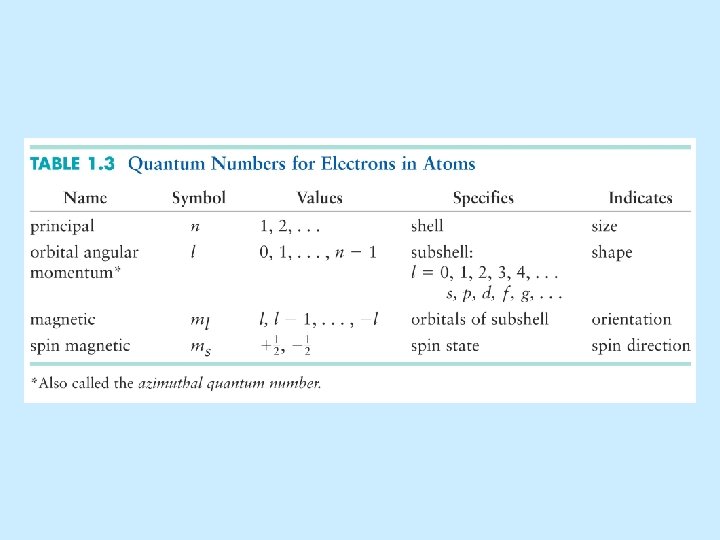
Now on to quantum numbers Quantum Numbers PRINCIPAL

Now on to quantum numbers…
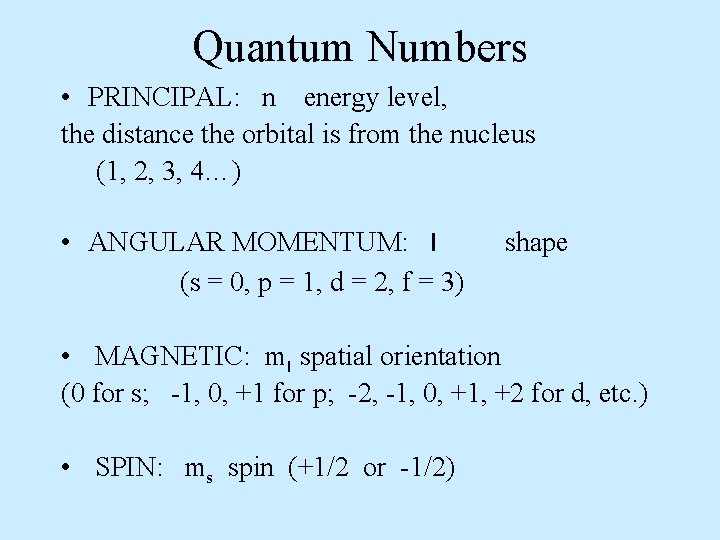
Quantum Numbers • PRINCIPAL: n energy level, the distance the orbital is from the nucleus (1, 2, 3, 4…) • ANGULAR MOMENTUM: l (s = 0, p = 1, d = 2, f = 3) shape • MAGNETIC: ml spatial orientation (0 for s; -1, 0, +1 for p; -2, -1, 0, +1, +2 for d, etc. ) • SPIN: ms spin (+1/2 or -1/2)

Review • Atomic number = # electrons • Electrons occupy orbitals defined by n, l, m • • Each orbital can hold two electrons Orbitals diffuse electron cloud “lower energy electron” closer to nucleus Outer electrons: “valence” most reactive
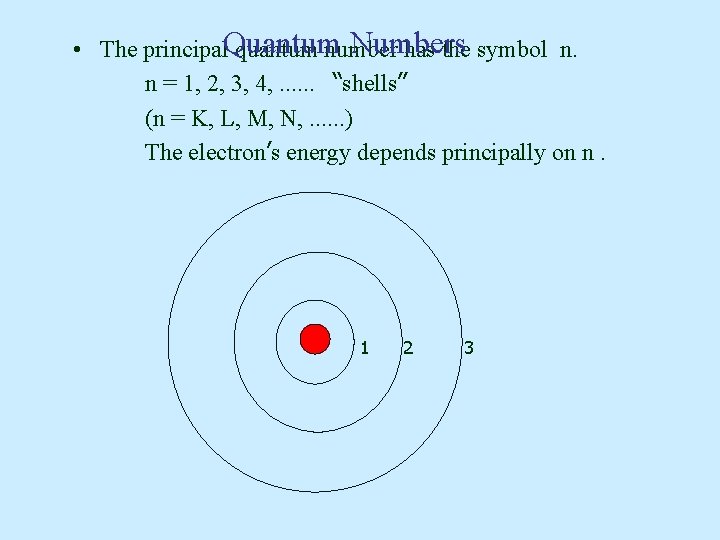
Numbers • The principal. Quantum quantum number has the symbol n. n = 1, 2, 3, 4, . . . “shells” (n = K, L, M, N, . . . ) The electron’s energy depends principally on n. 1 2 3

Quantum Numbers • The angular momentum quantum number has the symbol . = 0, 1, 2, 3, 4, 5, . . . . (n-1) = s, p, d, f, g, h, . . . . (n-1) • tells us the shape of the orbitals. • These orbitals are the volume around the atom that the electrons occupy 90 -95% of the time.
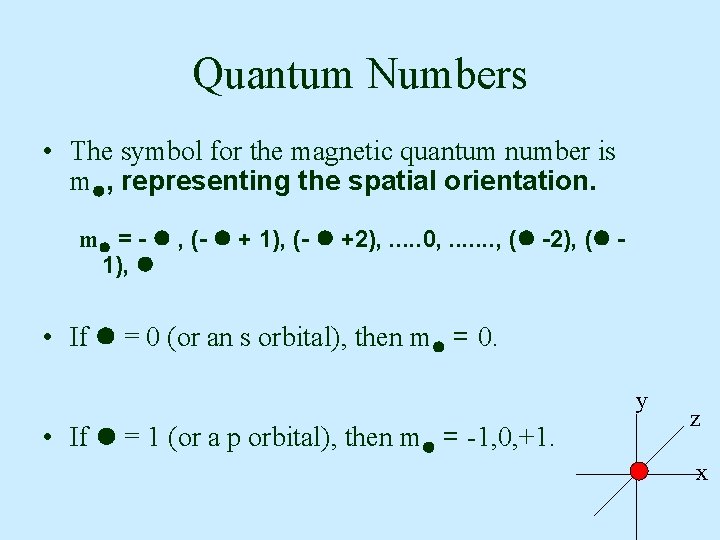
Quantum Numbers • The symbol for the magnetic quantum number is m , representing the spatial orientation. m = - , (- + 1), (- +2), . . . 0, . . . . , ( -2), ( 1), • If = 0 (or an s orbital), then m = 0. y • If = 1 (or a p orbital), then m = -1, 0, +1. z x

• If = 2 (or a d orbital), then m = -2, -1, 0, +1, +2. • If = 3 (or an f orbital), then m = -3, -2, 1, 0, +1, +2, +3. • Theoretically, this series continues on to g, h, i, etc
Spin quantum number • The last quantum number is the spin quantum number which has the symbol ms. • The spin quantum number only has two possible values. – ms = +1/2 or -1/2
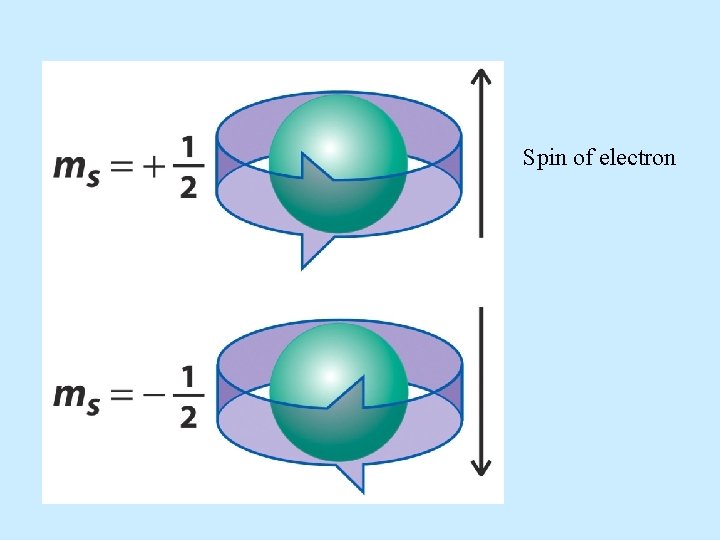
Spin of electron
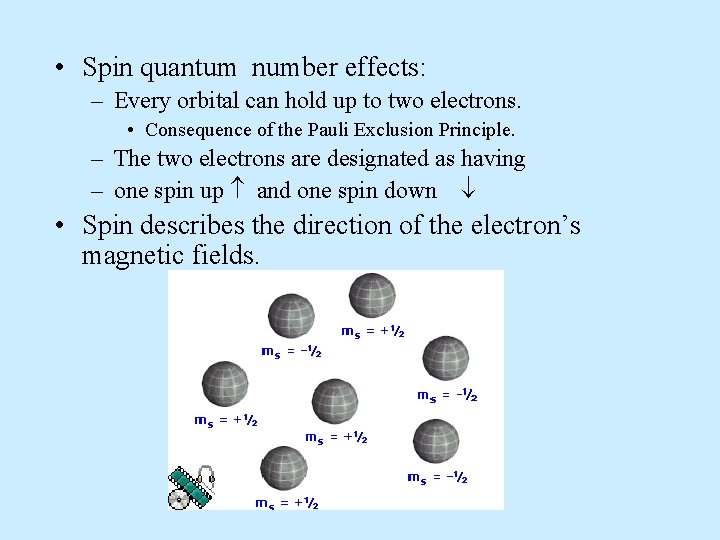
• Spin quantum number effects: – Every orbital can hold up to two electrons. • Consequence of the Pauli Exclusion Principle. – The two electrons are designated as having – one spin up and one spin down • Spin describes the direction of the electron’s magnetic fields.
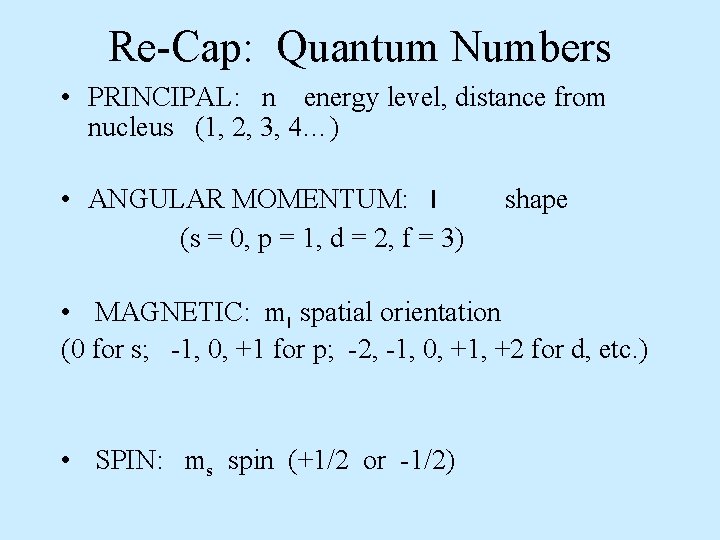
Re-Cap: Quantum Numbers • PRINCIPAL: n energy level, distance from nucleus (1, 2, 3, 4…) • ANGULAR MOMENTUM: l (s = 0, p = 1, d = 2, f = 3) shape • MAGNETIC: ml spatial orientation (0 for s; -1, 0, +1 for p; -2, -1, 0, +1, +2 for d, etc. ) • SPIN: ms spin (+1/2 or -1/2)

Atomic Orbitals: s, p, d, f • Atomic orbitals are regions of space where the probability of finding an electron about an atom is highest. • s orbital properties: – There is one s orbital per n level. =0 and only one value of m = 0
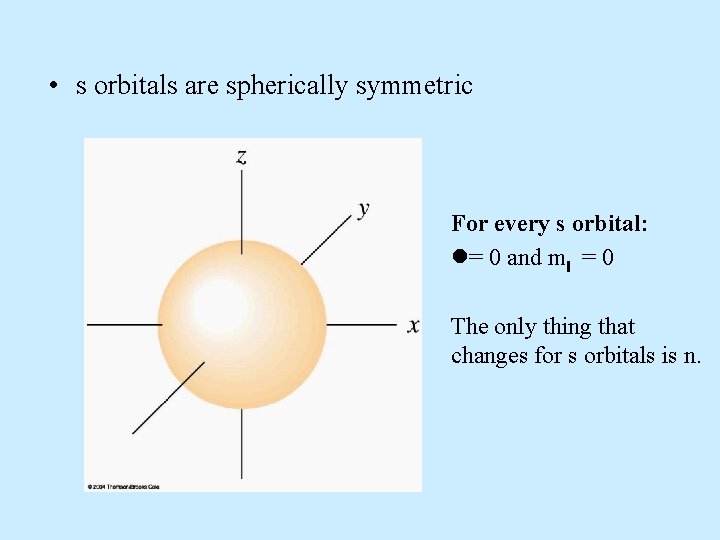
• s orbitals are spherically symmetric For every s orbital: l= 0 and ml = 0 The only thing that changes for s orbitals is n.
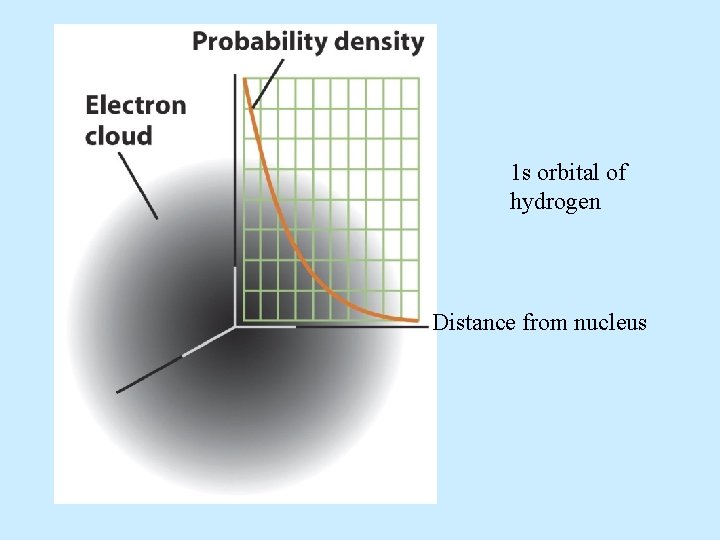
1 s orbital of hydrogen Distance from nucleus
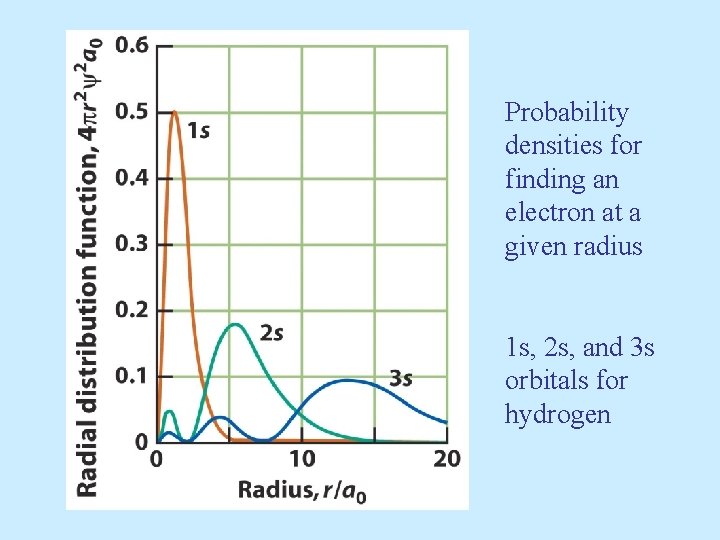
Probability densities for finding an electron at a given radius 1 s, 2 s, and 3 s orbitals for hydrogen
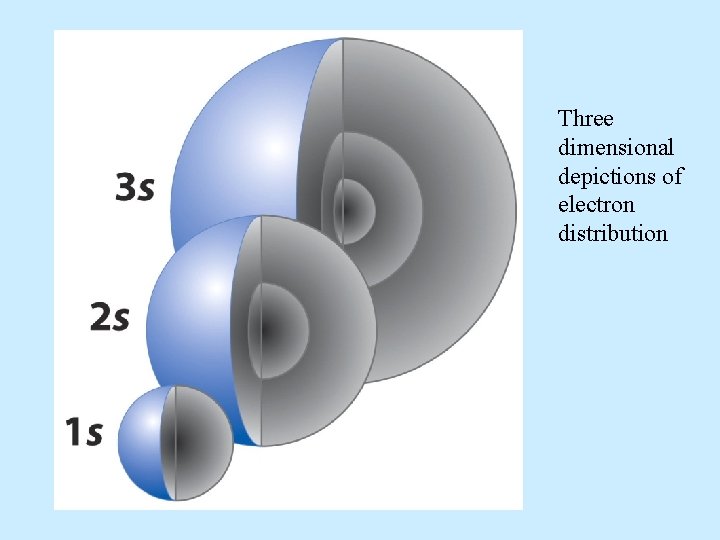
Three dimensional depictions of electron distribution

p orbitals • p orbital properties: – The first p orbitals appear in the n = 2 shell. • p orbitals are peanut or dumbbell shaped volumes. • There are 3 p orbitals per n level. – The three orbitals are named px, py, pz. = 1 for all p orbitals. m = -1, 0, +1 (designate three orientations)
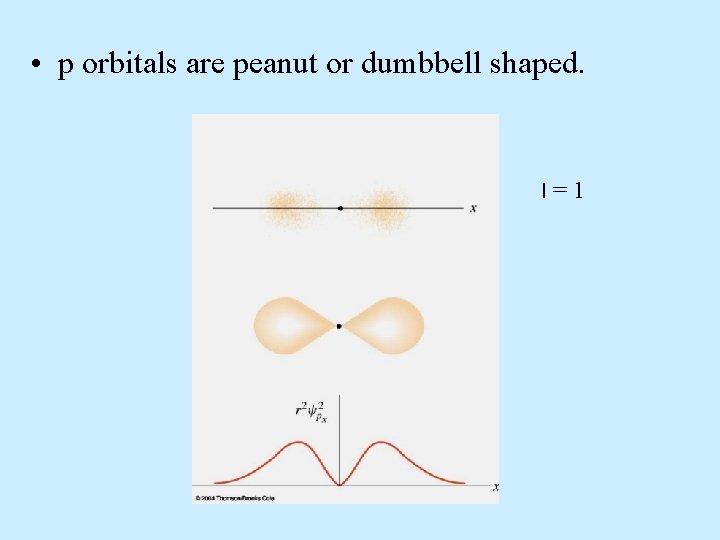
• p orbitals are peanut or dumbbell shaped. l=1
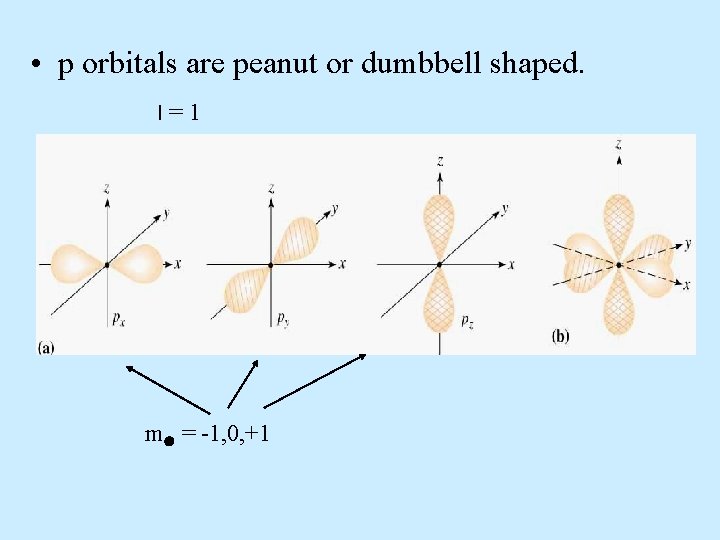
• p orbitals are peanut or dumbbell shaped. l=1 m = -1, 0, +1
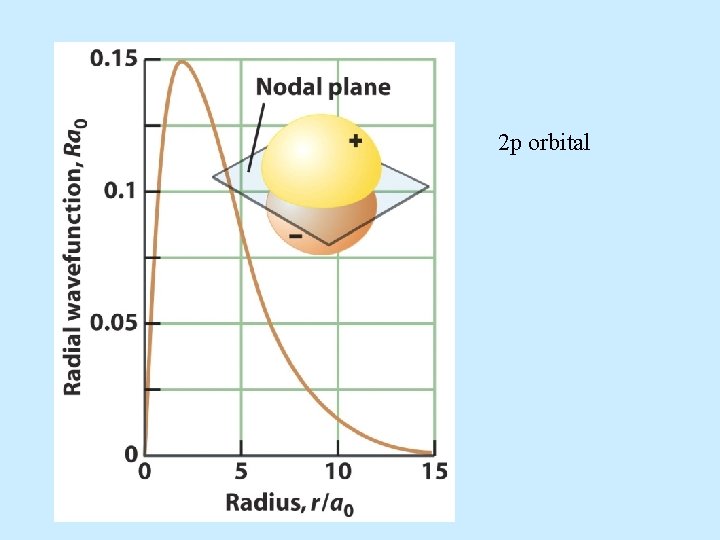
2 p orbital

d orbital properties: – The first d orbitals appear in the n = 3 shell. • The five d orbitals have two different shapes: – 4 are clover leaf shaped. – 1 is peanut shaped with a doughnut around it. – The orbitals lie directly on the Cartesian axes or are rotated 45 o from the axes. l. There –The are 5 d orbitals per n level. five orbitals are named: –They have an = 2. –m = -2, -1, 0, +1, +2 (5 values of m )
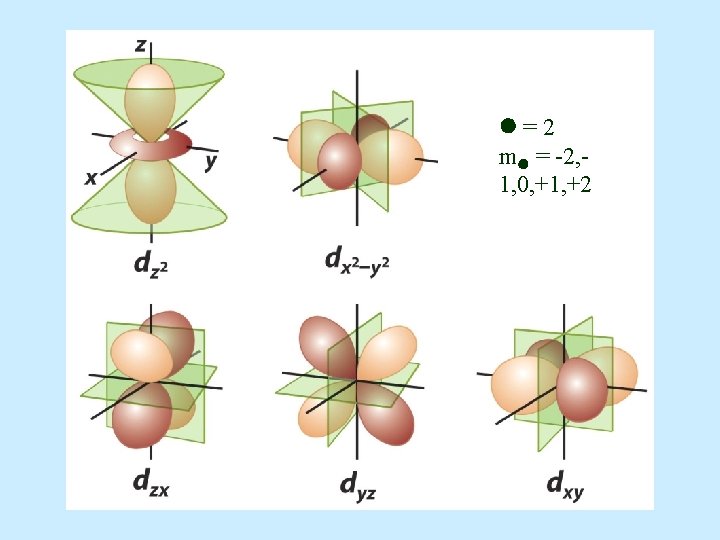
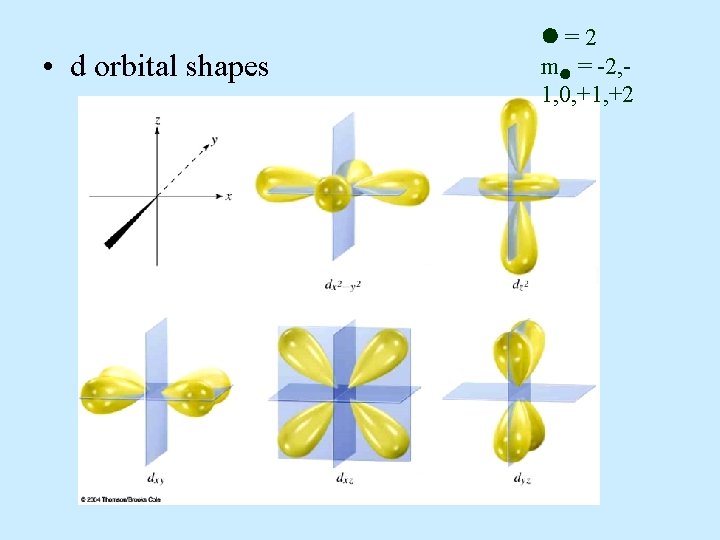
• d orbital shapes =2 m = -2, 1, 0, +1, +2
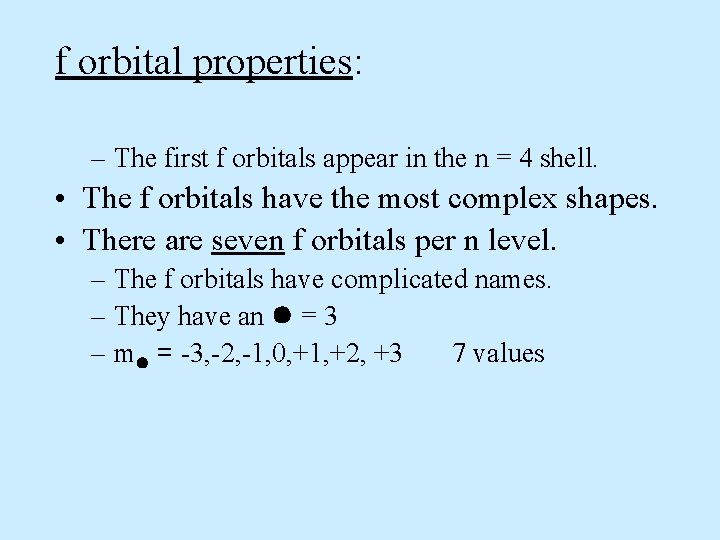
f orbital properties: – The first f orbitals appear in the n = 4 shell. • The f orbitals have the most complex shapes. • There are seven f orbitals per n level. – The f orbitals have complicated names. – They have an = 3 – m = -3, -2, -1, 0, +1, +2, +3 7 values
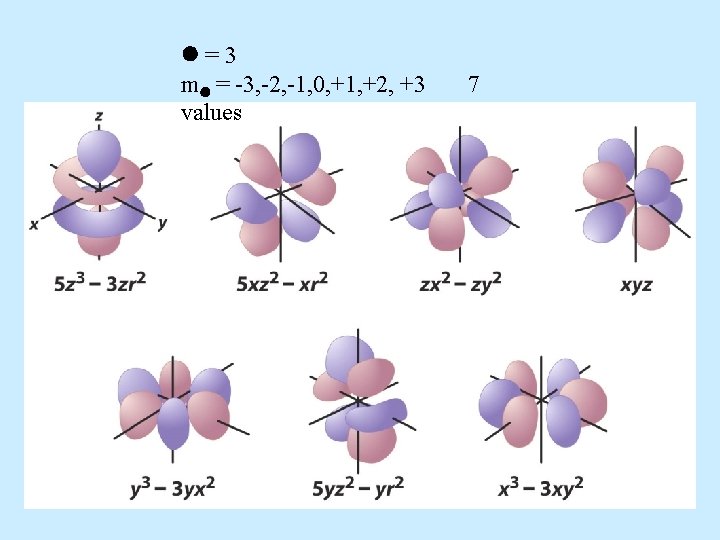
=3 m = -3, -2, -1, 0, +1, +2, +3 values 7
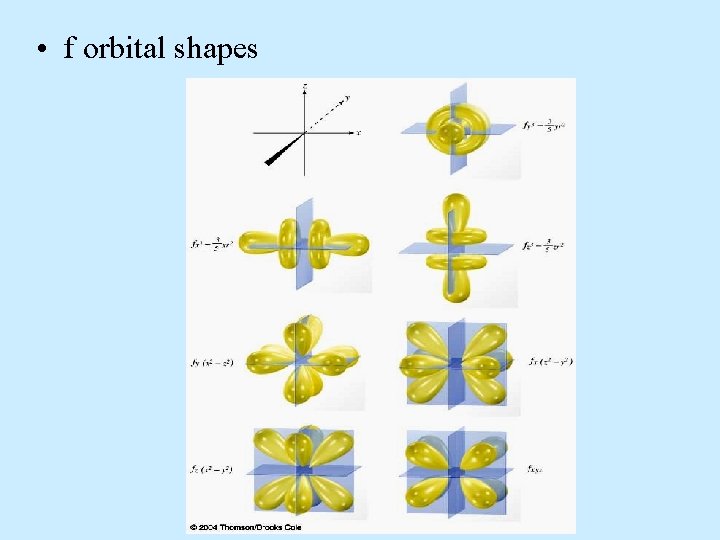
• f orbital shapes
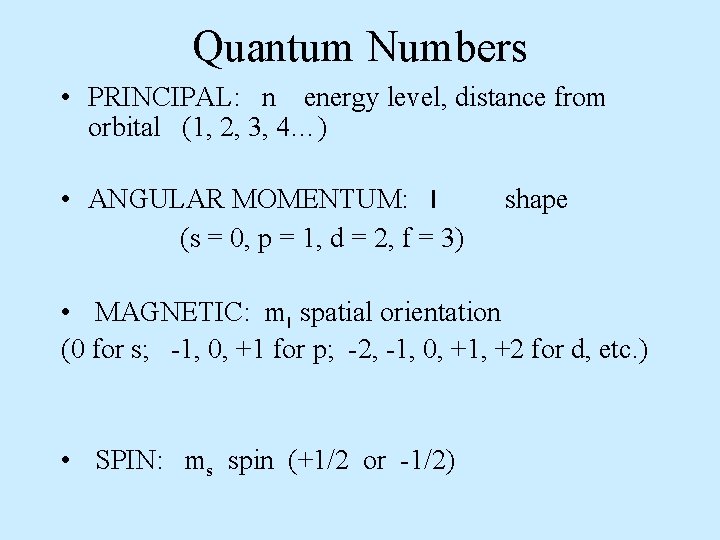
Quantum Numbers • PRINCIPAL: n energy level, distance from orbital (1, 2, 3, 4…) • ANGULAR MOMENTUM: l (s = 0, p = 1, d = 2, f = 3) shape • MAGNETIC: ml spatial orientation (0 for s; -1, 0, +1 for p; -2, -1, 0, +1, +2 for d, etc. ) • SPIN: ms spin (+1/2 or -1/2)
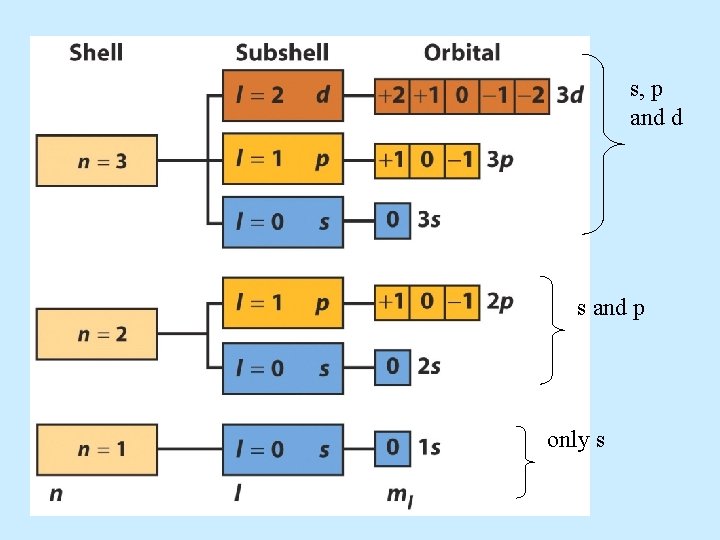
s, p and d s and p only s
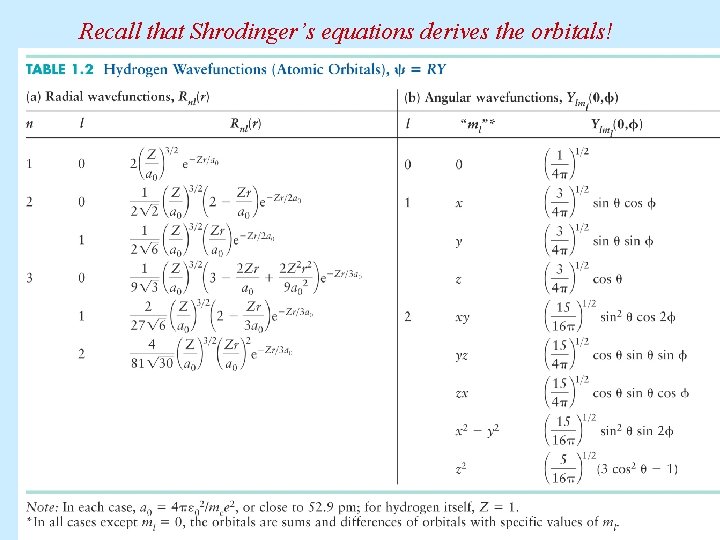
Recall that Shrodinger’s equations derives the orbitals!
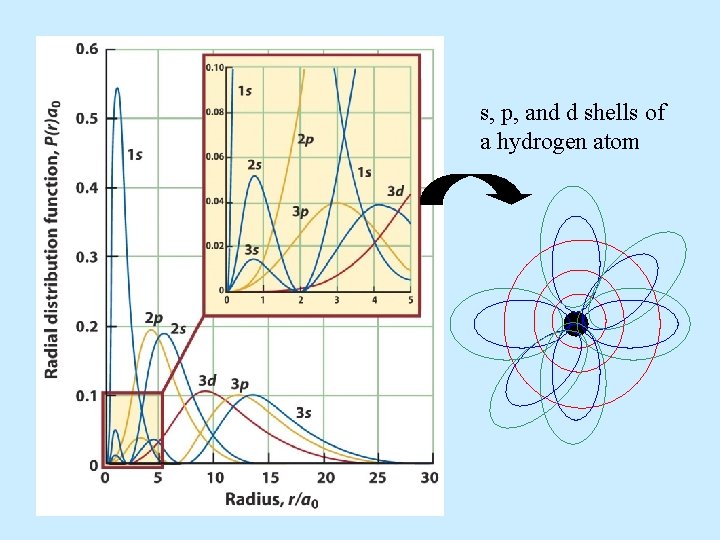
s, p, and d shells of a hydrogen atom
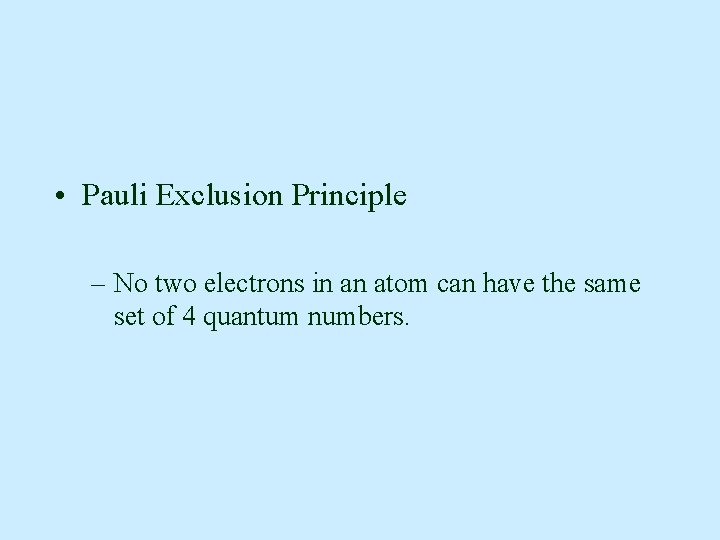
• Pauli Exclusion Principle – No two electrons in an atom can have the same set of 4 quantum numbers.
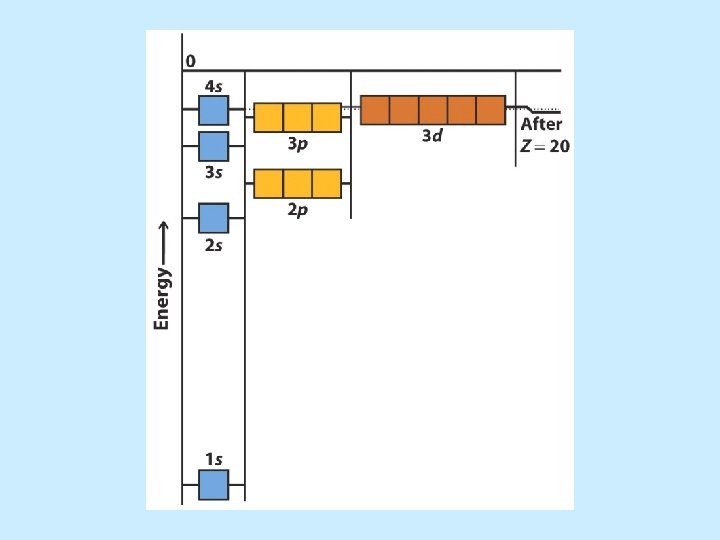
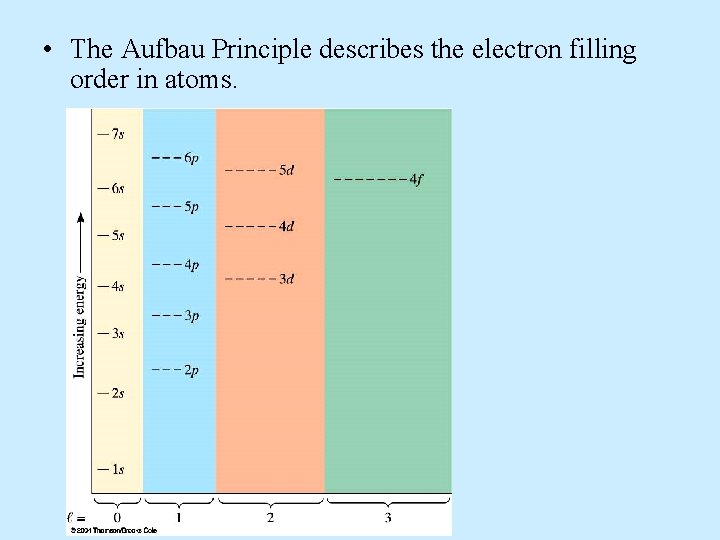
• The Aufbau Principle describes the electron filling order in atoms.
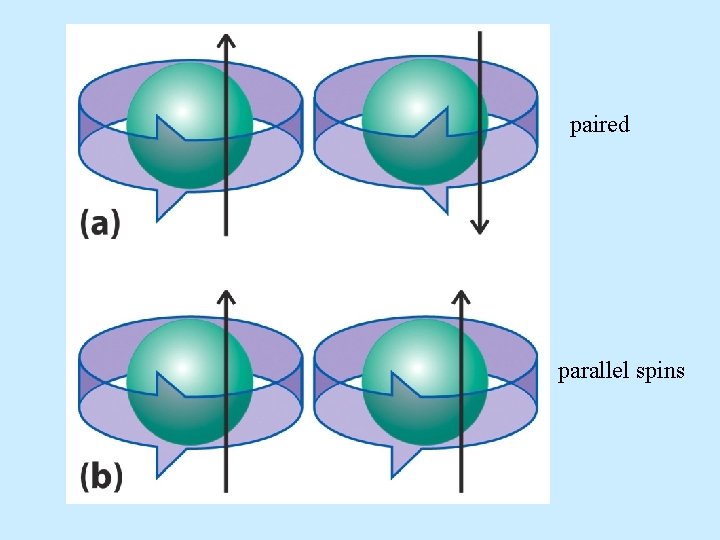
paired parallel spins

• Electron Configurations
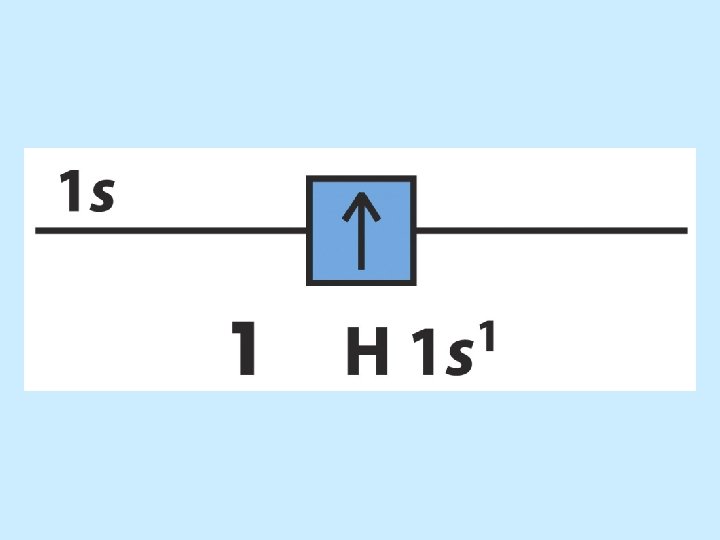
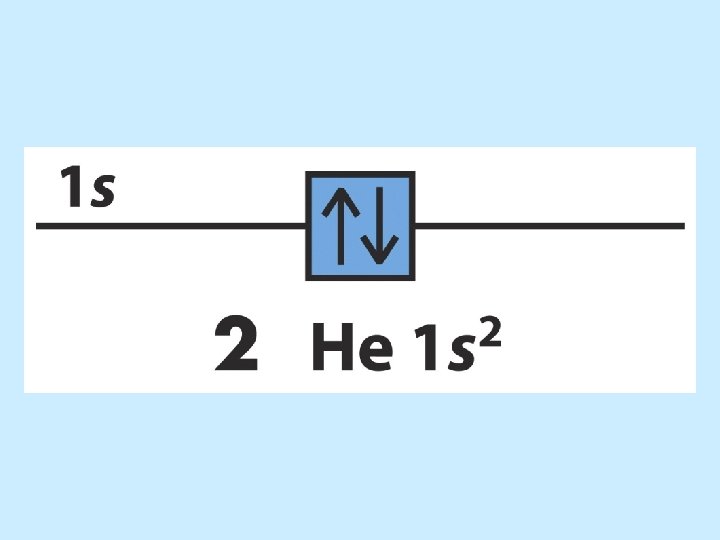
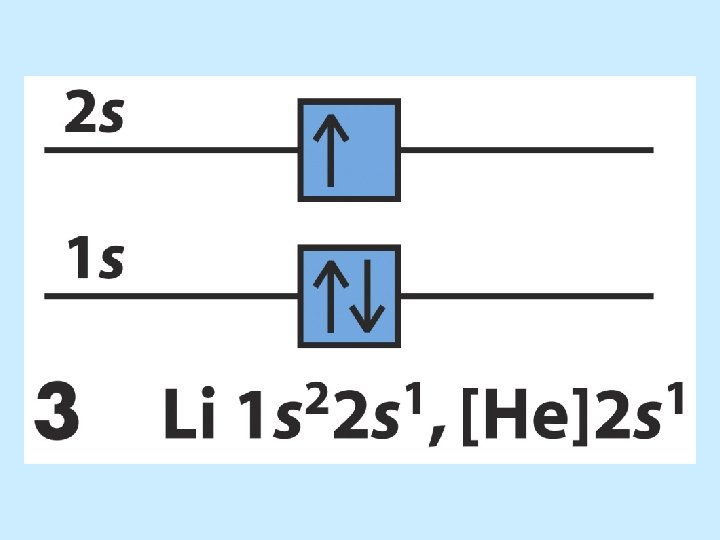
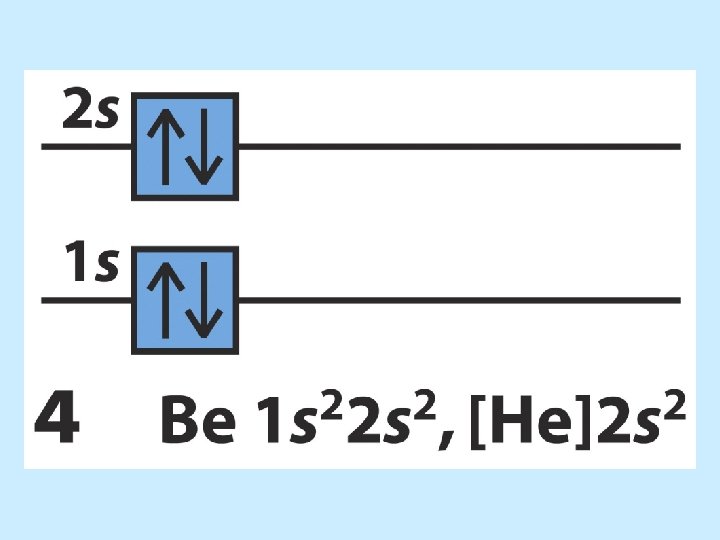
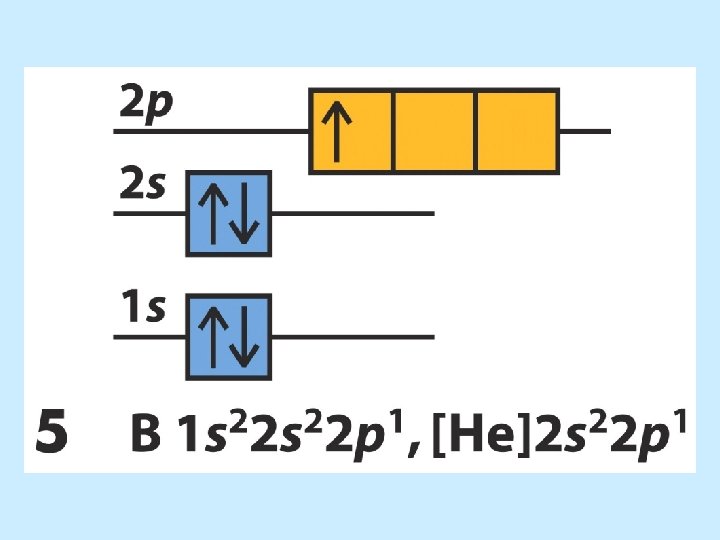
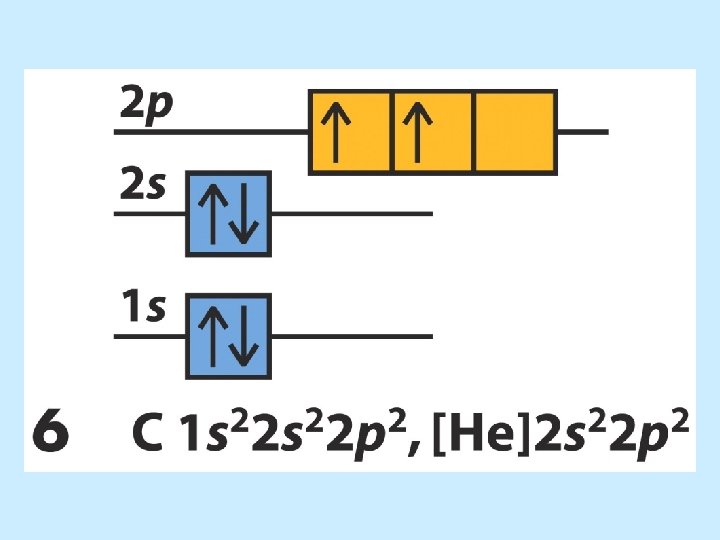
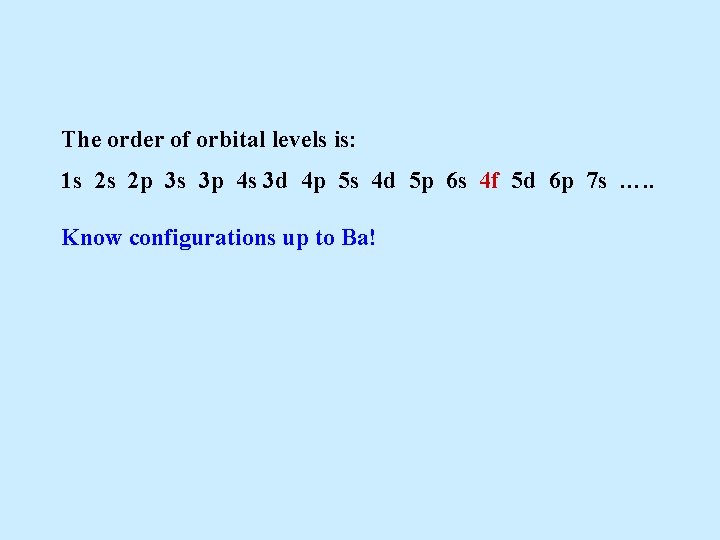
The order of orbital levels is: 1 s 2 s 2 p 3 s 3 p 4 s 3 d 4 p 5 s 4 d 5 p 6 s 4 f 5 d 6 p 7 s …. . Know configurations up to Ba!
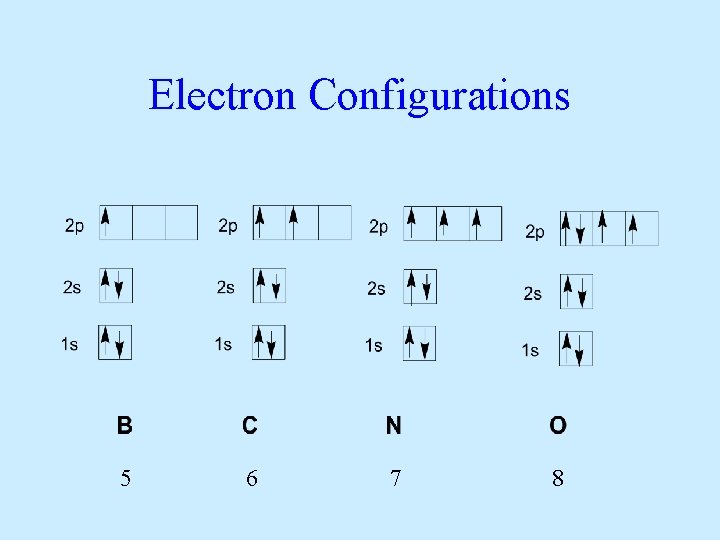
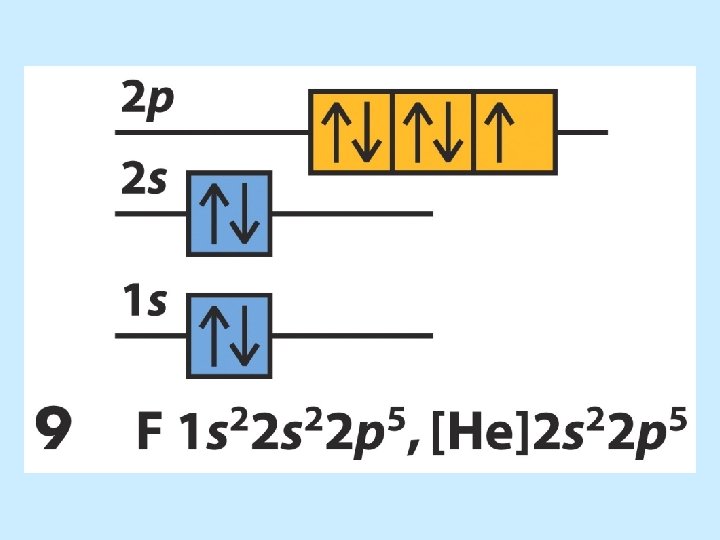
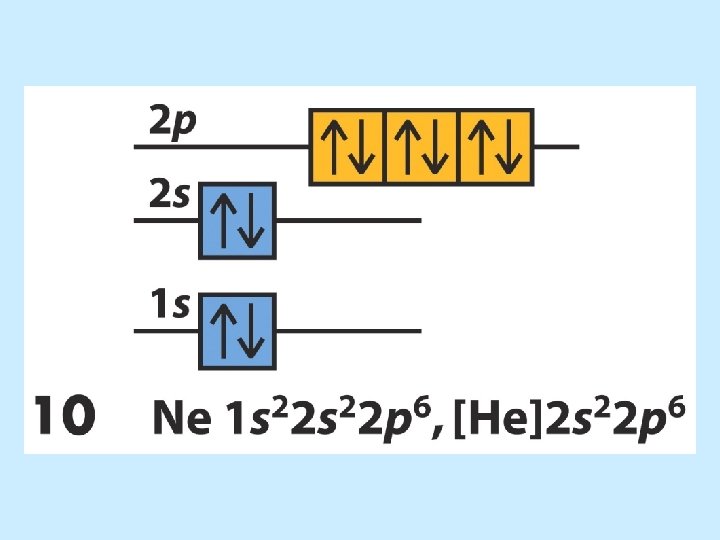
Electron Configurations 5 6 7 8
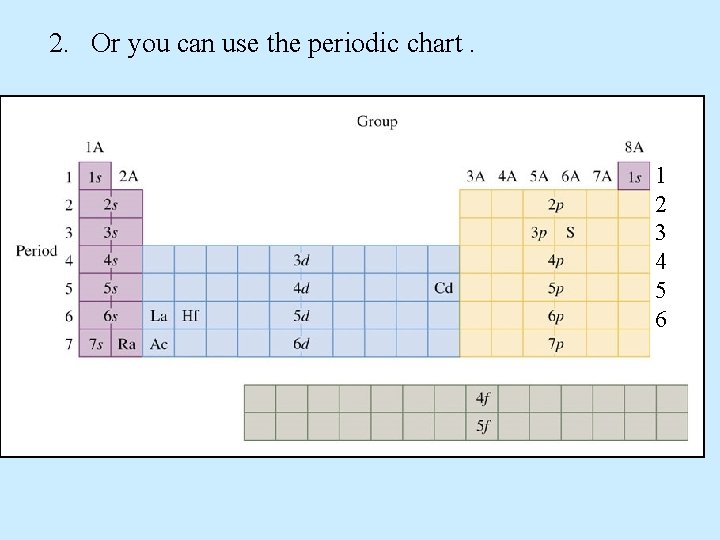
2. Or you can use the periodic chart. 1 2 3 4 5 6
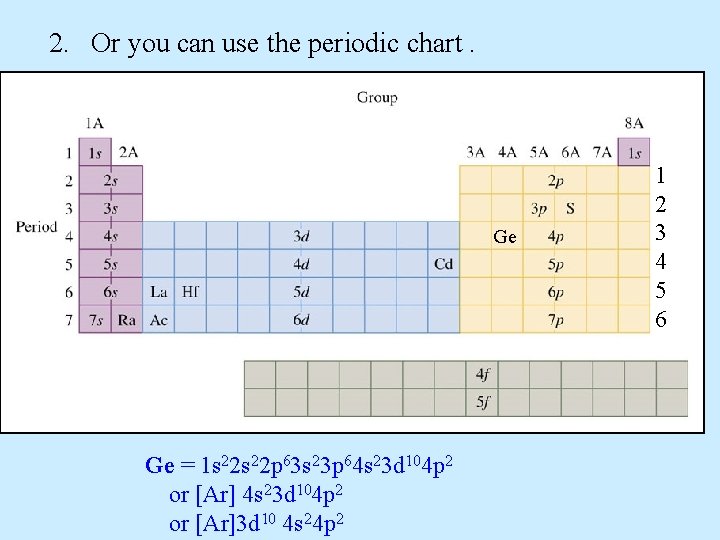
2. Or you can use the periodic chart. Ge Ge = 1 s 22 p 63 s 23 p 64 s 23 d 104 p 2 or [Ar]3 d 10 4 s 24 p 2 1 2 3 4 5 6
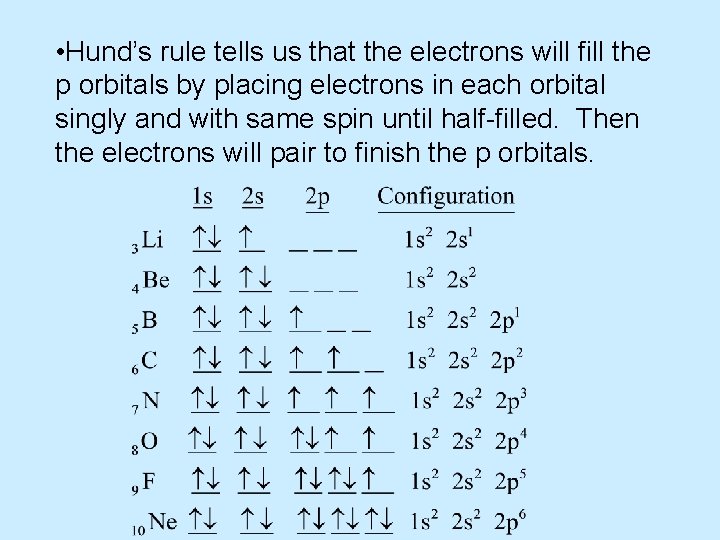
• Hund’s rule tells us that the electrons will fill the p orbitals by placing electrons in each orbital singly and with same spin until half-filled. Then the electrons will pair to finish the p orbitals.
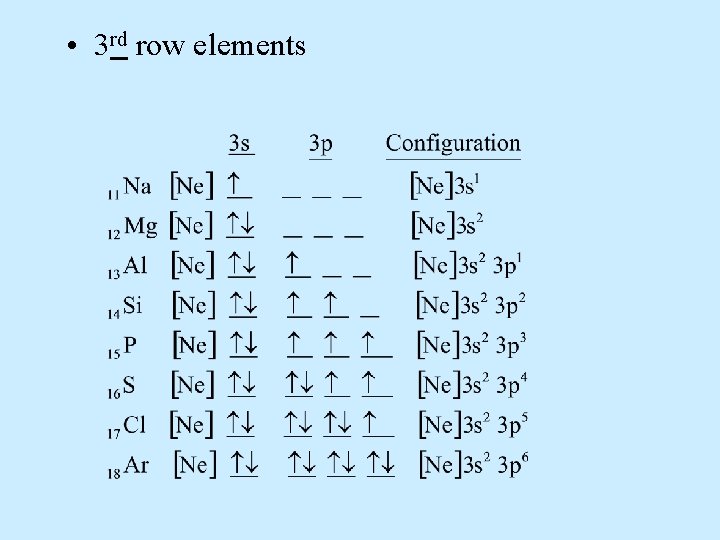
• 3 rd row elements
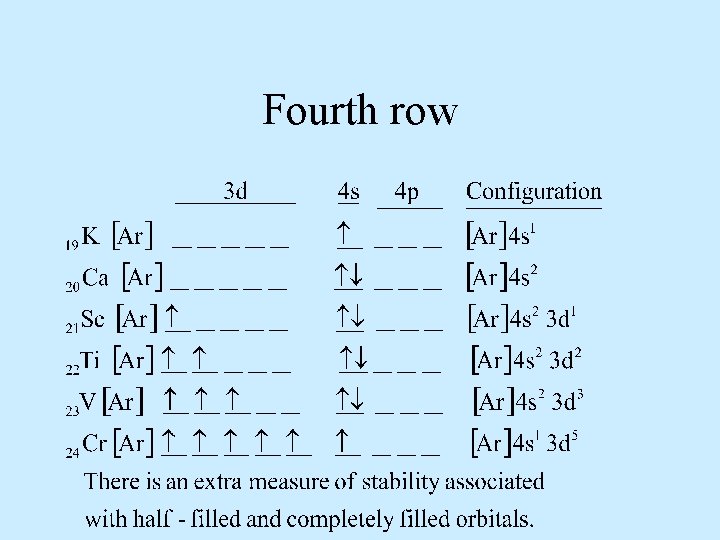
Fourth row
Fourth row
Fourth row
Fourth row
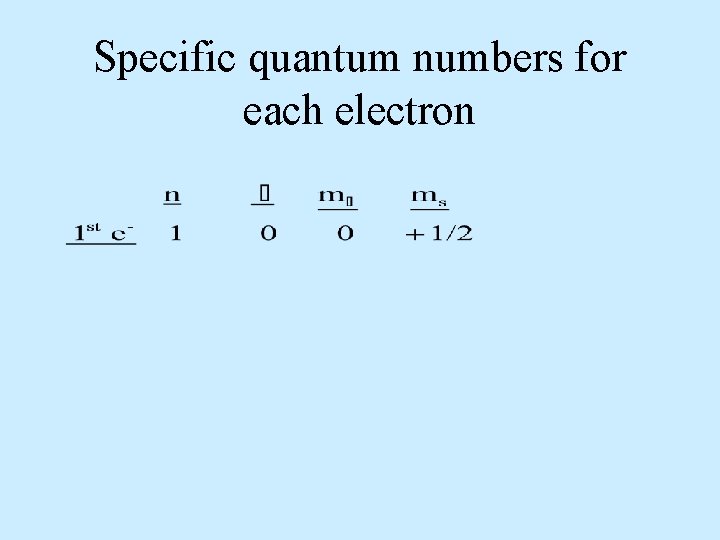
Specific quantum numbers for each electron
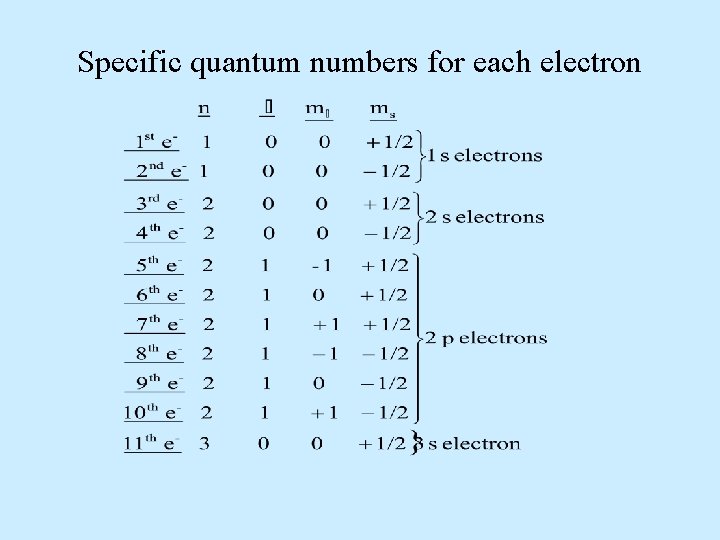
Specific quantum numbers for each electron
How to deal with ions? S vs S 2 - Cl vs Cl+

What type of ion would be expected to be favored for each element? Na F Na+ or Na. F+ or F- What are the electron configurations of the two C isotopes? 12 C 13 C
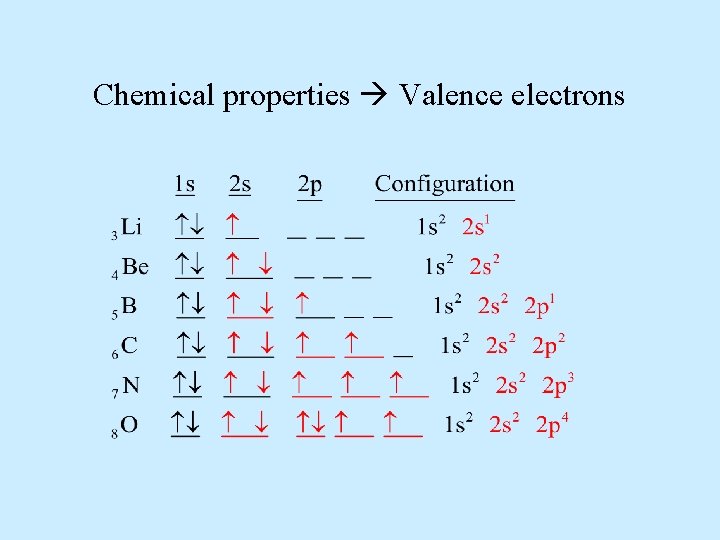
Chemical properties Valence electrons
- Slides: 61