Novel Surfactant Administration Strategies in Neonates Nemours Childrens













- Slides: 72

Novel Surfactant Administration Strategies in Neonates Nemours Children’s Hospital Grand Rounds June 10 th, 2015 Alan de Klerk, MBCh. B Division of Neonatology Nemours Children’s Hospital

FINANCIAL DISCLOSURE: Nothing to disclose Alan de Klerk, MBCh. B Division of Neonatology Nemours Children’s Hospital

Outline Ø Recent large RCTs Ø Novel approaches to surfactant administration: • CPAP/NIV With Less Invasive Surfactant Administration

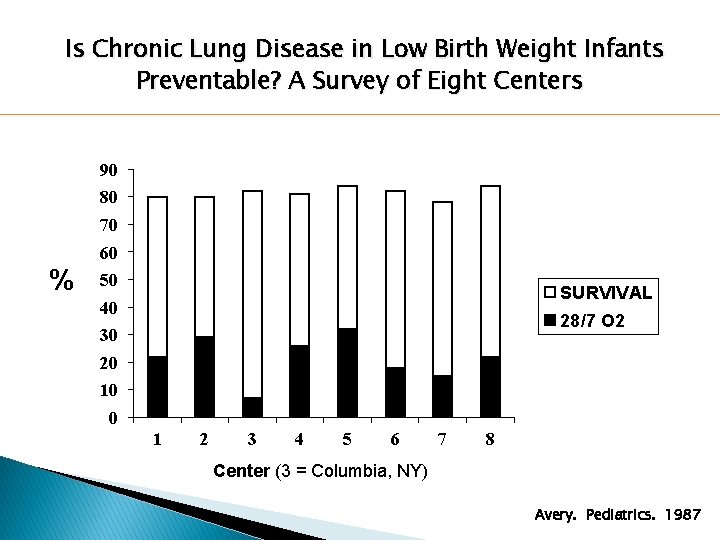
Is Chronic Lung Disease in Low Birth Weight Infants Preventable? A Survey of Eight Centers 90 80 70 60 % 50 SURVIVAL 40 28/7 O 2 30 20 10 0 1 2 3 4 5 6 7 8 Center (3 = Columbia, NY) Avery. Pediatrics. 1987

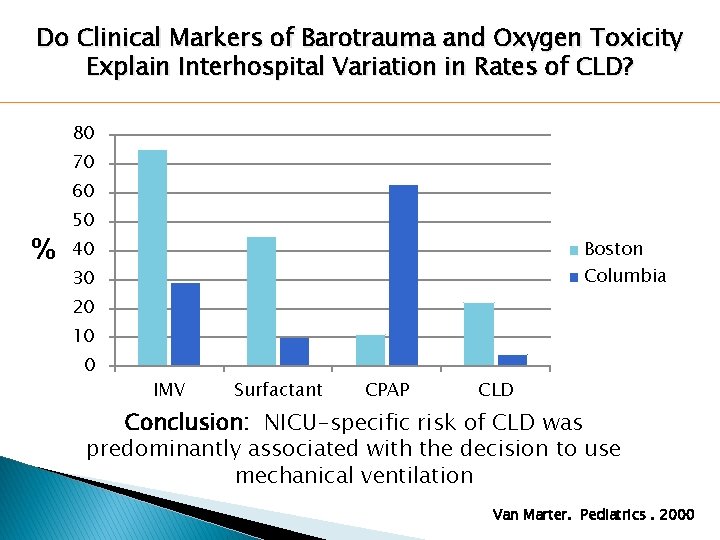
Do Clinical Markers of Barotrauma and Oxygen Toxicity Explain Interhospital Variation in Rates of CLD? 80 70 60 % 50 Boston 40 Columbia 30 20 10 0 IMV Surfactant CPAP CLD Conclusion: NICU-specific risk of CLD was predominantly associated with the decision to use mechanical ventilation Van Marter. Pediatrics. 2000

(Debatable) Generalizations! �Avoiding/limiting invasive ventilation (within reason) is desirable �Even when appropriately or optimally used, all forms of respiratory support are necessary evils �Inappropriately used they become unnecessary evils

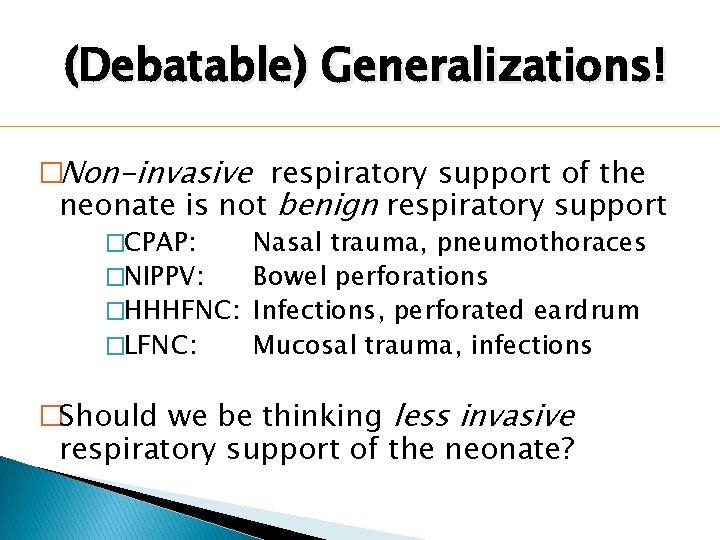
(Debatable) Generalizations! �Non-invasive respiratory support of the neonate is not benign respiratory support �CPAP: Nasal trauma, pneumothoraces �NIPPV: Bowel perforations �HHHFNC: Infections, perforated eardrum �LFNC: Mucosal trauma, infections �Should we be thinking less invasive respiratory support of the neonate?

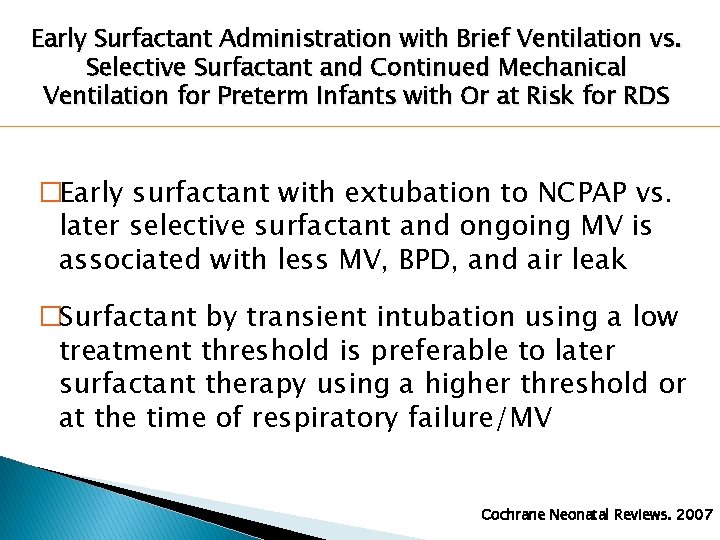
Early Surfactant Administration with Brief Ventilation vs. Selective Surfactant and Continued Mechanical Ventilation for Preterm Infants with Or at Risk for RDS �Early surfactant with extubation to NCPAP vs. later selective surfactant and ongoing MV is associated with less MV, BPD, and air leak �Surfactant by transient intubation using a low treatment threshold is preferable to later surfactant therapy using a higher threshold or at the time of respiratory failure/MV Cochrane Neonatal Reviews. 2007

Outline Ø Recent large RCTs Ø Novel approaches to surfactant administration: • CPAP With Less Invasive Surfactant Administration

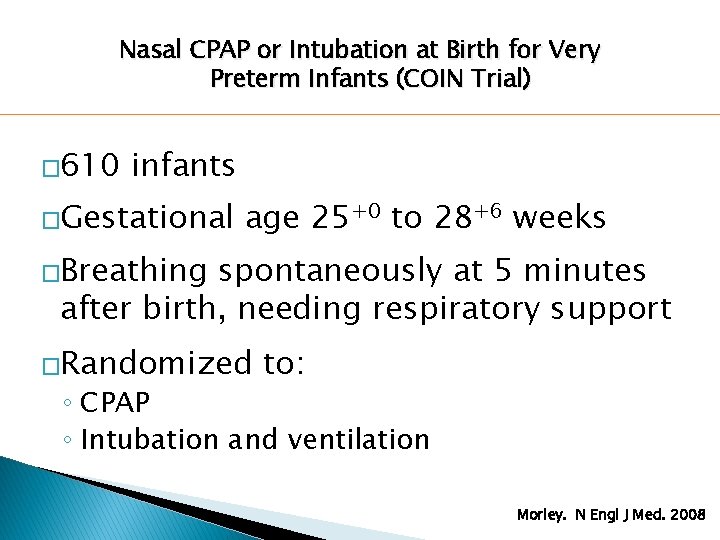
Nasal CPAP or Intubation at Birth for Very Preterm Infants (COIN Trial) � 610 infants �Gestational age 25+0 to 28+6 weeks �Breathing spontaneously at 5 minutes after birth, needing respiratory support �Randomized to: ◦ CPAP ◦ Intubation and ventilation Morley. N Engl J Med. 2008

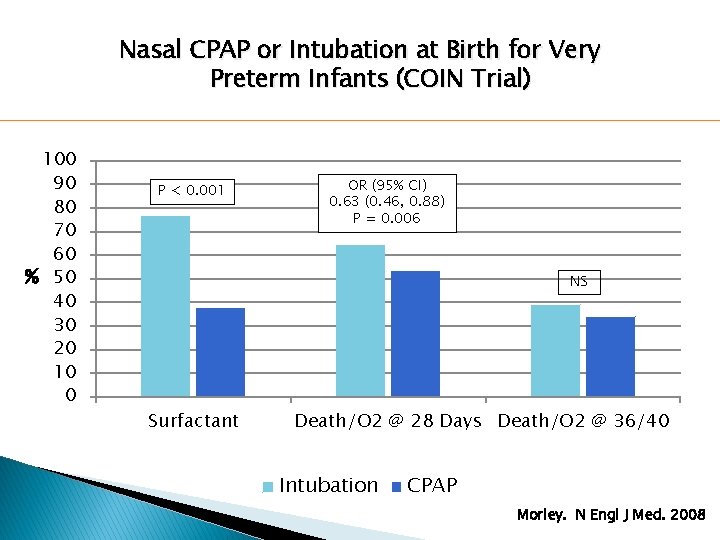
Nasal CPAP or Intubation at Birth for Very Preterm Infants (COIN Trial) 100 90 80 70 60 % 50 40 30 20 10 0 P < 0. 001 OR (95% CI) 0. 63 (0. 46, 0. 88) P = 0. 006 NS Surfactant Death/O 2 @ 28 Days Death/O 2 @ 36/40 Intubation CPAP Morley. N Engl J Med. 2008

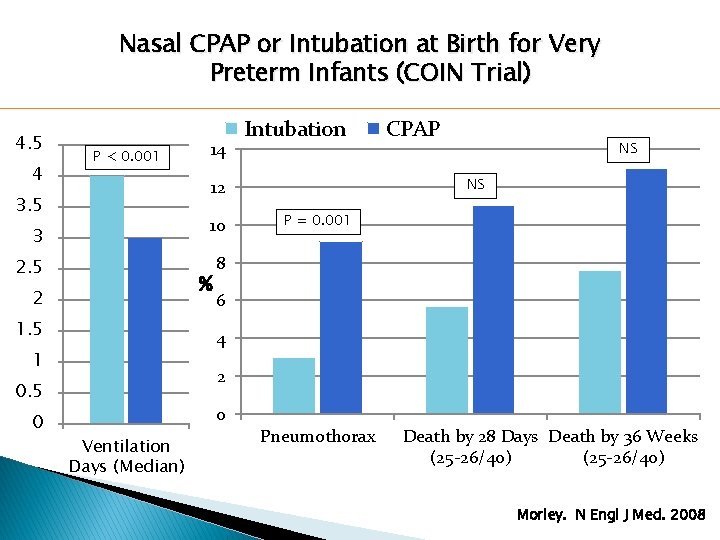
Nasal CPAP or Intubation at Birth for Very Preterm Infants (COIN Trial) 4. 5 4 P < 0. 001 14 3 10 2. 5 8 % 2 1. 5 CPAP NS NS 12 3. 5 P = 0. 001 6 4 1 2 0. 5 0 Intubation 0 Ventilation Days (Median) Pneumothorax Death by 28 Days Death by 36 Weeks (25 -26/40) Morley. N Engl J Med. 2008

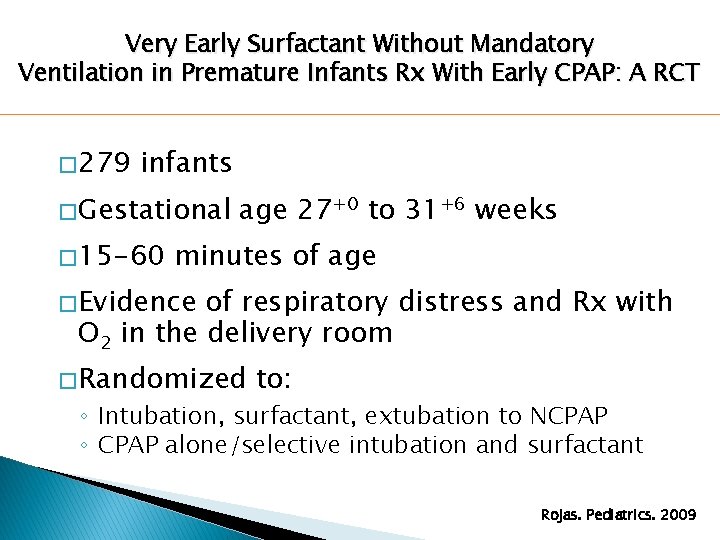
Very Early Surfactant Without Mandatory Ventilation in Premature Infants Rx With Early CPAP: A RCT � 279 infants � Gestational � 15 -60 age 27+0 to 31+6 weeks minutes of age � Evidence of respiratory distress and Rx with O 2 in the delivery room � Randomized to: ◦ Intubation, surfactant, extubation to NCPAP ◦ CPAP alone/selective intubation and surfactant Rojas. Pediatrics. 2009

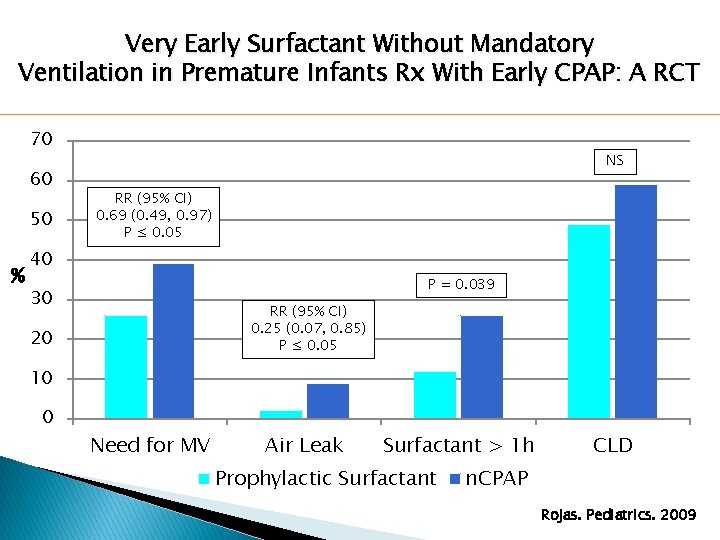
Very Early Surfactant Without Mandatory Ventilation in Premature Infants Rx With Early CPAP: A RCT 70 60 50 % NS RR (95% CI) 0. 69 (0. 49, 0. 97) P ≤ 0. 05 40 P = 0. 039 30 RR (95% CI) 0. 25 (0. 07, 0. 85) P ≤ 0. 05 20 10 0 Need for MV Air Leak Surfactant > 1 h Prophylactic Surfactant CLD n. CPAP Rojas. Pediatrics. 2009

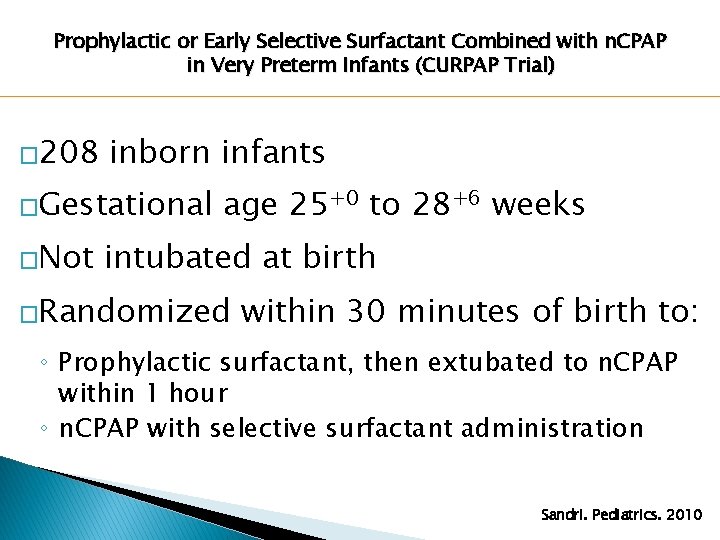
Prophylactic or Early Selective Surfactant Combined with n. CPAP in Very Preterm Infants (CURPAP Trial) � 208 inborn infants �Gestational �Not age 25+0 to 28+6 weeks intubated at birth �Randomized within 30 minutes of birth to: ◦ Prophylactic surfactant, then extubated to n. CPAP within 1 hour ◦ n. CPAP with selective surfactant administration Sandri. Pediatrics. 2010

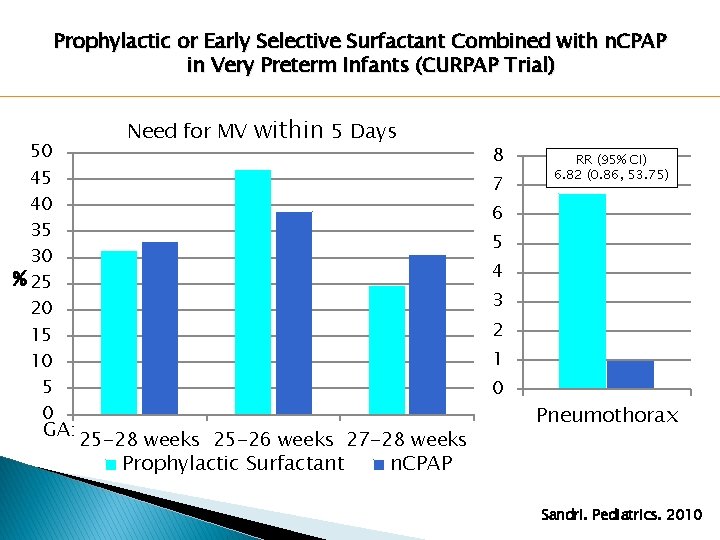
Prophylactic or Early Selective Surfactant Combined with n. CPAP in Very Preterm Infants (CURPAP Trial) 50 45 40 35 30 % 25 20 15 10 5 0 GA: Need for MV within 5 Days 8 7 RR (95% CI) 6. 82 (0. 86, 53. 75) 6 5 4 3 2 1 0 25 -28 weeks 25 -26 weeks 27 -28 weeks Prophylactic Surfactant Pneumothorax n. CPAP Sandri. Pediatrics. 2010

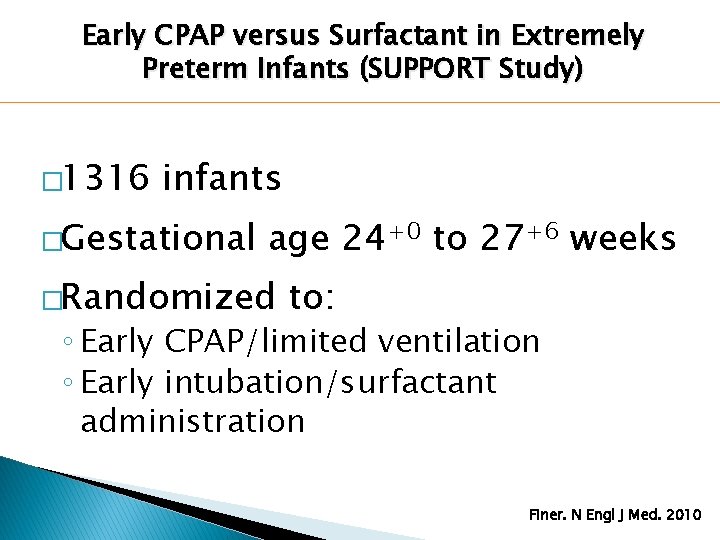
Early CPAP versus Surfactant in Extremely Preterm Infants (SUPPORT Study) � 1316 infants �Gestational age 24+0 to 27+6 weeks �Randomized to: ◦ Early CPAP/limited ventilation ◦ Early intubation/surfactant administration Finer. N Engl J Med. 2010

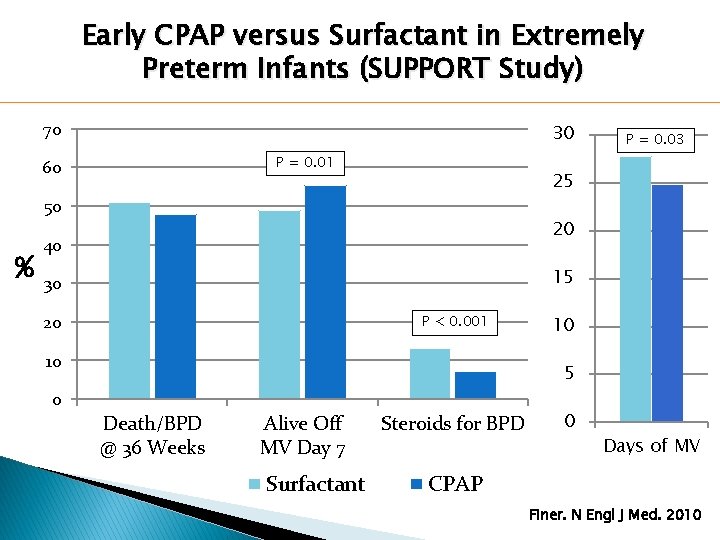
Early CPAP versus Surfactant in Extremely Preterm Infants (SUPPORT Study) 70 30 P = 0. 01 60 25 50 % P = 0. 03 20 40 15 30 20 P < 0. 001 10 10 5 0 Death/BPD @ 36 Weeks Alive Off MV Day 7 Surfactant Steroids for BPD 0 Days of MV CPAP Finer. N Engl J Med. 2010

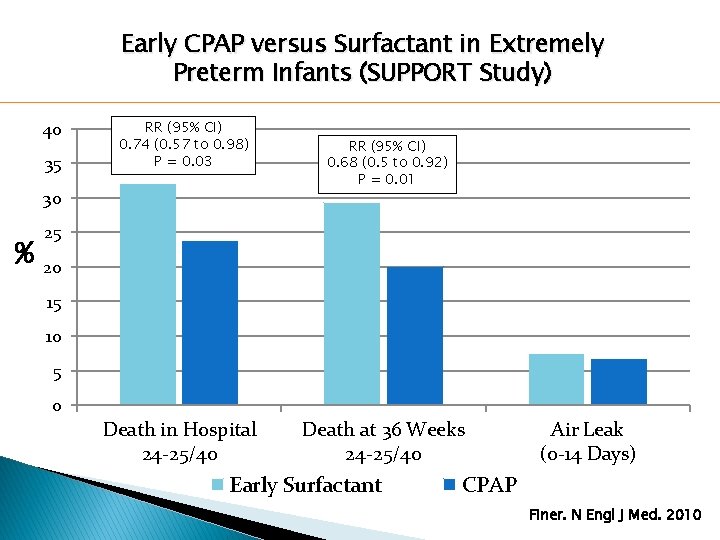
Early CPAP versus Surfactant in Extremely Preterm Infants (SUPPORT Study) 40 35 RR (95% CI) 0. 74 (0. 57 to 0. 98) P = 0. 03 30 % RR (95% CI) 0. 68 (0. 5 to 0. 92) P = 0. 01 25 20 15 10 5 0 Death in Hospital 24 -25/40 Death at 36 Weeks 24 -25/40 Early Surfactant Air Leak (0 -14 Days) CPAP Finer. N Engl J Med. 2010

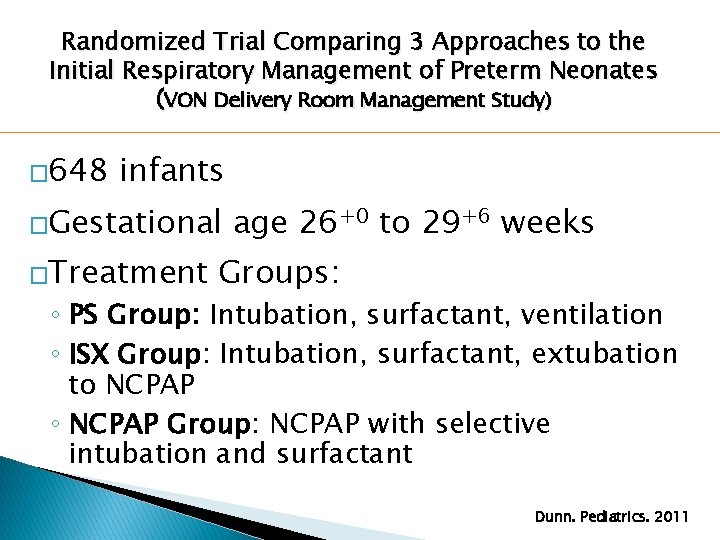
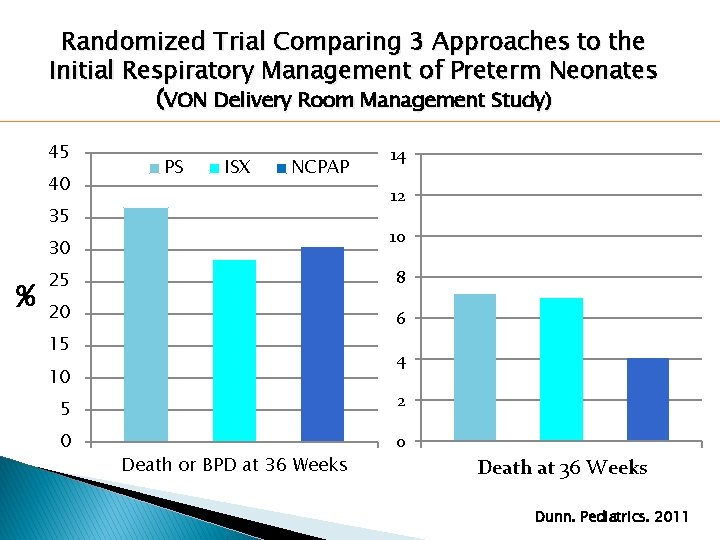
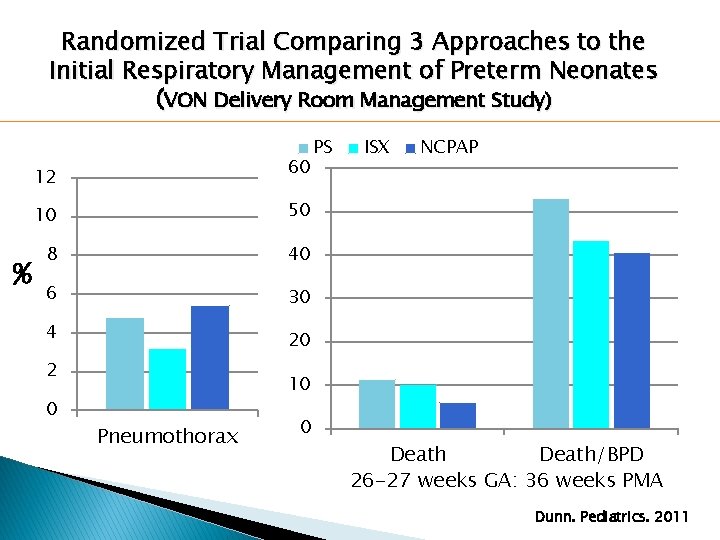
Randomized Trial Comparing 3 Approaches to the Initial Respiratory Management of Preterm Neonates (VON Delivery Room Management Study) � 648 infants �Gestational �Treatment age 26+0 to 29+6 weeks Groups: ◦ PS Group: Intubation, surfactant, ventilation ◦ ISX Group: Intubation, surfactant, extubation to NCPAP ◦ NCPAP Group: NCPAP with selective intubation and surfactant Dunn. Pediatrics. 2011

Randomized Trial Comparing 3 Approaches to the Initial Respiratory Management of Preterm Neonates (VON Delivery Room Management Study) 45 40 PS ISX NCPAP 12 35 10 30 % 14 25 8 20 6 15 4 10 5 2 0 0 Death or BPD at 36 Weeks Death at 36 Weeks Dunn. Pediatrics. 2011

Randomized Trial Comparing 3 Approaches to the Initial Respiratory Management of Preterm Neonates (VON Delivery Room Management Study) 60 12 % 10 50 8 40 6 30 4 20 2 0 PS ISX NCPAP 10 Pneumothorax 0 Death/BPD 26 -27 weeks GA: 36 weeks PMA Dunn. Pediatrics. 2011

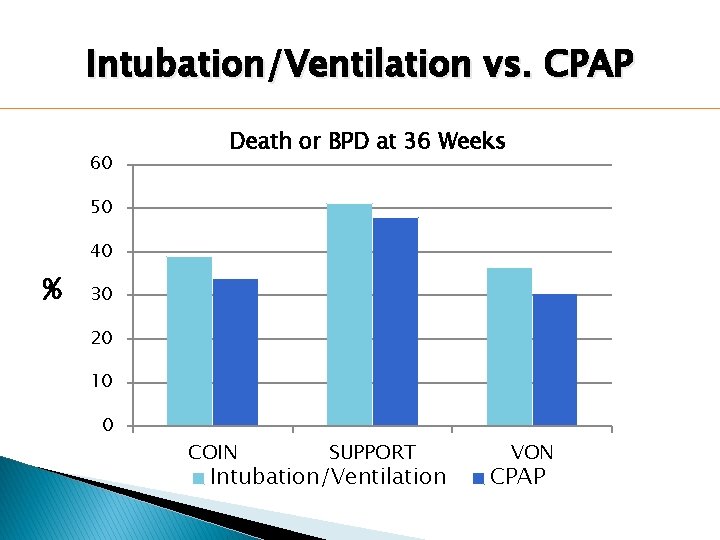
Intubation/Ventilation vs. CPAP 60 Death or BPD at 36 Weeks 50 40 % 30 20 10 0 COIN SUPPORT Intubation/Ventilation VON CPAP

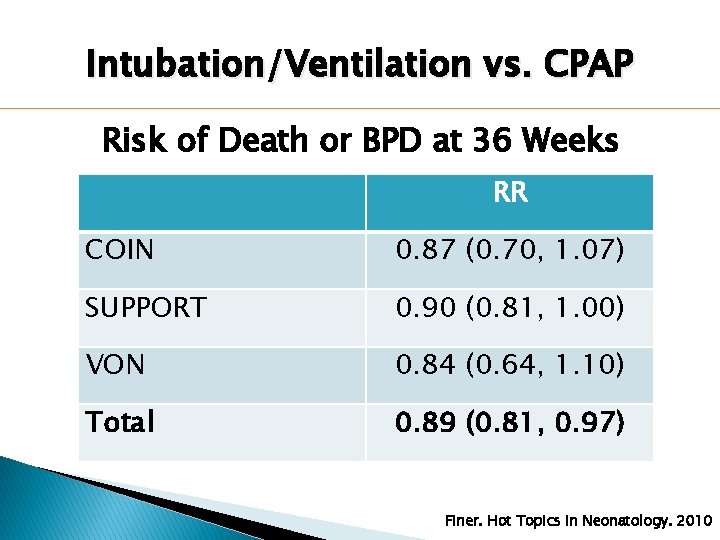
Intubation/Ventilation vs. CPAP Risk of Death or BPD at 36 Weeks RR COIN 0. 87 (0. 70, 1. 07) SUPPORT 0. 90 (0. 81, 1. 00) VON 0. 84 (0. 64, 1. 10) Total 0. 89 (0. 81, 0. 97) Finer. Hot Topics in Neonatology. 2010

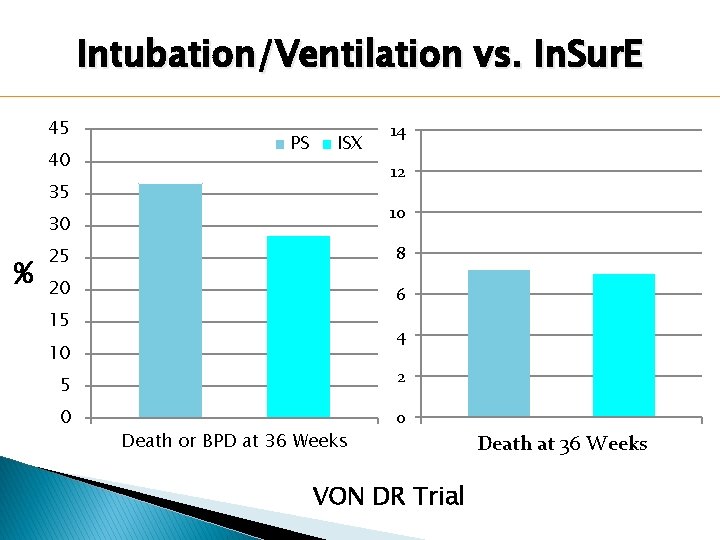
Intubation/Ventilation vs. In. Sur. E 45 40 PS ISX 12 35 10 30 % 14 25 8 20 6 15 4 10 5 2 0 0 Death or BPD at 36 Weeks VON DR Trial Death at 36 Weeks

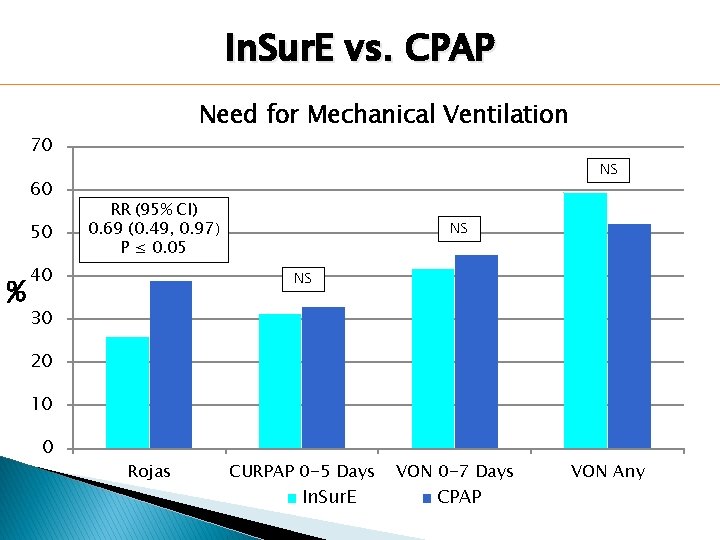
In. Sur. E vs. CPAP Need for Mechanical Ventilation 70 60 50 % NS RR (95% CI) 0. 69 (0. 49, 0. 97) P ≤ 0. 05 40 NS NS 30 20 10 0 Rojas CURPAP 0 -5 Days In. Sur. E VON 0 -7 Days CPAP VON Any

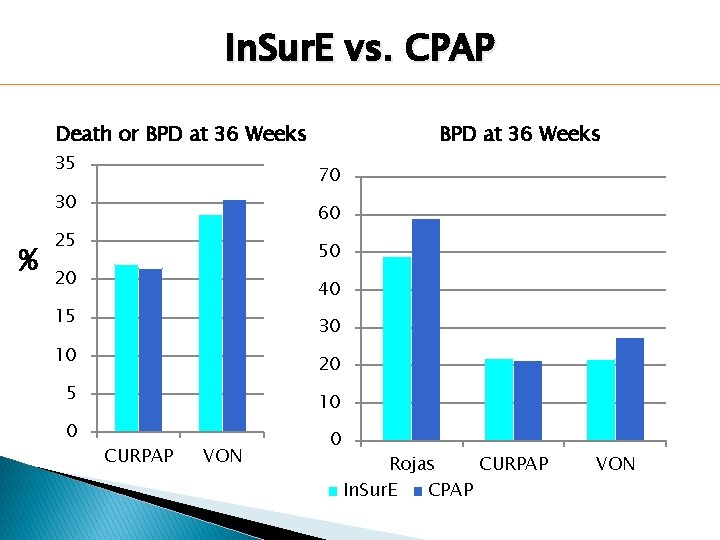
In. Sur. E vs. CPAP Death or BPD at 36 Weeks 35 70 30 % BPD at 36 Weeks 60 25 50 20 40 15 30 10 20 5 10 0 0 CURPAP VON Rojas CURPAP In. Sur. E CPAP VON

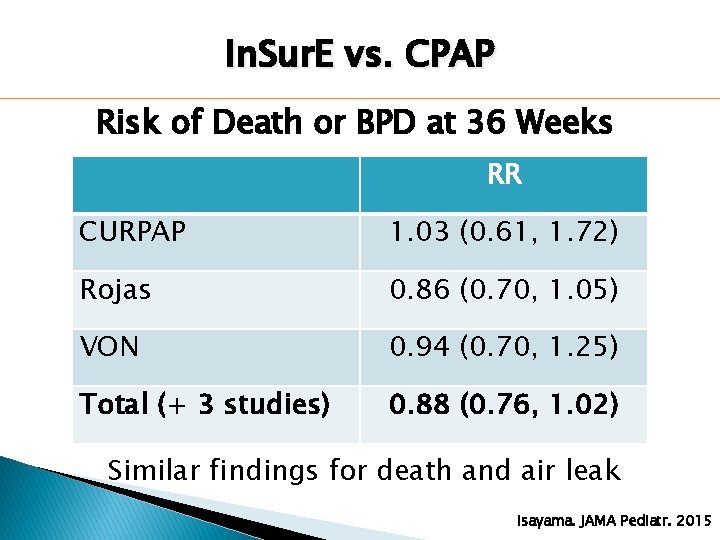
In. Sur. E vs. CPAP Risk of Death or BPD at 36 Weeks RR CURPAP 1. 03 (0. 61, 1. 72) Rojas 0. 86 (0. 70, 1. 05) VON 0. 94 (0. 70, 1. 25) Total (+ 3 studies) 0. 88 (0. 76, 1. 02) Similar findings for death and air leak Isayama. JAMA Pediatr. 2015

Conclusions �Non-invasive support (CPAP or In. Sur. E) is at least as effective as invasive support �In. Sur. E is not consistently superior to CPAP alone �Long term outcomes are needed

Hypothesis �Would even less invasive surfactant administration to a spontaneously breathing neonate improve outcomes further?

CPAP With Less Invasive Surfactant Administration �Intrapartum/pharyngeal administration �Administration airway (LMA) �Aerosolized �Thin via laryngeal mask surfactant catheter administration

CPAP With Less Invasive Surfactant Administration �Intrapartum/pharyngeal administration �Administration airway (LMA) �Aerosolized �Thin via laryngeal mask surfactant catheter administration

Ten Centre Trial of Artificial Surfactant (Artificial Lung Expanding Compound) in Very Premature Babies �RCT of 328 preterm infants ◦ 25 - 29 weeks gestation �Procedure: ◦ Pharyngeal ALEC or normal saline at birth ◦ Up to 3 more doses if intubated DOL #1 Ten Centre Study Group. BMJ. 1987

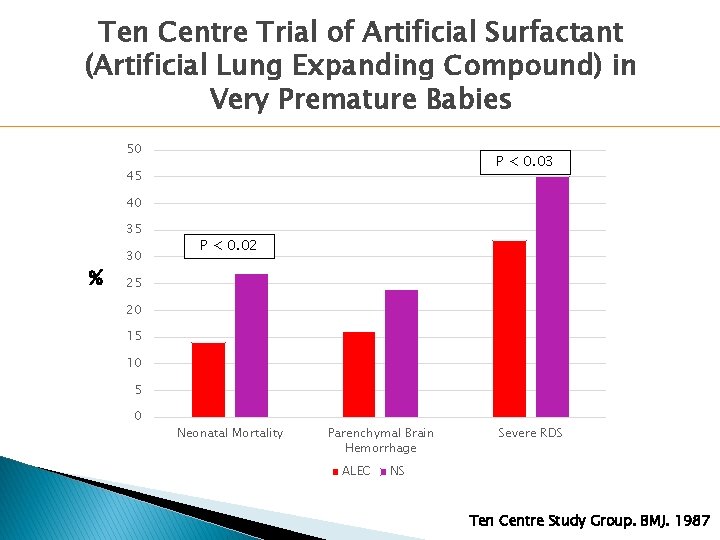
Ten Centre Trial of Artificial Surfactant (Artificial Lung Expanding Compound) in Very Premature Babies 50 P < 0. 03 45 40 35 % 30 P < 0. 02 25 20 15 10 5 0 Neonatal Mortality Parenchymal Brain Hemorrhage ALEC Severe RDS NS Ten Centre Study Group. BMJ. 1987

Technique for Intrapartum Administration of Surfactant without Requirement for an ETT �Non-randomized � 23 feasibility study preterm infants ◦ 27 - 30 weeks gestation, 560 – 1804 g �Procedure: ◦ Nasopharyngeal airway suctioned ◦ Infasurf instilled into nasopharynx before delivery of shoulders ◦ CPAP by mask as breathing initiated, then maintained on CPAP for 48+ hours Kattwinkel. J Perinatol. 2004

Technique for Intrapartum Administration of Surfactant without Requirement for an ETT � Results: ◦ 13 of 15 vaginally delivered babies weaned quickly to room air with no further surfactant or intubation ◦ 5 of 8 C-section babies required intubation, 2 received surfactant � Conclusion: ◦ NP surfactant at birth appears relatively safe and simple, especially for vaginal births Kattwinkel. J Perinatol. 2004

CPAP With Less Invasive Surfactant Administration �Intrapartum/pharyngeal administration �Administration airway (LMA) �Aerosolized �Thin via laryngeal mask surfactant catheter administration

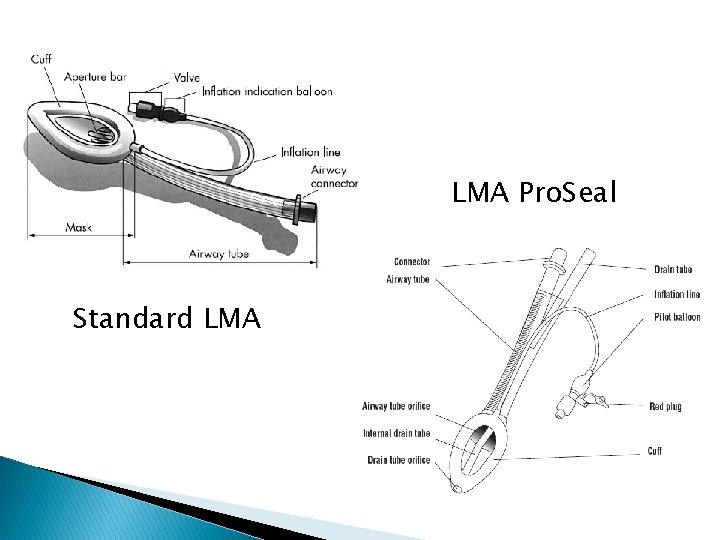
LMA Pro. Seal Standard LMA

LMA Used as a Delivery Conduit for the Administration of Surfactant to Preterm Infants with RDS �Feasibility trial of 8 infants ◦ Median GA: 31 weeks (28 -35 weeks) ◦ Median BW: 1700 g (880 -2520 g) ◦ RDS treated with nasal CPAP �Procedure: ◦ Surfactant administered via LMA without sedation/analgesia Trevisanuto. Biol Neonate. 2005

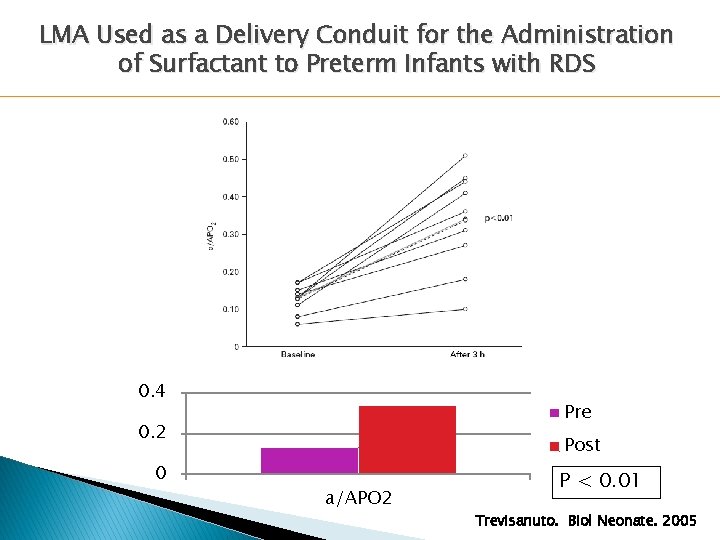
LMA Used as a Delivery Conduit for the Administration of Surfactant to Preterm Infants with RDS 0. 4 Pre 0. 2 0 Post a/APO 2 P < 0. 01 Trevisanuto. Biol Neonate. 2005

Laryngeal Mask Airway for Surfactant Administration in a Newborn Animal Model � RCT of newborn piglets with lung injury induced by normal saline surfactant washout � On CPAP via short binasal prongs � Randomized to: ◦ Surfactant via ETT ◦ Surfactant via LMA ◦ LMA, no surfactant � Returned (n = 8) to CPAP after 5 minutes Roberts. Ped Research. 2010

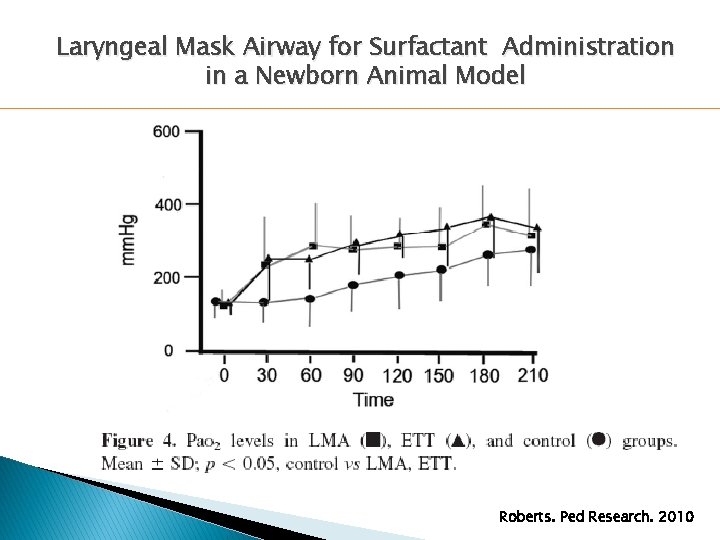
Laryngeal Mask Airway for Surfactant Administration in a Newborn Animal Model Roberts. Ped Research. 2010

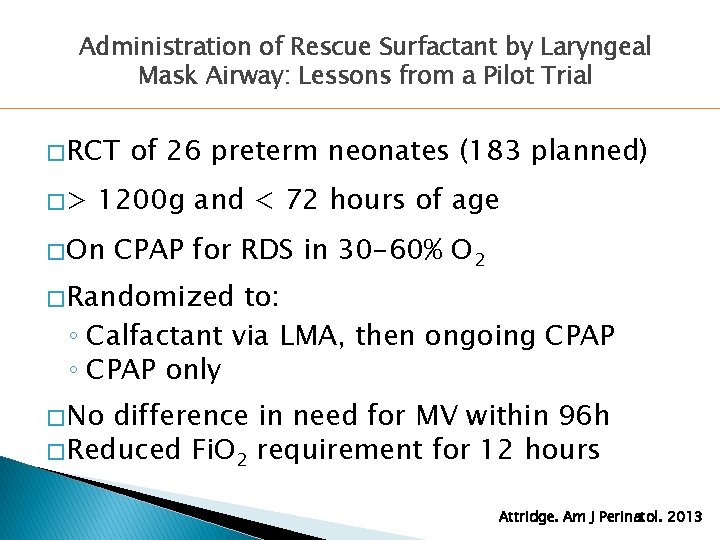
Administration of Rescue Surfactant by Laryngeal Mask Airway: Lessons from a Pilot Trial � RCT �> of 26 preterm neonates (183 planned) 1200 g and < 72 hours of age � On CPAP for RDS in 30 -60% O 2 � Randomized to: ◦ Calfactant via LMA, then ongoing CPAP ◦ CPAP only � No difference in need for MV within 96 h � Reduced Fi. O 2 requirement for 12 hours Attridge. Am J Perinatol. 2013

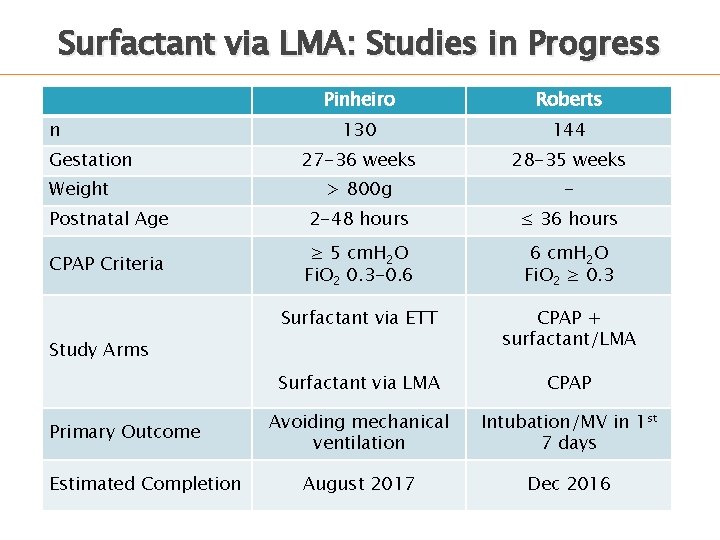
Surfactant via LMA: Studies in Progress Pinheiro Roberts 130 144 27 -36 weeks 28 -35 weeks > 800 g - Postnatal Age 2 -48 hours ≤ 36 hours CPAP Criteria ≥ 5 cm. H 2 O Fi. O 2 0. 3 -0. 6 6 cm. H 2 O Fi. O 2 ≥ 0. 3 Surfactant via ETT CPAP + surfactant/LMA Surfactant via LMA CPAP Avoiding mechanical ventilation Intubation/MV in 1 st 7 days August 2017 Dec 2016 n Gestation Weight Study Arms Primary Outcome Estimated Completion

CPAP With Less Invasive Surfactant Administration �Intrapartum/pharyngeal administration �Administration airway (LMA) �Aerosolized �Thin via laryngeal mask surfactant catheter administration

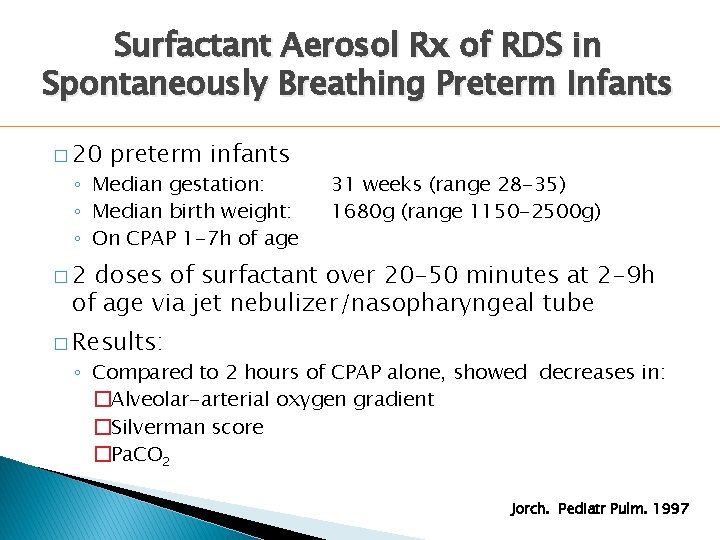
Surfactant Aerosol Rx of RDS in Spontaneously Breathing Preterm Infants � 20 preterm infants ◦ Median gestation: ◦ Median birth weight: ◦ On CPAP 1 -7 h of age 31 weeks (range 28 -35) 1680 g (range 1150 -2500 g) � 2 doses of surfactant over 20 -50 minutes at 2 -9 h of age via jet nebulizer/nasopharyngeal tube � Results: ◦ Compared to 2 hours of CPAP alone, showed decreases in: �Alveolar-arterial oxygen gradient �Silverman score �Pa. CO 2 Jorch. Pediatr Pulm. 1997

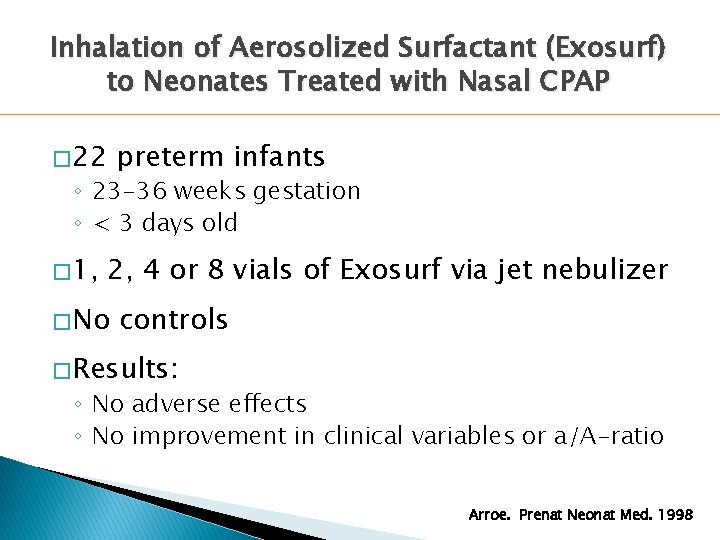
Inhalation of Aerosolized Surfactant (Exosurf) to Neonates Treated with Nasal CPAP � 22 preterm infants ◦ 23 -36 weeks gestation ◦ < 3 days old � 1, 2, 4 or 8 vials of Exosurf via jet nebulizer � No controls � Results: ◦ No adverse effects ◦ No improvement in clinical variables or a/A-ratio Arroe. Prenat Neonat Med. 1998

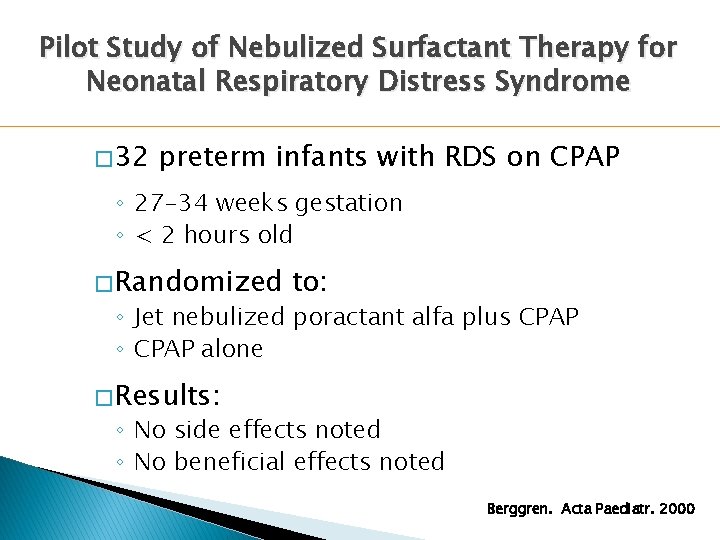
Pilot Study of Nebulized Surfactant Therapy for Neonatal Respiratory Distress Syndrome � 32 preterm infants with RDS on CPAP ◦ 27 -34 weeks gestation ◦ < 2 hours old � Randomized to: ◦ Jet nebulized poractant alfa plus CPAP ◦ CPAP alone � Results: ◦ No side effects noted ◦ No beneficial effects noted Berggren. Acta Paediatr. 2000

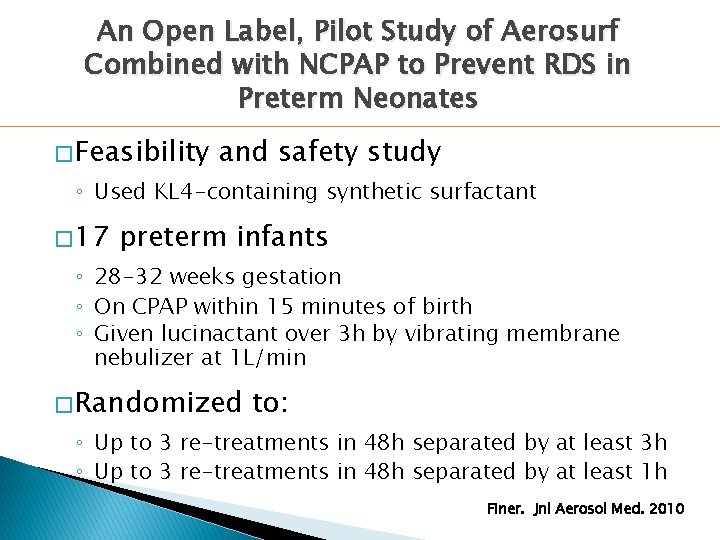
An Open Label, Pilot Study of Aerosurf Combined with NCPAP to Prevent RDS in Preterm Neonates � Feasibility and safety study ◦ Used KL 4 -containing synthetic surfactant � 17 preterm infants ◦ 28 -32 weeks gestation ◦ On CPAP within 15 minutes of birth ◦ Given lucinactant over 3 h by vibrating membrane nebulizer at 1 L/min � Randomized to: ◦ Up to 3 re-treatments in 48 h separated by at least 3 h ◦ Up to 3 re-treatments in 48 h separated by at least 1 h Finer. Jnl Aerosol Med. 2010

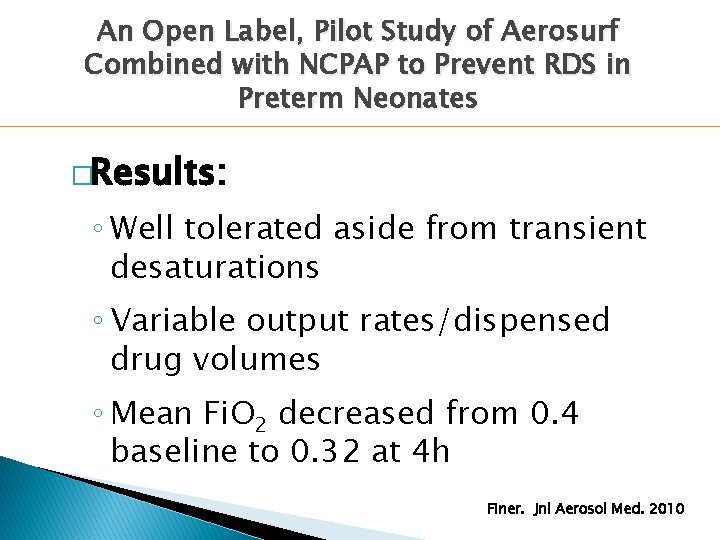
An Open Label, Pilot Study of Aerosurf Combined with NCPAP to Prevent RDS in Preterm Neonates �Results: ◦ Well tolerated aside from transient desaturations ◦ Variable output rates/dispensed drug volumes ◦ Mean Fi. O 2 decreased from 0. 4 baseline to 0. 32 at 4 h Finer. Jnl Aerosol Med. 2010

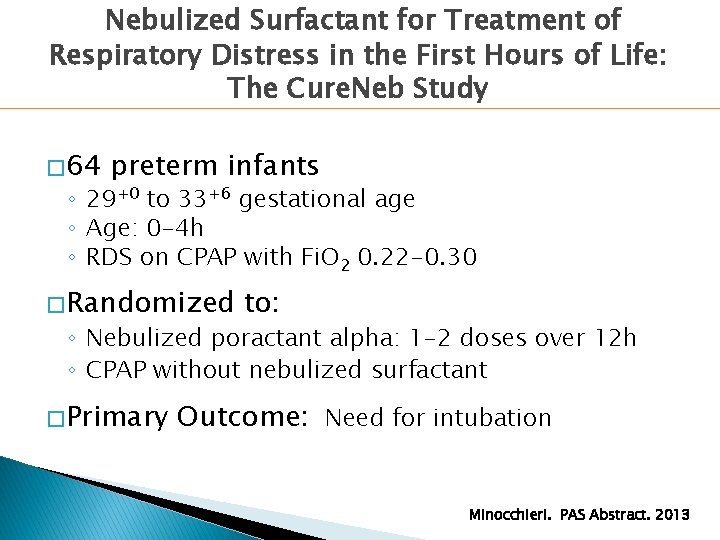
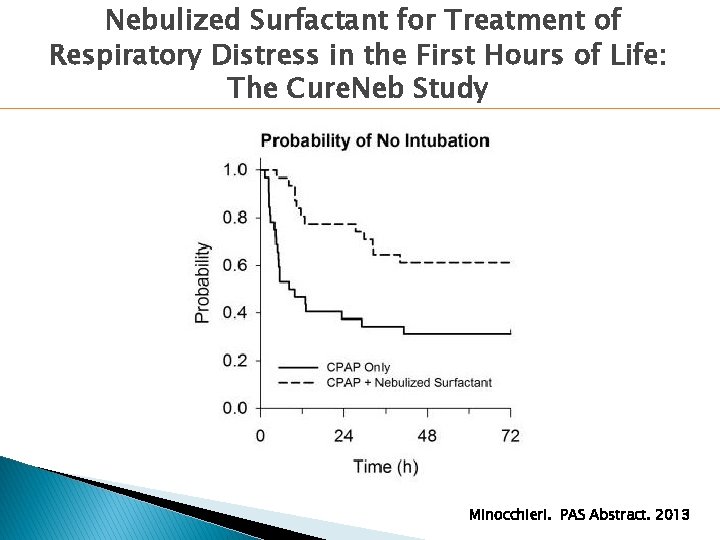
Nebulized Surfactant for Treatment of Respiratory Distress in the First Hours of Life: The Cure. Neb Study � 64 preterm infants ◦ 29+0 to 33+6 gestational age ◦ Age: 0 -4 h ◦ RDS on CPAP with Fi. O 2 0. 22 -0. 30 � Randomized to: ◦ Nebulized poractant alpha: 1 -2 doses over 12 h ◦ CPAP without nebulized surfactant � Primary Outcome: Need for intubation Minocchieri. PAS Abstract. 2013

Nebulized Surfactant for Treatment of Respiratory Distress in the First Hours of Life: The Cure. Neb Study Minocchieri. PAS Abstract. 2013

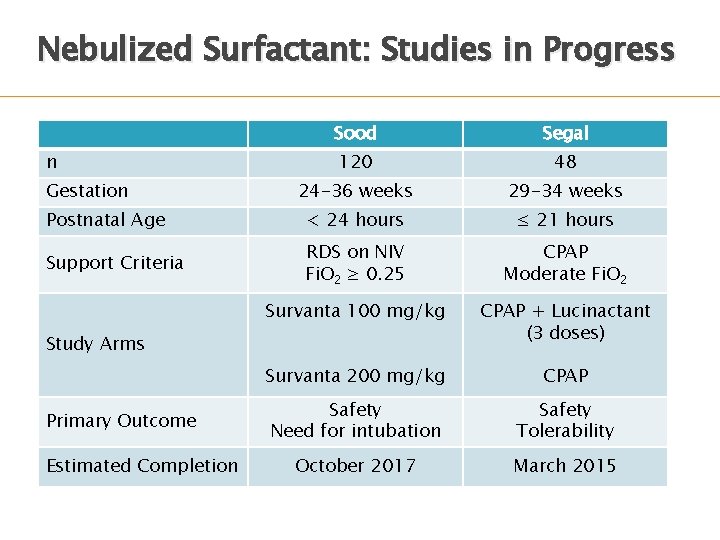
Nebulized Surfactant: Studies in Progress Sood Segal 120 48 24 -36 weeks 29 -34 weeks Postnatal Age < 24 hours ≤ 21 hours Support Criteria RDS on NIV Fi. O 2 ≥ 0. 25 CPAP Moderate Fi. O 2 Survanta 100 mg/kg CPAP + Lucinactant (3 doses) Survanta 200 mg/kg CPAP Safety Need for intubation Safety Tolerability October 2017 March 2015 n Gestation Study Arms Primary Outcome Estimated Completion

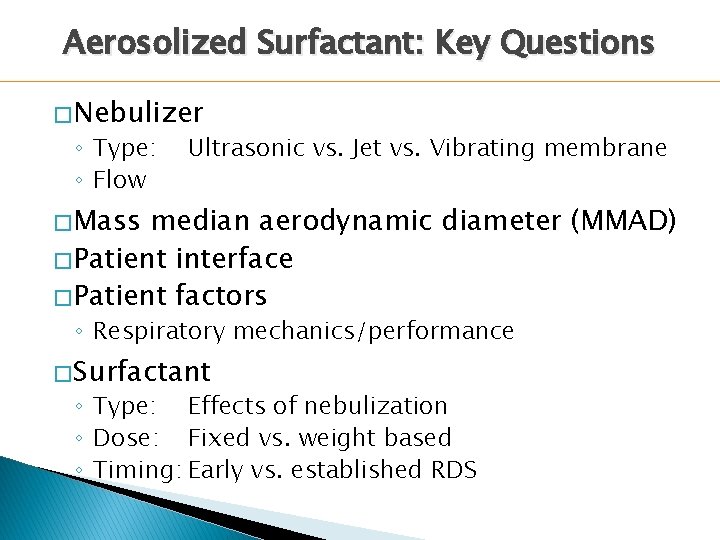
Aerosolized Surfactant: Key Questions � Nebulizer ◦ Type: ◦ Flow Ultrasonic vs. Jet vs. Vibrating membrane � Mass median aerodynamic diameter (MMAD) � Patient interface � Patient factors ◦ Respiratory mechanics/performance � Surfactant ◦ Type: Effects of nebulization ◦ Dose: Fixed vs. weight based ◦ Timing: Early vs. established RDS

CPAP With Less Invasive Surfactant Administration �Intrapartum/pharyngeal administration �Administration airway (LMA) �Aerosolized �Thin via laryngeal mask surfactant catheter administration

MIST Study - Technique Dargaville

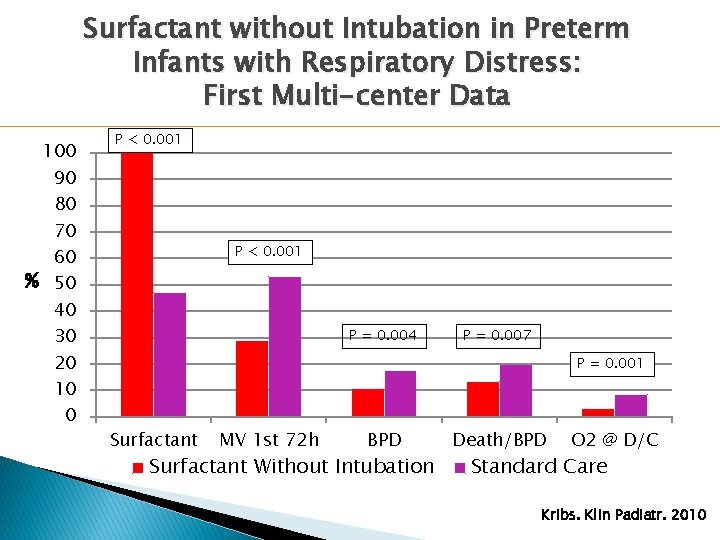
Surfactant without Intubation in Preterm Infants with Respiratory Distress: First Multi-center Data � Observational data comparing “surfactant without intubation” with standard care from 2003 - 2007 � 1542 preterm infants < 1500 g and ≤ 30 weeks GA � Procedure: ◦ Performed on n. CPAP ◦ No mandatory sedation/analgesia ◦ Surfactant given over 1 -5 minutes via thin catheter placed in trachea under direct laryngoscopy Kribs. Klin Padiatr. 2010

Surfactant without Intubation in Preterm Infants with Respiratory Distress: First Multi-center Data 100 90 80 70 60 % 50 40 30 20 10 0 P < 0. 001 P = 0. 004 P = 0. 007 P = 0. 001 Surfactant MV 1 st 72 h BPD Surfactant Without Intubation Death/BPD O 2 @ D/C Standard Care Kribs. Klin Padiatr. 2010

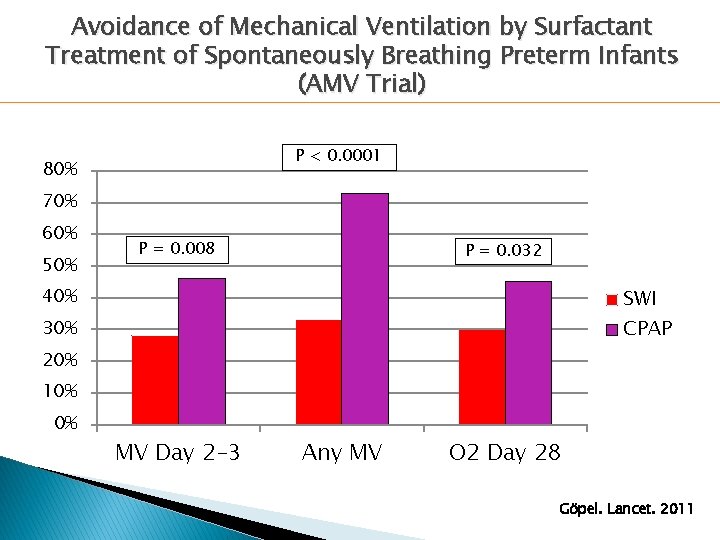
Avoidance of Mechanical Ventilation by Surfactant Treatment of Spontaneously Breathing Preterm Infants (AMV Trial) � 220 ◦ ◦ infants Gestational age 26+0 to 28+6 weeks < 1500 g birth weight < 12 hours of age RDS on CPAP with Fi. O 2 > 0. 30 � Randomized to: ◦ Surfactant via thin ET catheter on CPAP ◦ CPAP with rescue intubation/surfactant if indicated � Primary Outcome: Need for mechanical ventilation OR p. CO 2 > 65 or Fi. O 2 > 0. 6 for 2+ hours Göpel. Lancet. 2011

Avoidance of Mechanical Ventilation by Surfactant Treatment of Spontaneously Breathing Preterm Infants (AMV Trial) P < 0. 0001 80% 70% 60% 50% P = 0. 008 P = 0. 032 40% SWI CPAP 30% 20% 10% 0% MV Day 2 -3 Any MV O 2 Day 28 Göpel. Lancet. 2011

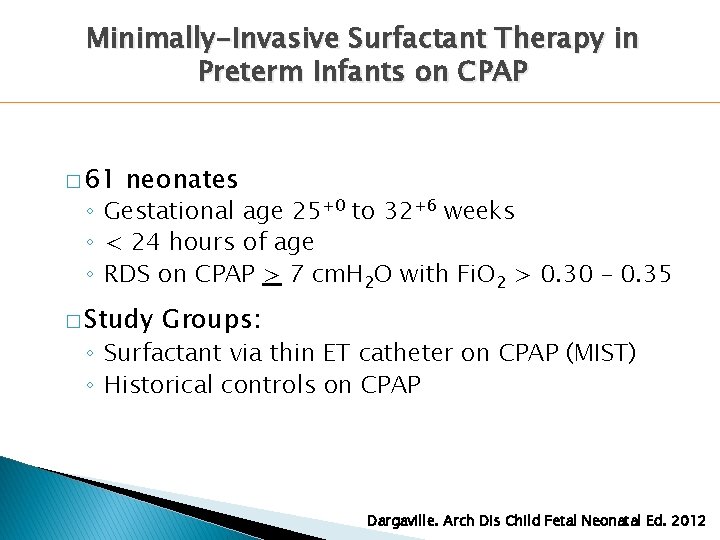
Minimally-Invasive Surfactant Therapy in Preterm Infants on CPAP � 61 neonates ◦ Gestational age 25+0 to 32+6 weeks ◦ < 24 hours of age ◦ RDS on CPAP > 7 cm. H 2 O with Fi. O 2 > 0. 30 – 0. 35 � Study Groups: ◦ Surfactant via thin ET catheter on CPAP (MIST) ◦ Historical controls on CPAP Dargaville. Arch Dis Child Fetal Neonatal Ed. 2012

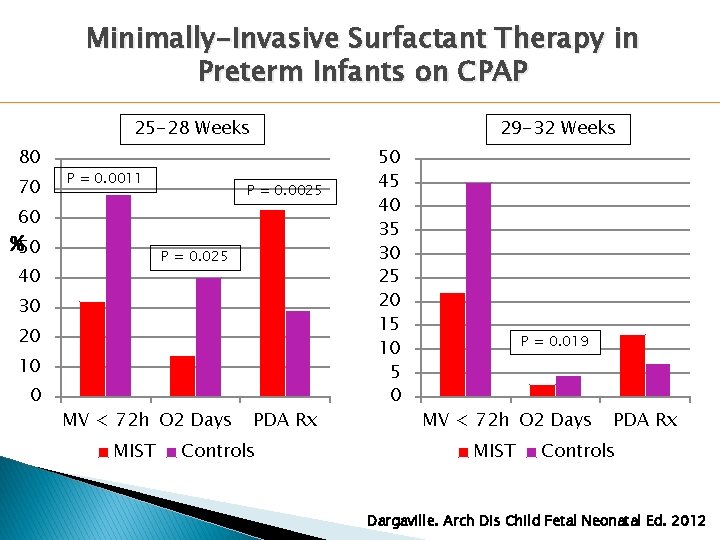
Minimally-Invasive Surfactant Therapy in Preterm Infants on CPAP 25 -28 Weeks 80 70 P = 0. 0011 29 -32 Weeks P = 0. 0025 60 %50 P = 0. 025 40 30 20 10 0 MV < 72 h O 2 Days MIST PDA Rx Controls 50 45 40 35 30 25 20 15 10 5 0 P = 0. 019 MV < 72 h O 2 Days MIST PDA Rx Controls Dargaville. Arch Dis Child Fetal Neonatal Ed. 2012

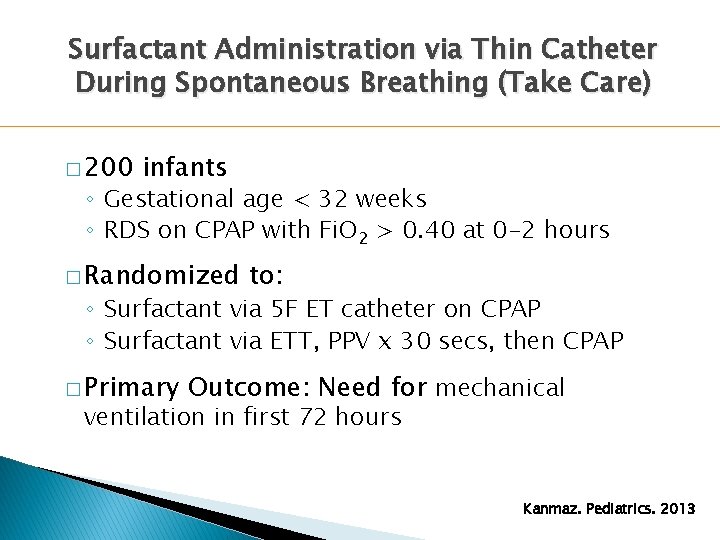
Surfactant Administration via Thin Catheter During Spontaneous Breathing (Take Care) � 200 infants ◦ Gestational age < 32 weeks ◦ RDS on CPAP with Fi. O 2 > 0. 40 at 0 -2 hours � Randomized to: ◦ Surfactant via 5 F ET catheter on CPAP ◦ Surfactant via ETT, PPV x 30 secs, then CPAP � Primary Outcome: Need for mechanical ventilation in first 72 hours Kanmaz. Pediatrics. 2013

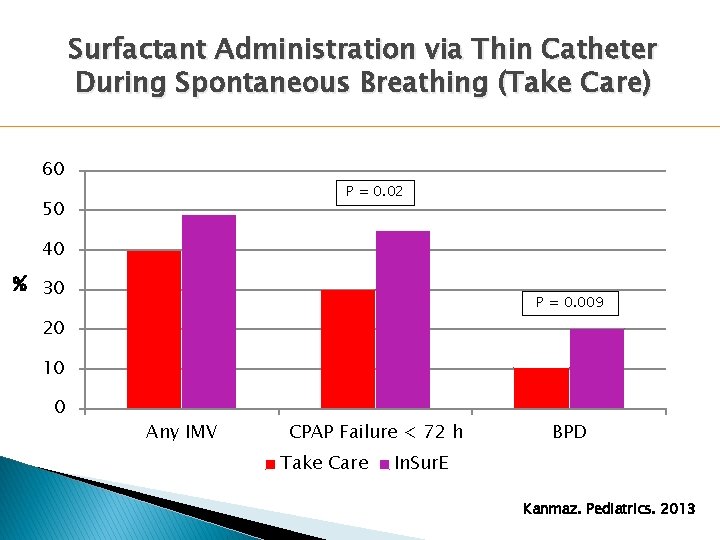
Surfactant Administration via Thin Catheter During Spontaneous Breathing (Take Care) 60 P = 0. 02 50 40 % 30 P = 0. 009 20 10 0 Any IMV CPAP Failure < 72 h Take Care BPD In. Sur. E Kanmaz. Pediatrics. 2013

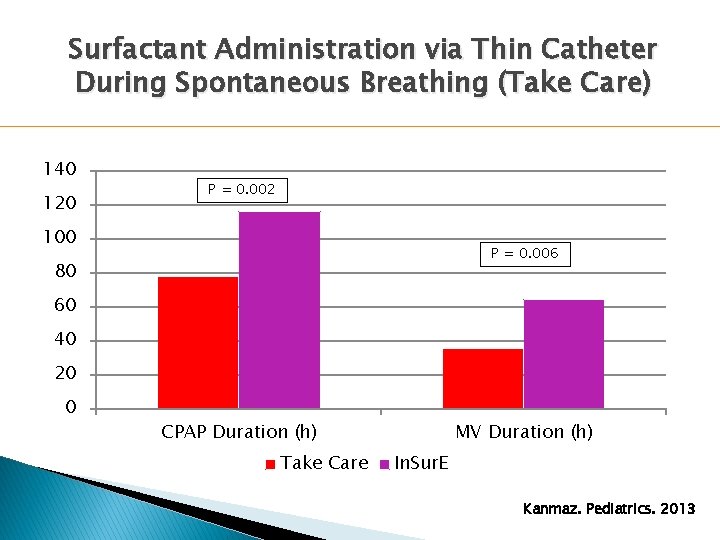
Surfactant Administration via Thin Catheter During Spontaneous Breathing (Take Care) 140 120 P = 0. 002 100 P = 0. 006 80 60 40 20 0 CPAP Duration (h) Take Care MV Duration (h) In. Sur. E Kanmaz. Pediatrics. 2013

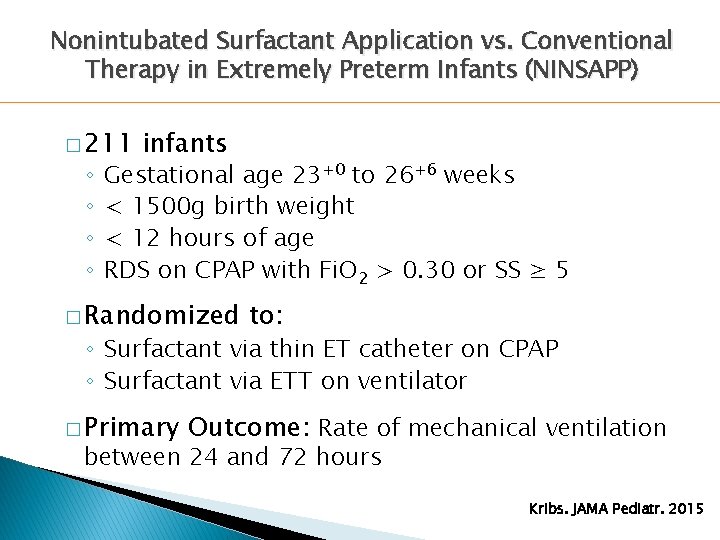
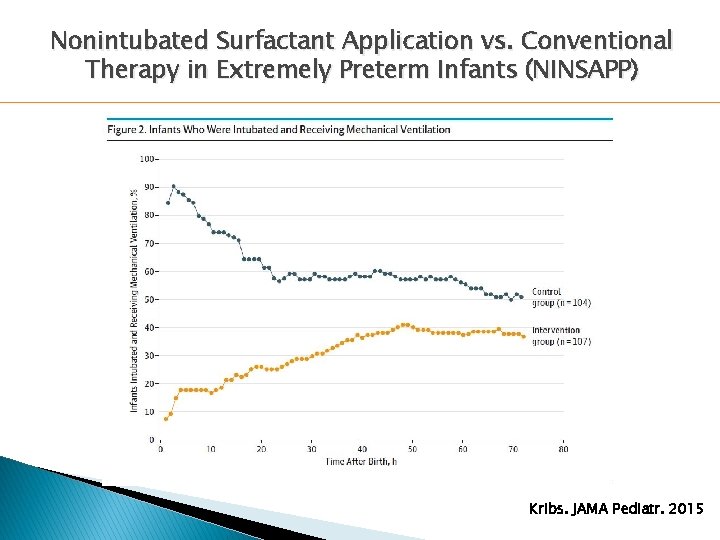
Nonintubated Surfactant Application vs. Conventional Therapy in Extremely Preterm Infants (NINSAPP) � 211 ◦ ◦ infants Gestational age 23+0 to 26+6 weeks < 1500 g birth weight < 12 hours of age RDS on CPAP with Fi. O 2 > 0. 30 or SS ≥ 5 � Randomized to: ◦ Surfactant via thin ET catheter on CPAP ◦ Surfactant via ETT on ventilator � Primary Outcome: Rate of mechanical ventilation between 24 and 72 hours Kribs. JAMA Pediatr. 2015

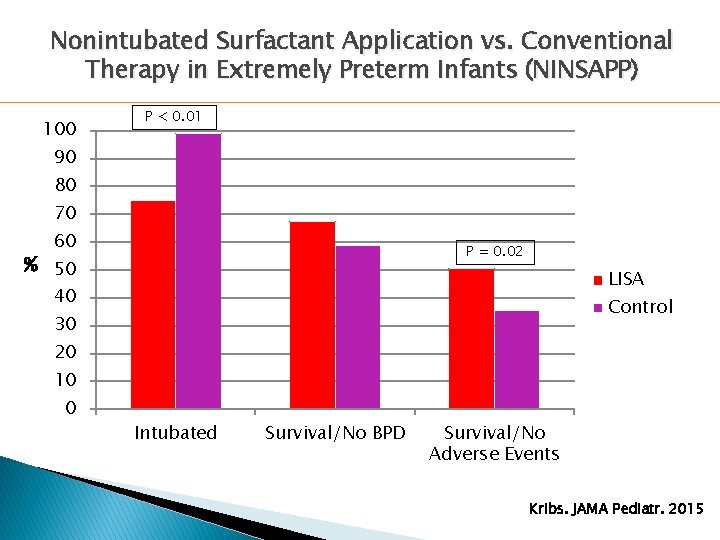
Nonintubated Surfactant Application vs. Conventional Therapy in Extremely Preterm Infants (NINSAPP) 100 P < 0. 01 90 80 70 60 P = 0. 02 % 50 LISA 40 Control 30 20 10 0 Intubated Survival/No BPD Survival/No Adverse Events Kribs. JAMA Pediatr. 2015

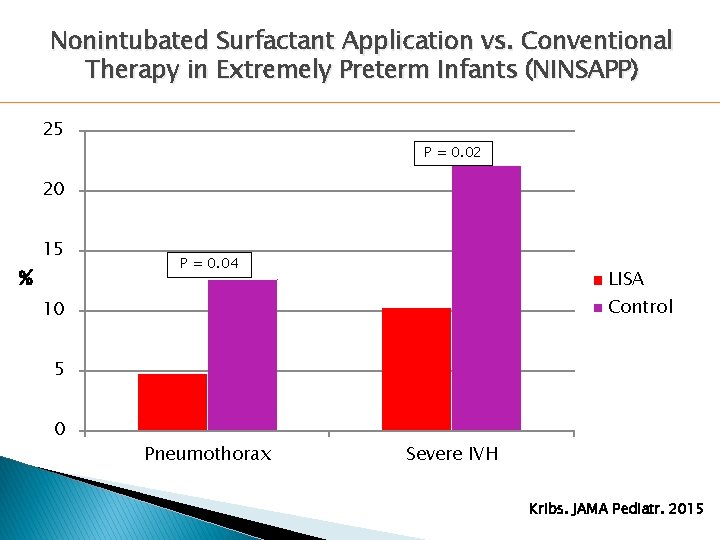
Nonintubated Surfactant Application vs. Conventional Therapy in Extremely Preterm Infants (NINSAPP) 25 P = 0. 02 20 % 15 P = 0. 04 LISA Control 10 5 0 Pneumothorax Severe IVH Kribs. JAMA Pediatr. 2015

Nonintubated Surfactant Application vs. Conventional Therapy in Extremely Preterm Infants (NINSAPP) Kribs. JAMA Pediatr. 2015

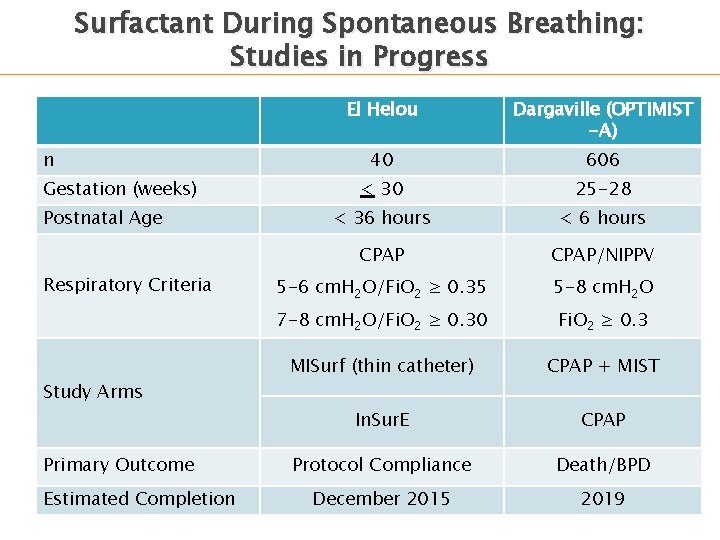
Surfactant During Spontaneous Breathing: Studies in Progress n Gestation (weeks) Postnatal Age Respiratory Criteria Study Arms Primary Outcome Estimated Completion El Helou Dargaville (OPTIMIST -A) 40 606 < 30 25 -28 < 36 hours < 6 hours CPAP/NIPPV 5 -6 cm. H 2 O/Fi. O 2 ≥ 0. 35 5 -8 cm. H 2 O 7 -8 cm. H 2 O/Fi. O 2 ≥ 0. 30 Fi. O 2 ≥ 0. 3 MISurf (thin catheter) CPAP + MIST In. Sur. E CPAP Protocol Compliance Death/BPD December 2015 2019

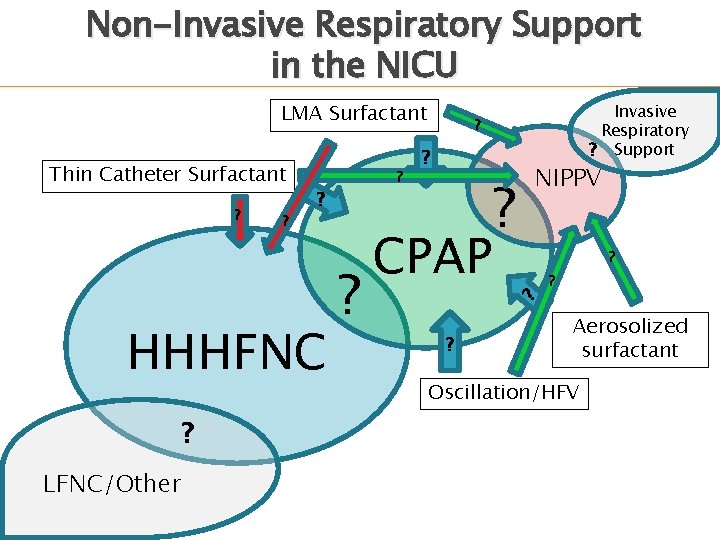
Non-Invasive Respiratory Support in the NICU LMA Surfactant ? ? ? HHHFNC ? LFNC/Other ? ? ? CPAP ? NIPPV ? ? ? Thin Catheter Surfactant Invasive Respiratory ? Support ? ? Aerosolized surfactant Oscillation/HFV

Thank you! Questions?