November 2 What is the difference between evaporation

November 2 What is the difference between evaporation and boiling? Objectives : * Liquids * Vapor pressure * Identify the factors that affect vapor presure * Learn to use table H * Intermolecular attractions * Evaporation * Boiling

LIQUIDS

LIQUID • A form of matter that has definite volume but no definite shape. • A liquid takes the shape of the container is in. • Particles are hold together by forces of attractions that are called intermolecular forces.

VAPOR • The gas phase of a substance that is ordinarily a solid of liquid at that temperature. • The vapor above the surface of a liquid exerts a characteristic pressure called vapor pressure.

EVAPORATION • It happens at ALL • temperatures and only at the surface of the liquid. The molecules at the surface that can escape the liquid inside a closed container produce the vapor pressure.
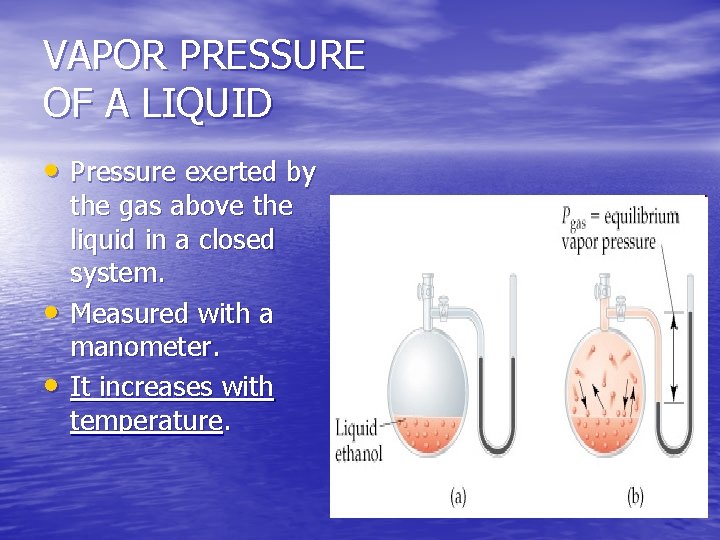
VAPOR PRESSURE OF A LIQUID • Pressure exerted by • • the gas above the liquid in a closed system. Measured with a manometer. It increases with temperature.
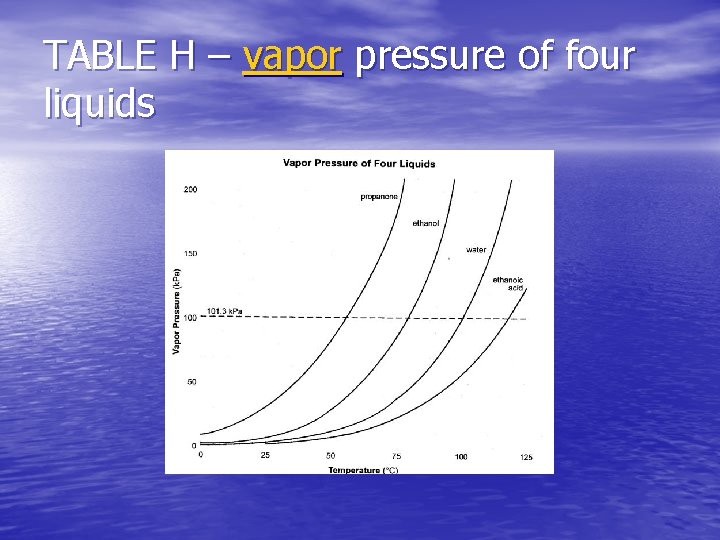
TABLE H – vapor pressure of four liquids

• *Evaporation is an endothermic process. When a liquid evaporates absorbs heat from the surroundings. • If the liquid is evaporating over our skin we feel cold. • Transpiration is a cooling process. Explain why.

STRONG INTERMOLECULAR ATTRACTIONS • If a liquid has STRONG intermolecular attractions the molecules tend to stay together as liquid. • A lot of energy is needed to separate the molecules. The liquid will have • * LOW VAPOR PRESSURE and • * HIGH BOILING POINT

• Liquids with weak forces of attraction will have • *high vapor pressure and • *low boiling points.

Boiling • A liquid boils when the vapor pressure of the liquid equals the external pressure. • The temperature at which the liquid boils is the boiling point.

NORMAL BOILING POINT • The temperature at which the vapor pressure of the liquid is equal to 1 atm or 101. 3 k. Pa. • For water is 1000 C. • Its vapor pressure at that temperature is 101. 3 k. Pa. • (Table H)

CONDENSATION • the phase change from liquid to gas. It is an exothermic process.

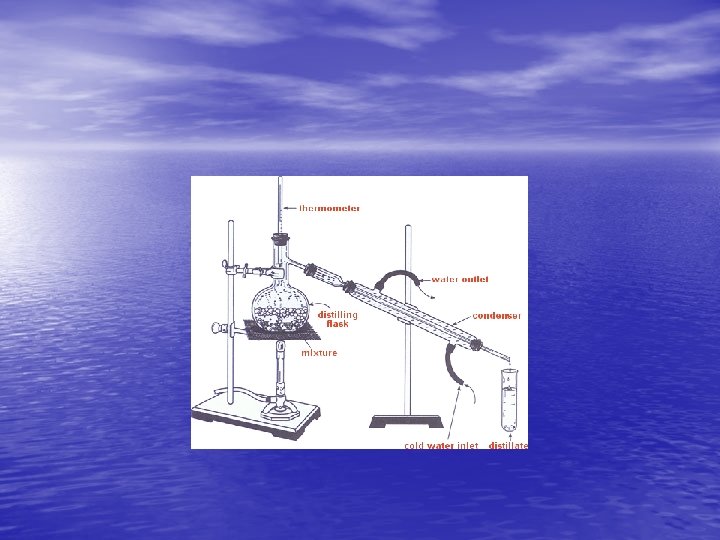
DISTILLATION • A method of separating homogeneous mixtures of solids in liquids or different liquids. • By boiling and condensing the vapors, the mixtures can be separated. • When a mixture of different liquids is heated up the liquid with weaker intermolecular attractions will boil first. The vapors can be condensed to separate the liquids.

Pressure Cooker • it works by increasing the pressure on top of the water. The BP at higher T will be higher and the food cooks faster.

HEAT OF VAPORIZATION The heat needed to completely vaporize 1 gram of liquid at is BP.
SOLIDS * Have definite shape and volume. * Particles are close together (packed). * Particles have 2 types of movement rotation and vibration in their places * Particles are arranged in a crystalline structure, that is a geometrical pattern that repeats itself.

AMORPHOUS SOLIDS • Lack an ordered internal structure, don’t have crystalline structure. • Do not have a definite melting point. • Example glass, asphalt, rubber

SUBLIMATION • Change of state from solid to gas without going through the liquid state. Endothermic • Examples: • Dry Ice • Iodine • Moth balls

DEPOSITION • Phase change from gas directly to solid.
- Slides: 21