Novocure NVCR overview updated August 2018 forwardlooking statements









- Slides: 22

Novocure (NVCR) overview updated August 2018

forward-looking statements This presentation contains certain forward-looking statements with respect to the business of Novocure and certain of its plans and objectives, including with respect to the development and commercialization of its lead product candidate, Optune, for a number of oncology indications. These forward-looking statements can be identified in this presentation by the fact that they do not relate only to historical or current facts. Forward-looking statements often use words “expect”, “intend”, “anticipate”, “plan”, “may”, “should”, “would”, “could” or other words of similar meaning. These statements are based on assumptions and assessments made by Novocure in light of industry experience and perception of historical trends, current conditions, expected future developments and other appropriate factors. By their nature, forward-looking statements involve risk and uncertainty, and Novocure's performance and financial results could differ materially from those expressed or implied in these forward-looking statements due to general financial, economic, regulatory and political conditions as well as more specific risks and uncertainties facing Novocure such as those set forth in its Annual Report on Form 10 -K filed on February 22, 2018, or in subsequent quarterly filings with the U. S. Securities and Exchange Commission. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those described in this presentation. Novocure assumes no obligation to update or correct the information contained in this presentation, whether as a result of new information, future events or otherwise, except to the extent legally required. The statements contained in this presentation are made as at the date of this presentation, unless some other time is specified in relation to them, and service of this presentation shall not give rise to any implication that there has been no change in the facts set out in this presentation since such date. Nothing contained in this presentation shall be deemed to be a forecast, projection or estimate of the future financial performance of Novocure, except where expressly stated. As of the date of this presentation, Optune is only FDA-approved for the treatment of adults with supratentorial glioblastoma, or GBM, and its approval for other indications is not certain. Novocure can provide no assurances regarding market acceptance of Optune or its successful commercialization, and can provide no assurances regarding the company’s results of operations or financial condition in the future. This presentation is for informational purposes only and may not be relied upon in connection with the purchase or sale of any security. © Novocure 2018 2

Optune® indications for use and important safety information INDICATIONS • Optune is intended as a treatment for adult patients (22 years of age or older) with histologically-confirmed glioblastoma multiforme (GBM). • Optune with temozolomide is indicated for the treatment of adult patients with newly diagnosed, supratentorial glioblastoma following maximal debulking surgery, and completion of radiation therapy together with concomitant standard of care chemotherapy. • For the treatment of recurrent GBM, Optune is indicated following histologically-or radiologically-confirmed recurrence in the supratentorial region of the brain after receiving chemotherapy. The device is intended to be used as a monotherapy, and is intended as an alternative to standard medical therapy for GBM after surgical and radiation options have been exhausted. CONTRAINDICATIONS • Do not use Optune in patients with an active implanted medical device, a skull defect (such as, missing bone with no replacement), or bullet fragments. Use of Optune together with implanted electronic devices has not been tested and may theoretically lead to malfunctioning of the implanted device. Use of Optune together with skull defects or bullet fragments has not been tested and may possibly lead to tissue damage or render Optune ineffective. • Do not use Optune in patients that are known to be sensitive to conductive hydrogels. In this case, skin contact with the gel used with Optune may commonly cause increased redness and itching, and rarely may even lead to severe allergic reactions such as shock and respiratory failure. © Novocure 2018 3

Optune® indications for use and important safety information WARNINGS AND PRECAUTIONS • Optune can only be prescribed by a healthcare provider that has completed the required certification training provided by Novocure (the device manufacturer). • Do not prescribe Optune for patients that are pregnant, you think might be pregnant or are trying to get pregnant, as the safety and effectiveness of Optune in these populations have not been established. • The most common (≥ 10%) adverse events involving Optune in combination with temozolomide were thrombocytopenia, nausea, constipation, vomiting, fatigue, medical device site reaction, headache, convulsions, and depression. • The most common (≥ 10%) adverse events seen with Optune monotherapy were medical device site reaction and headache. • The following adverse reactions were considered related to Optune when used as monotherapy: medical device site reaction, headache, malaise, muscle twitching, fall and skin ulcer. • Use of Optune in patients with an inactive implanted medical device in the brain has not been studied for safety and effectiveness, and use of Optune in these patients could lead to tissue damage or lower the chance of Optune being effective. • If the patient has an underlying serious skin condition on the scalp, evaluate whether this may prevent or temporarily interfere with Optune treatment. © Novocure 2018 4

a global oncology company with a proprietary platform GROWING COMMERCIAL BUSINESS SIGNIFICANT UPSIDE POTENTIAL • More than 2, 100 patients on therapy • 14 consecutive quarters of patient growth • $217 million trailing twelve month revenues • Increase adoption and average reimbursement in GBM • Advance clinical pipeline in five additional solid tumor indications Information above as of June 30, 2018 © Novocure 2018 5

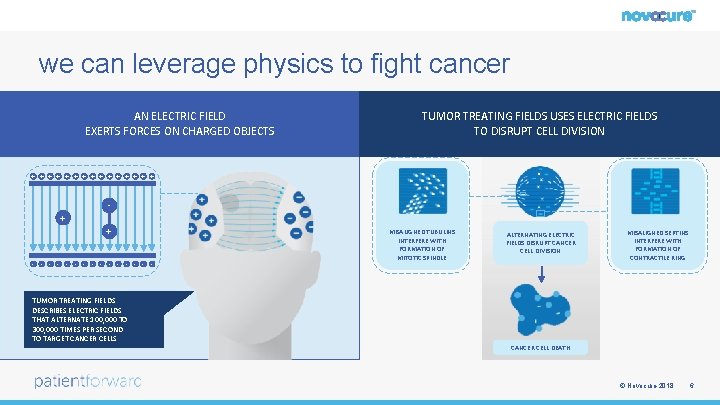
we can leverage physics to fight cancer AN ELECTRIC FIELD EXERTS FORCES ON CHARGED OBJECTS TUMOR TREATING FIELDS USES ELECTRIC FIELDS TO DISRUPT CELL DIVISION + + + + + - - - - TUMOR TREATING FIELDS DESCRIBES ELECTRIC FIELDS THAT ALTERNATE 100, 000 TO 300, 000 TIMES PER SECOND TO TARGET CANCER CELLS MISALIGNED TUBULINS INTERFERE WITH FORMATION OF MITOTIC SPINDLE ALTERNATING ELECTRIC FIELDS DISRUPT CANCER CELL DIVISION MISALIGNED SEPTINS INTERFERE WITH FORMATION OF CONTRACTILE RING CANCER CELL DEATH © Novocure 2018 6

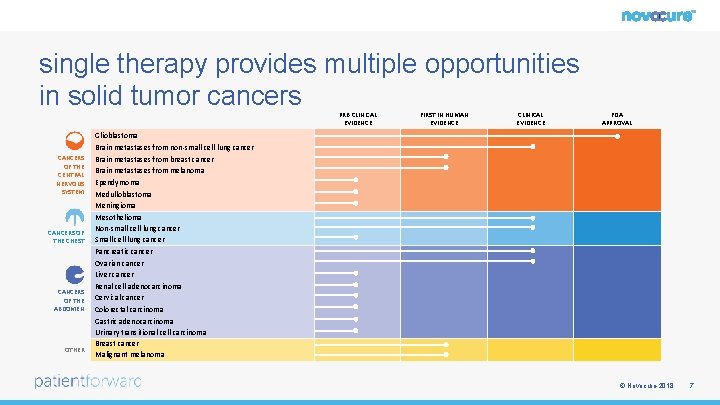
single therapy provides multiple opportunities in solid tumor cancers PRE-CLINICAL EVIDENCE CANCERS OF THE CENTRAL NERVOUS SYSTEM CANCERS OF THE CHEST CANCERS OF THE ABDOMEN OTHER FIRST IN HUMAN EVIDENCE CLINICAL EVIDENCE FDA APPROVAL Glioblastoma Brain metastases from non-small cell lung cancer Brain metastases from breast cancer Brain metastases from melanoma Ependymoma Medulloblastoma Meningioma Mesothelioma Non-small cell lung cancer Small cell lung cancer Pancreatic cancer Ovarian cancer Liver cancer Renal cell adenocarcinoma Cervical cancer Colorectal carcinoma Gastric adenocarcinoma Urinary transitional cell carcinoma Breast cancer Malignant melanoma © Novocure 2018 7

the Optune® system ELECTRIC FIELD GENERATOR The portable field generator can be carried with you to generate Tumor Treating Fields as you go about your day. TRANSDUCER ARRAY Sterile, single-use transducer arrays are connected to the electric field generator to deliver therapy. Transducer arrays should be changed at least 2 times per week (every 4 days at most). Steve is an Optune user © Novocure 2018 8

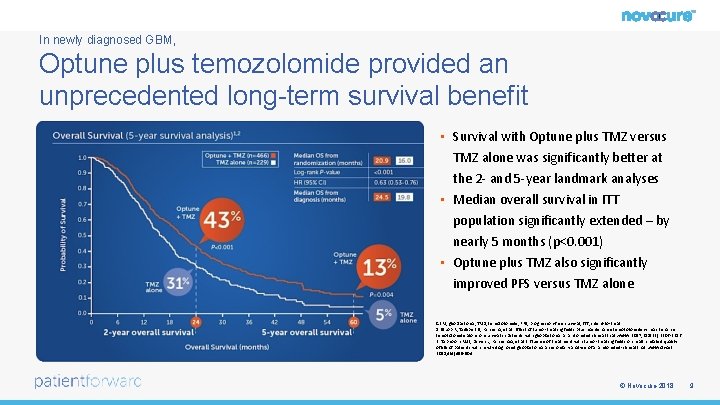
In newly diagnosed GBM, Optune plus temozolomide provided an unprecedented long-term survival benefit • In Newly diagnosed GBM, • Survival with Optune plus TMZ versus TMZ alone was significantly better at the 2 - and 5 -year landmark analyses • Median overall survival in ITT population significantly extended – by nearly 5 months (p<0. 001) • Optune plus TMZ also significantly improved PFS versus TMZ alone GBM, glioblastoma; TMZ, temozolomide; PFS, progression-free survival; ITT, intent-to-treat 1. Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017; 318(23): 2306 -2316. 2. Taphoorn MJB, Dirven L, Kanner AA, et al. Influence of treatment with tumor-treating fields on health-related quality of life of patients with newly diagnosed glioblastoma: a secondary analysis of a randomized-clinical trial. JAMA Oncol 2018; 4(4)495 -504. © Novocure 2018 9

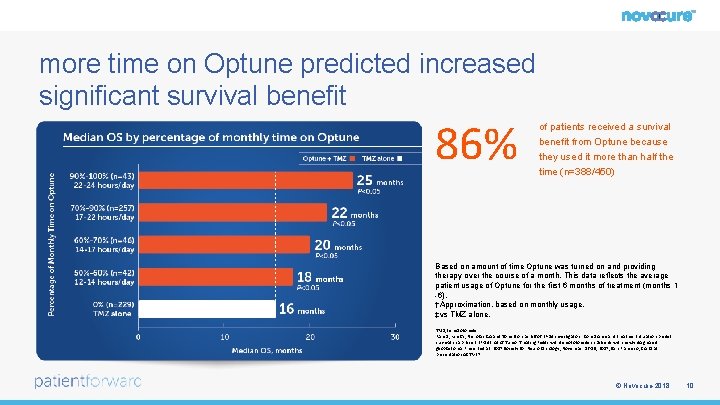
more time on Optune predicted increased significant survival benefit 86% of patients received a survival benefit from Optune because they used it more than half the time (n=388/450) Based on amount of time Optune was turned on and providing therapy over the course of a month. This data reflects the average patient usage of Optune for the first 6 months of treatment (months 1 -6). †Approximation, based on monthly usage. ‡vs TMZ alone. TMZ, temozolomide Ram Z, Kim CY, Nicholas GA and Toms S on behalf of EF-14 investigators. Compliance and treatment duration predict survival in a phase 3 EF-14 trial of Tumor Treating Fields with temozolomide in patients with newly diagnosed glioblastoma. Presented at: 2017 Society for Neuro Oncology; November 16 -19, 2017; San Francisco, CA. Oral presentation ACTR-27. © Novocure 2018 10

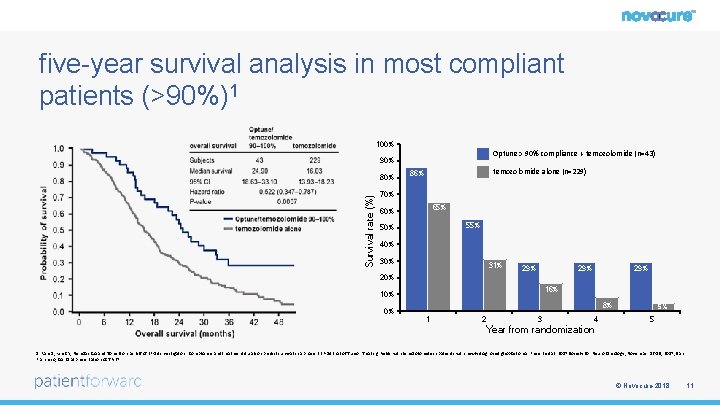
five-year survival analysis in most compliant patients (>90%)1 100% Optune > 90% compliance + temozolomide (n=43) 90% Survival rate (%) 80% temozolomide alone (n=229) 86% 70% 65% 60% 55% 50% 40% 31% 29% 29% 20% 16% 10% 8% 0% 1 2 3 4 5% 5 Year from randomization 1. Ram Z, Kim CY, Nicholas GA and Toms S on behalf of EF-14 investigators. Compliance and treatment duration predict survival in a phase 3 EF-14 trial of Tumor Treating Fields with temozolomide in patients with newly diagnosed glioblastoma. Presented at: 2017 Society for Neuro Oncology; November 16 -19, 2017; San Francisco, CA. Oral presentation ACTR-27. © Novocure 2018 11

established commercial operations in six markets across three regions EMEA † UNITED STATES 30% estimated penetration EMEA † 20% 3, 450 eligible patient population estimated penetration JAPAN 10% UNITED STATES 9, 300 eligible patient population estimated penetration JAPAN 1, 100 eligible patient population Information above as of June 30, 2018 †Considers currently active markets: Germany, Switzerland, Austria and Israel © Novocure 2018 12

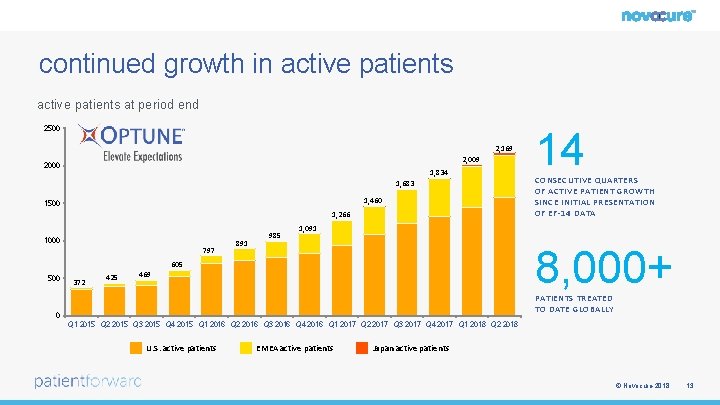
continued growth in active patients at period end 2500 2, 169 2, 009 2000 1, 834 1, 683 1, 460 1500 1, 266 1000 797 891 985 372 425 CONSECUTIVE QUARTERS OF ACTIVE PATIENT GROWTH SINCE INITIAL PRESENTATION OF EF-14 DATA 1, 091 8, 000+ 605 500 14 469 PATIENTS TREATED TO DATE GLOBALLY 0 Q 1 2015 Q 2 2015 Q 3 2015 Q 4 2015 Q 1 2016 Q 2 2016 Q 3 2016 Q 4 2016 Q 1 2017 Q 2 2017 Q 3 2017 Q 4 2017 Q 1 2018 Q 2 2018 U. S. active patients EMEA active patients Japan active patients © Novocure 2018 13

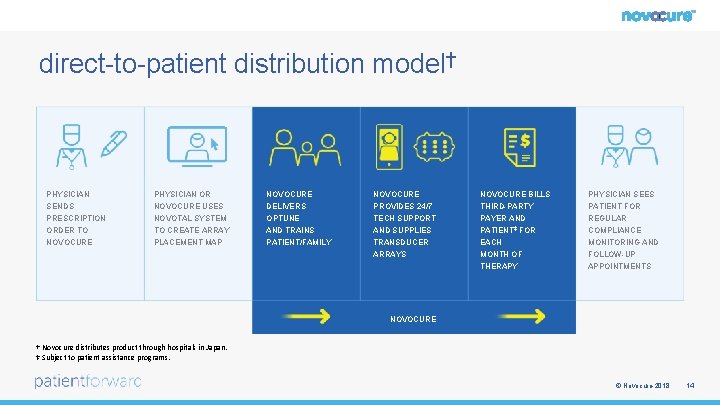
direct-to-patient distribution model† PHYSICIAN SENDS PHYSICIAN OR NOVOCURE USES NOVOCURE DELIVERS NOVOCURE PROVIDES 24/7 NOVOCURE BILLS THIRD-PARTY PHYSICIAN SEES PATIENT FOR PRESCRIPTION ORDER TO NOVOCURE NOVOTAL SYSTEM TO CREATE ARRAY PLACEMENT MAP OPTUNE AND TRAINS PATIENT/FAMILY TECH SUPPORT AND SUPPLIES TRANSDUCER ARRAYS PAYER AND PATIENT‡ FOR EACH MONTH OF THERAPY REGULAR COMPLIANCE MONITORING AND FOLLOW-UP APPOINTMENTS NOVOCURE † Novocure distributes product through hospitals in Japan. ‡ Subject to patient assistance programs. © Novocure 2018 14

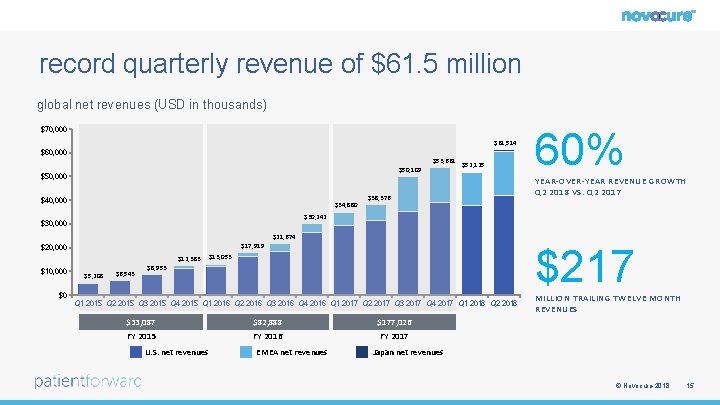
record quarterly revenue of $61. 5 million global net revenues (USD in thousands) $70, 000 $61, 514 $60, 000 $53, 661 $50, 109 $50, 000 $40, 000 $34, 880 $52, 125 $38, 376 60% YEAR-OVER-YEAR REVENUE GROWTH Q 2 2018 VS. Q 2 2017 $30, 242 $30, 000 $21, 674 $17, 919 $20, 000 $12, 383 $10, 000 $5, 208 $6, 543 $217 $13, 053 $8, 953 $0 Q 1 2015 Q 2 2015 Q 3 2015 Q 4 2015 Q 1 2016 Q 2 2016 Q 3 2016 Q 4 2016 Q 1 2017 Q 2 2017 Q 3 2017 Q 4 2017 Q 1 2018 Q 2 2018 $33, 087 $82, 888 $177, 026 FY 2015 FY 2016 FY 2017 U. S. net revenues EMEA net revenues MILLION TRAILING TWELVE MONTH REVENUES Japan net revenues © Novocure 2018 15

multiple levers for revenue growth in GBM OPPORTUNITY TO INCREASE GBM PENETRATION • Category 1 NCCN guidelines® recommendation • Steady growth in prescriptions for newly diagnosed GBM OPPORTUNITY TO INCREASE NET REVENUES REALIZATION • Step-function improvements with national reimbursements, e. g. Medicare • Incremental improvements with ongoing market access initiatives OPPORTUNITY TO STRATEGICALLY EXPAND INTO ADDITIONAL MARKETS OVER TIME • Current commercial efforts focused on six markets • Ability to leverage established organization across three regions © Novocure 2018 16

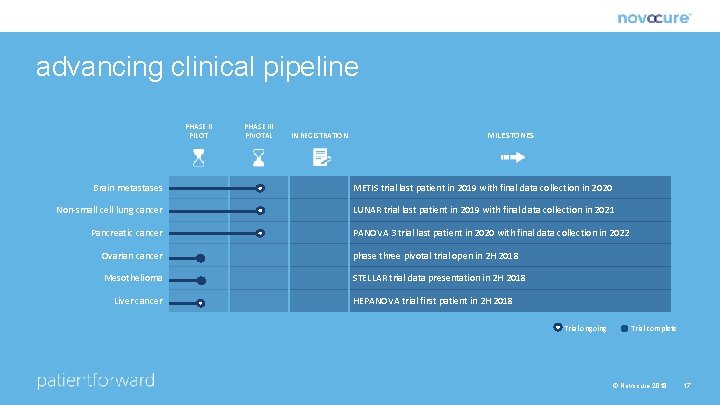
advancing clinical pipeline PHASE II PILOT PHASE III PIVOTAL IN REGISTRATION MILESTONES Brain metastases METIS trial last patient in 2019 with final data collection in 2020 Non-small cell lung cancer LUNAR trial last patient in 2019 with final data collection in 2021 Pancreatic cancer PANOVA 3 trial last patient in 2020 with final data collection in 2022 Ovarian cancer phase three pivotal trial open in 2 H 2018 Mesothelioma STELLAR trial data presentation in 2 H 2018 Liver cancer HEPANOVA trial first patient in 2 H 2018 Trial ongoing Trial complete © Novocure 2018 17

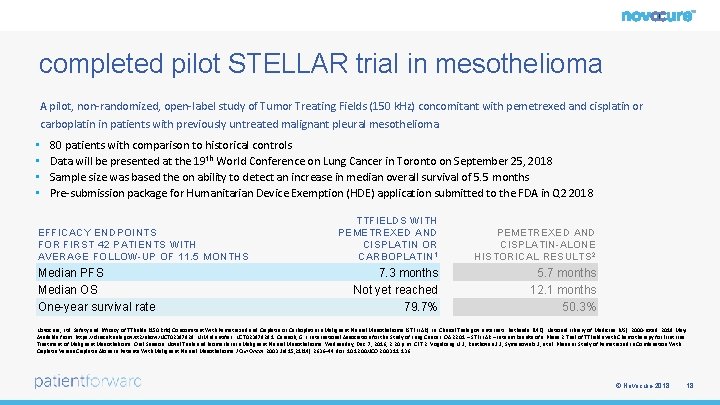
completed pilot STELLAR trial in mesothelioma A pilot, non-randomized, open-label study of Tumor Treating Fields (150 k. Hz) concomitant with pemetrexed and cisplatin or carboplatin in patients with previously untreated malignant pleural mesothelioma • • 80 patients with comparison to historical controls Data will be presented at the 19 th World Conference on Lung Cancer in Toronto on September 25, 2018 Sample size was based the on ability to detect an increase in median overall survival of 5. 5 months Pre-submission package for Humanitarian Device Exemption (HDE) application submitted to the FDA in Q 2 2018 EFFICACY ENDPOINTS FOR FIRST 42 PATIENTS WITH AVERAGE FOLLOW-UP OF 11. 5 MONTHS Median PFS Median OS One-year survival rate TTFIELDS WITH PEMETREXED AND CISPLATIN OR CARBOPLATIN 1 PEMETREXED AND CISPLATIN-ALONE HISTORICAL RESULTS 2 7. 3 months Not yet reached 79. 7% 5. 7 months 12. 1 months 50. 3% Novocure, Ltd. Safety and Efficacy of TTFields (150 k. Hz) Concomitant With Pemetrexed and Cisplatin or Carboplatin in Malignant Pleural Mesothelioma (STELLAR) In: Clinical. Trials. gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 -[cited 2018 May]. Available from: https: //clinicaltrials. gov/ct 2/show/NCT 02397928. NLM Identifier: NCT 02397928 1. Cerasoli, G. L. International Association for the Study of Lung Cancer. OA 22. 01 – STELLAR – Interim Results of a Phase 2 Trial of TTFields with Chemotherapy for First Line Treatment of Malignant Mesothelioma. Oral Session: Novel Trials and Biomarkers in Malignant Pleural Mesothelioma. Wednesday, Dec. 7, 2016, 2: 20 p. m. CET 2. Vogelzang N. J. , Rusthoven J. J. , Symanowski J. , et al. Phase III Study of Pemetrexed in Combination With Cisplatin Versus Cisplatin Alone in Patients With Malignant Pleural Mesothelioma J Clin Oncol. 2003 Jul 15; 21(14): 2636– 44. doi: 10. 1200/JCO. 2003. 11. 136 © Novocure 2018 18

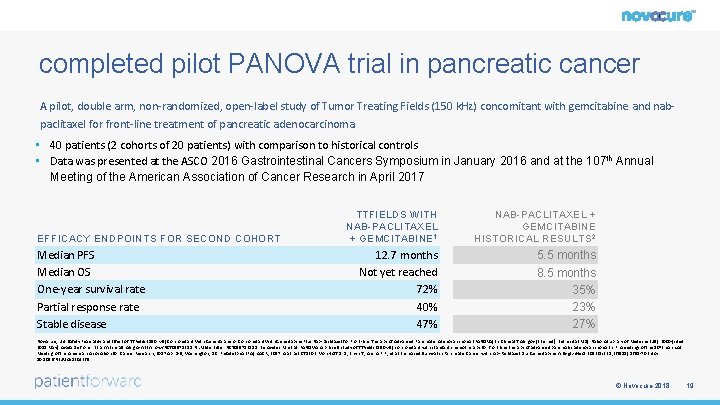
completed pilot PANOVA trial in pancreatic cancer A pilot, double arm, non-randomized, open-label study of Tumor Treating Fields (150 k. Hz) concomitant with gemcitabine and nabpaclitaxel for front-line treatment of pancreatic adenocarcinoma • 40 patients (2 cohorts of 20 patients) with comparison to historical controls • Data was presented at the ASCO 2016 Gastrointestinal Cancers Symposium in January 2016 and at the 107 th Annual Meeting of the American Association of Cancer Research in April 2017 EFFICACY ENDPOINTS FOR SECOND COHORT Median PFS Median OS One-year survival rate Partial response rate Stable disease TTFIELDS WITH NAB-PACLITAXEL + GEMCITABINE 1 12. 7 months Not yet reached 72% 40% 47% NAB-PACLITAXEL + GEMCITABINE HISTORICAL RESULTS 2 5. 5 months 8. 5 months 35% 23% 27% Novocure, Ltd. Safety Feasibility and Effect of TTFields (150 k. Hz) Concomitant With Gemcitabine or Concomitant With Gemcitabine Plus Nab-paclitaxel for Front-line Therapy of Advanced Pancreatic Adenocarcinoma (PANOVA) In: Clinical. Trials. gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 -[cited 2018 May]. Available from: https: //clinicaltrials. gov/ct 2/show/NCT 01971281. NLM Identifier: NCT 01971281 1. Benavides M. et. al. PANOVA: A phase II study of TTFields (150 k. Hz) concomitant with standard chemotherapy for front line therapy of advanced pancreatic adenocarcinoma In: Proceedings of the 107 th Annual Meeting of the American Association for Cancer Research; 2017 Apr 1 -5; Washington, DC. Philadelphia (PA): AACR; 2017. Abstract CT 130. 2. Von Hoff D. D. , Ervin T. , Arena F. P. , et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N Engl J Med. 2013 Oct 31; 369(18): 1691 -703. doi: 10. 1056/NEJMoa 1304369 © Novocure 2018 19

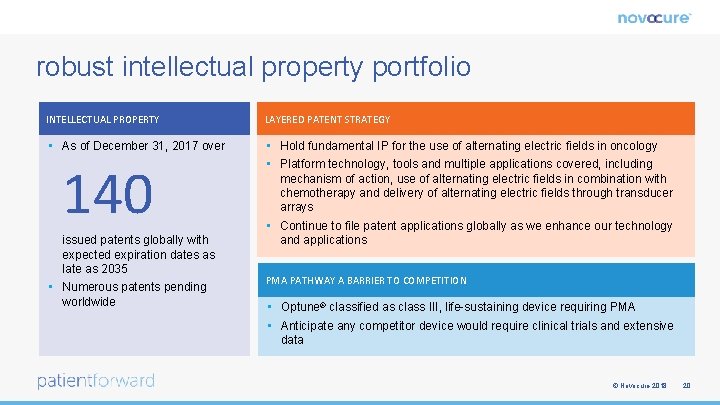
robust intellectual property portfolio INTELLECTUAL PROPERTY LAYERED PATENT STRATEGY • As of December 31, 2017 over • Hold fundamental IP for the use of alternating electric fields in oncology • Platform technology, tools and multiple applications covered, including mechanism of action, use of alternating electric fields in combination with chemotherapy and delivery of alternating electric fields through transducer arrays • Continue to file patent applications globally as we enhance our technology and applications 140 issued patents globally with expected expiration dates as late as 2035 • Numerous patents pending worldwide PMA PATHWAY A BARRIER TO COMPETITION • Optune® classified as class III, life-sustaining device requiring PMA • Anticipate any competitor device would require clinical trials and extensive data © Novocure 2018 20

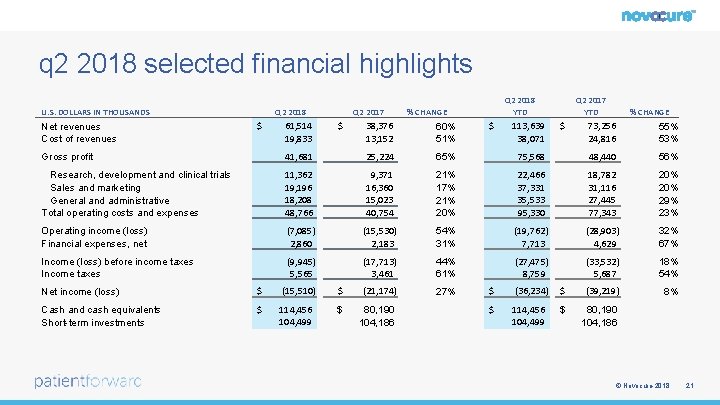
q 2 2018 selected financial highlights U. S. DOLLARS IN THOUSANDS Net revenues Cost of revenues Q 2 2018 $ 61, 514 19, 833 Q 2 2017 $ Q 2 2018 YTD % CHANGE 38, 376 13, 152 60% 51% $ 113, 639 38, 071 Q 2 2017 YTD $ % CHANGE 73, 256 24, 816 55% 53% Gross profit 41, 681 25, 224 65% 75, 568 48, 440 56% Research, development and clinical trials Sales and marketing General and administrative Total operating costs and expenses 11, 362 19, 196 18, 208 48, 766 9, 371 16, 360 15, 023 40, 754 21% 17% 21% 20% 22, 466 37, 331 35, 533 95, 330 18, 782 31, 116 27, 445 77, 343 20% 29% 23% Operating income (loss) Financial expenses, net (7, 085) 2, 860 (15, 530) 2, 183 54% 31% (19, 762) 7, 713 (28, 903) 4, 629 32% 67% Income (loss) before income taxes Income taxes (9, 945) 5, 565 (17, 713) 3, 461 44% 61% (27, 475) 8, 759 (33, 532) 5, 687 18% 54% 27% 8% Net income (loss) $ (15, 510) $ (21, 174) Cash and cash equivalents Short-term investments $ 114, 456 104, 499 $ 80, 190 104, 186 $ (36, 234) $ (39, 219) $ 114, 456 104, 499 $ 80, 190 104, 186 © Novocure 2018 21

© Novocure 2018 22