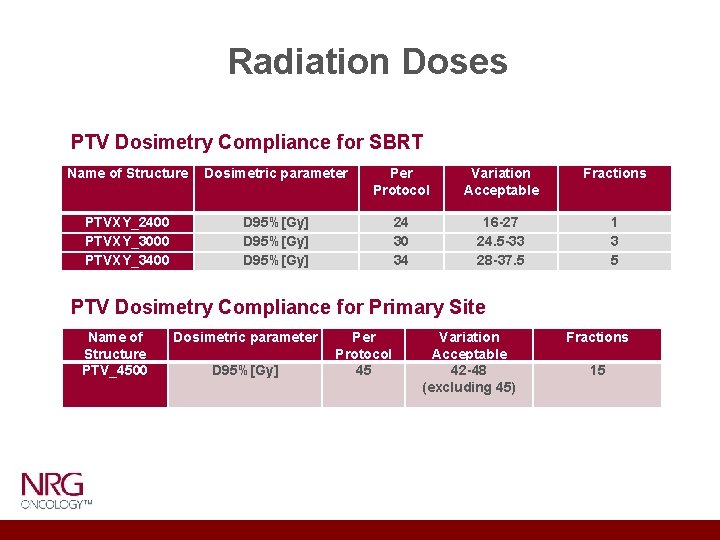
Radiation Doses PTV Dosimetry Compliance for SBRT Name of Structure Dosimetric parameter Protocol Variation Acceptable Fractions PTVXY_2400 PTVXY_3000 PTVXY_3400 D 95%[Gy] 24 30 34 16 -27 24. 5 -33 28 -37. 5 1 3 5 PTV Dosimetry Compliance for Primary Site Name of Structure PTV_4500 Dosimetric parameter D 95%[Gy] Per Protocol 45 Variation Acceptable 42 -48 (excluding 45) Fractions 15

Systemic Therapy Maintenance therapy → Systemic therapy protocol treatment ideally begun either: • within 2 weeks of registration on the maintenance systemic therapy (Arm 1) • within 2 weeks of the completion of LCT (Arm 2) • tailored to patient/histology Maintenance drugs: – Pemetrexed, Docetaxel, Gemcitabine – Pembrolizumab, Pembrolizumab/Pemetrexed NOTE: Must refer to the package insert for complete details on safety and treatment information. Premedications should be given according to the package insert and institutional guidelines for each agent.

Previous amendment: 1) FDA approved IO allowed as first line and in maintenance as single agent or in combination therapy. To date, that includes Pembrolizumab single agent, Pembro/Pemetrexed/ Platinum, and Pembro/Platinum/Paclitaxel in induction and Pembro or Pembro/Pemetrexed in maintenance. Cannot include atezolizumab since its FDA approval is with bev, not allowed by NCI. 2) Surgery allowed as option for consolidation of mets. One site of disease will still need to receive radiation. Drs. Donington and Swisher are Surgical Co-Chairs. 3) With modified inclusion criteria and stratification for immunotherapy, approximately 378 patients will need to be accrued in 2: 1 randomization favoring local therapy arm. 4) Patients can receive radiation for palliation and still be enrolled on study as long as there areas to consolidate after induction systemic therapy.

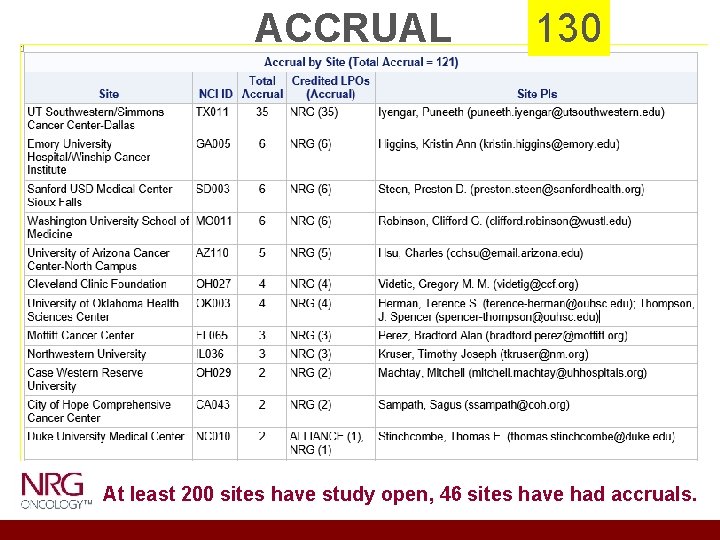
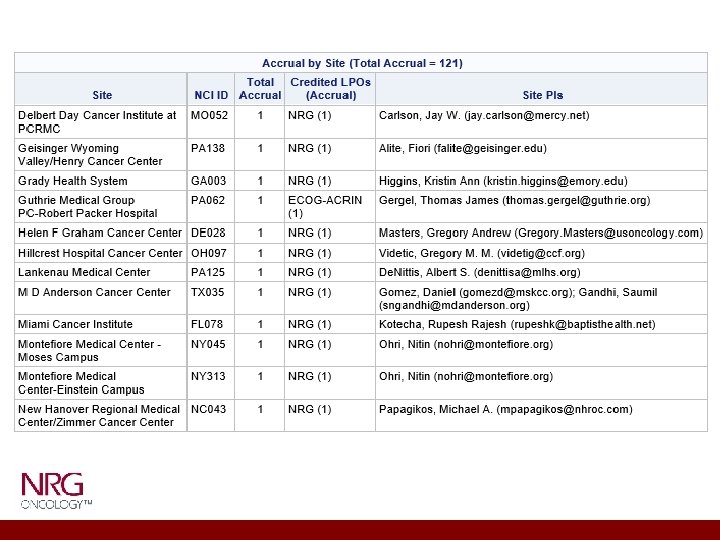
ACCRUAL 130 At least 200 sites have study open, 46 sites have had accruals.




AMENDMENT TIMELINE – PROPOSED Update permitted IO regimens. Allow more flexibility for timing of enrollment. July 2020 - Draft the amendment August 2020 - Submit the amendment to CTEP Amendment to CIRB review Final CTEP approval Broadcast the amendment

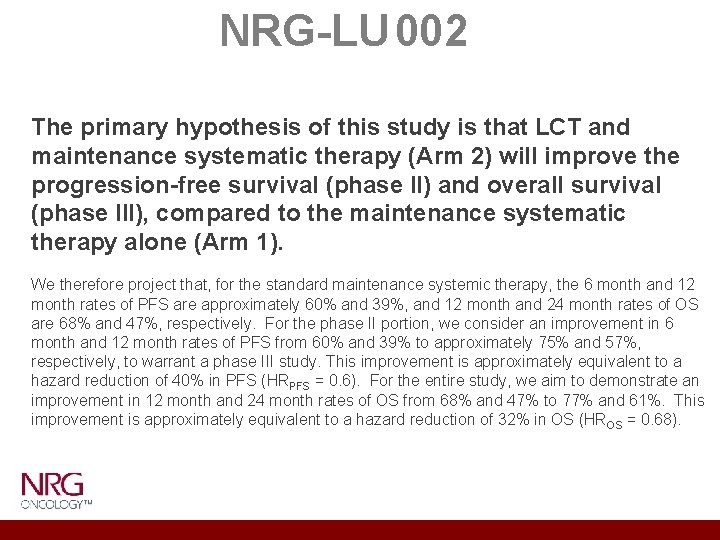
NRG-LU 002 The primary hypothesis of this study is that LCT and maintenance systematic therapy (Arm 2) will improve the progression-free survival (phase II) and overall survival (phase III), compared to the maintenance systematic therapy alone (Arm 1). We therefore project that, for the standard maintenance systemic therapy, the 6 month and 12 month rates of PFS are approximately 60% and 39%, and 12 month and 24 month rates of OS are 68% and 47%, respectively. For the phase II portion, we consider an improvement in 6 month and 12 month rates of PFS from 60% and 39% to approximately 75% and 57%, respectively, to warrant a phase III study. This improvement is approximately equivalent to a hazard reduction of 40% in PFS (HRPFS = 0. 6). For the entire study, we aim to demonstrate an improvement in 12 month and 24 month rates of OS from 68% and 47% to 77% and 61%. This improvement is approximately equivalent to a hazard reduction of 32% in OS (HROS = 0. 68).
 Nrg lu 002
Nrg lu 002 Arpana iyengar
Arpana iyengar The art of choosing ted talk
The art of choosing ted talk Iyengar frases
Iyengar frases Iyengar framing
Iyengar framing Venugopal iyengar
Venugopal iyengar Radha iyengar
Radha iyengar Classification of executive
Classification of executive 001 002 003
001 002 003 Um objeto com massa de 10kg e volume de 0 002
Um objeto com massa de 10kg e volume de 0 002 Elevator flowchart
Elevator flowchart Semt.001
Semt.001 002
002 001 002 003
001 002 003 002
002 Dtu p 06-002
Dtu p 06-002 Um objeto com massa de 10kg e volume de 0 002
Um objeto com massa de 10kg e volume de 0 002 Cip 002-009
Cip 002-009 002
002 Semt.002
Semt.002 Um objeto com massa de 10kg e volume de 0 002
Um objeto com massa de 10kg e volume de 0 002 Mfc-002
Mfc-002 Gmas-002
Gmas-002 002
002 002
002