Novel Oral Anticoagulants NOACS J Barnett MD A

Novel Oral Anticoagulants (NOACS) J Barnett MD A Gutman MD prehospitalmd@gmail. com
Objectives • Overview of the commercially available novel oral anticoagulants (NOACs) – Dabigatran (Pradaxa®) – Rivaroxaban (Xarelto®) – Apixaban (Eliquis®) • Perioperative management of anticoagulants • Management of acute hemorrhage

Introduction • Warfarin previously the standard of care medication for management of atrial fibrillation (AF) & venous thromboembolism (VTE) with many treatment advantages & disadvantages • Novel oral anticoagulants (NOACs) changing management of patients with these common, chronic conditions • Challenges for peri- & post-operative management, & when bleeding complications occur
Scope of the Problem • Prevalence of atrial fibrillation (AF) – 3. 03 million in 2005 – 7. 56 million by 2050 • Incidence of venous thomboembolism (VTE) – 900, 000/yr in US • 1 -2% of adults take warfarin – 15% all patients on warfarin have >1 minor bleed annually, 2 -8% major bleeds, 0 -5% fatal bleeds
Warfarin • 1920 s: Outbreak of hemorrhagic disease in cattle in US & Canada from eating sweet clover plants containing molecule coumarin • 1933: Isolated by Karl Link – WARF-arin: Wisconsin Alumni Research Foundation • 1948: Used as a rodenticide • 1954: Approved in humans for clot prevention

Is Warfarin Obsolete? ADVANTAGES • Preferred agent for: – – – Mechanical valves Rheumatic mitral valve disease Renal failure Thrombophilias Antiphospholipid antibody syndrome – Oncology patients (if no LMWH) • Inexpensive DISADVANTAGES • • Bridging Drug and food interactions Long half-life Close monitoring required – Variable dose – INR affected by diet, illness, medications – Narrow therapeutic index
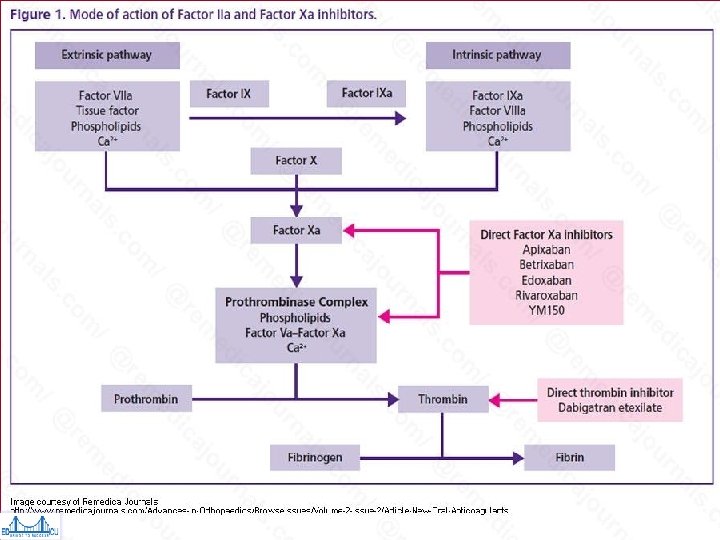
NOAC Categories • Direct Thrombin Inhibitors (DTIs) – Dabigatran • Target Specific Oral Anticoagulants (TSOACS) – Dabigatran (Pradaxa®) – Rivaroxaban (Xarelto®) – Apixaban (Eliquis®)
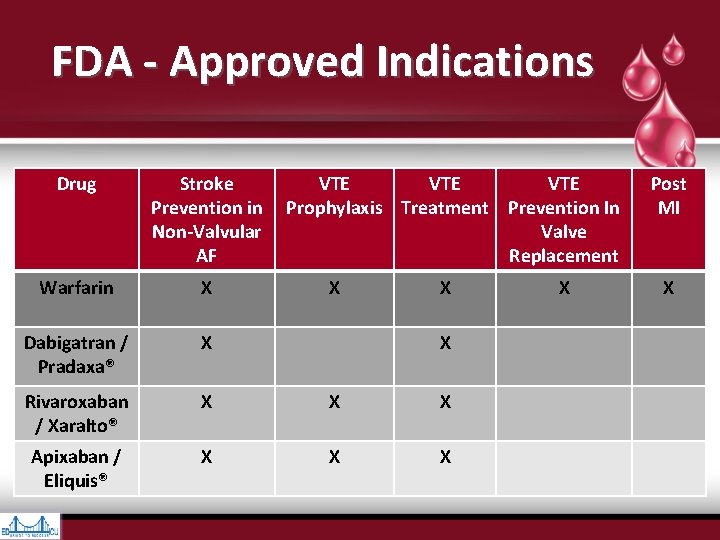
FDA - Approved Indications Drug Stroke VTE VTE Prevention in Prophylaxis Treatment Prevention In Non-Valvular Valve AF Replacement Warfarin X X X Dabigatran / Pradaxa® X Rivaroxaban / Xaralto® X X X Apixaban / Eliquis® X X X Post MI X

Why A NOAC? • Poor INR control on warfarin despite good compliance • Monitoring barriers • Warfarin allergy • Non-hemorrhagic adverse events on warfarin • Stroke on warfarin
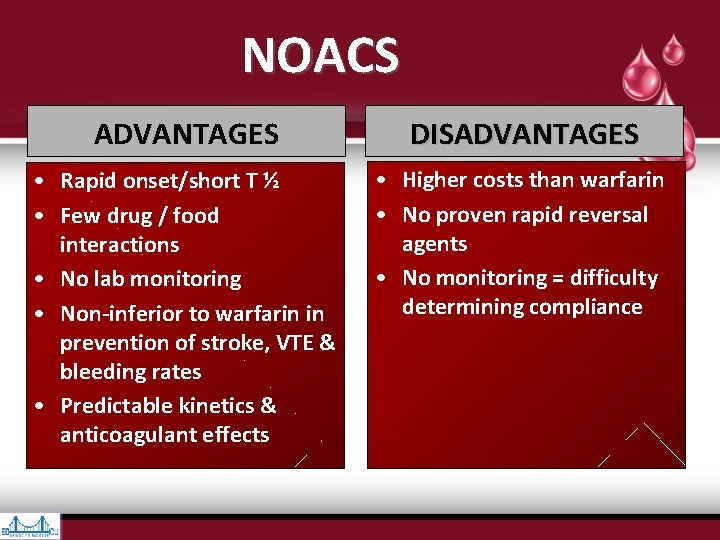
NOACS ADVANTAGES DISADVANTAGES • Rapid onset/short T ½ • Few drug / food interactions • No lab monitoring • Non-inferior to warfarin in prevention of stroke, VTE & bleeding rates • Predictable kinetics & anticoagulant effects • Higher costs than warfarin • No proven rapid reversal agents • No monitoring = difficulty determining compliance
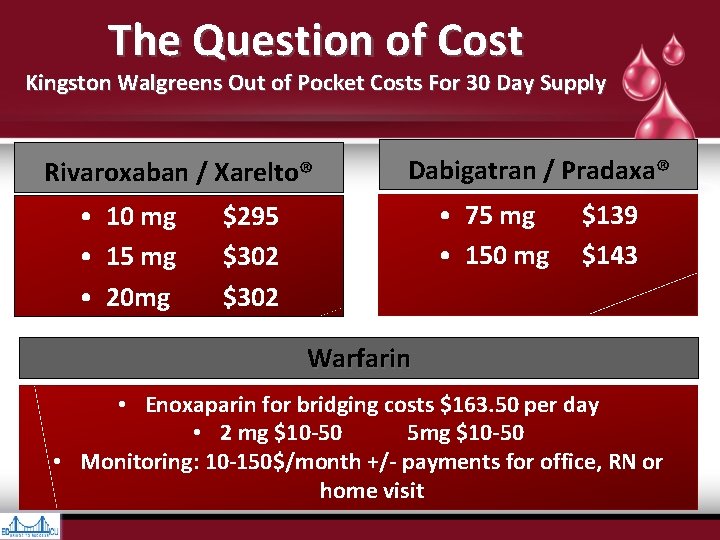
The Question of Cost Kingston Walgreens Out of Pocket Costs For 30 Day Supply Rivaroxaban / Xarelto® • 10 mg • 15 mg • 20 mg Dabigatran / Pradaxa® • 75 mg $139 • 150 mg $143 $295 $302 Warfarin • Enoxaparin for bridging costs $163. 50 per day • 2 mg $10 -50 5 mg $10 -50 • Monitoring: 10 -150$/month +/- payments for office, RN or home visit
Do NOACs Actually Work?
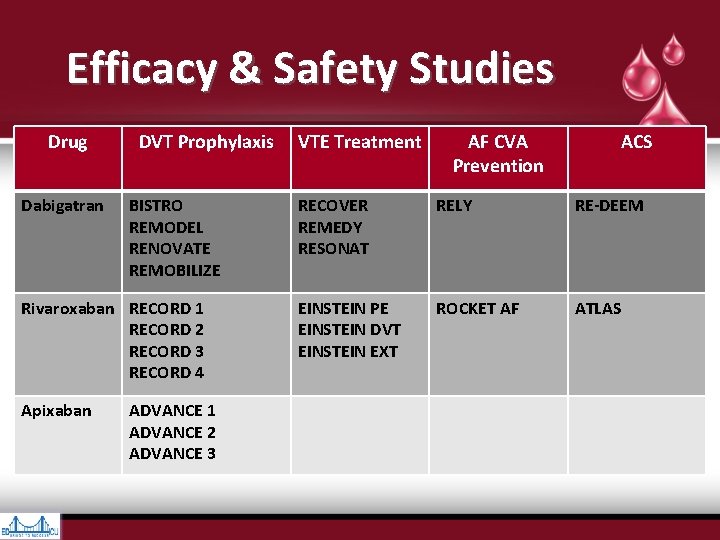
Efficacy & Safety Studies Drug Dabigatran DVT Prophylaxis BISTRO REMODEL RENOVATE REMOBILIZE Rivaroxaban RECORD 1 RECORD 2 RECORD 3 RECORD 4 Apixaban ADVANCE 1 ADVANCE 2 ADVANCE 3 VTE Treatment AF CVA Prevention ACS RECOVER REMEDY RESONAT RELY RE-DEEM EINSTEIN PE EINSTEIN DVT EINSTEIN EXT ROCKET AF ATLAS
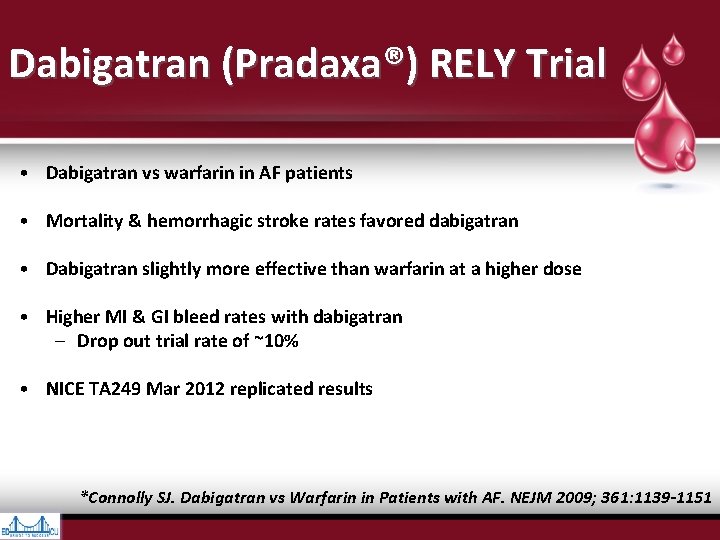
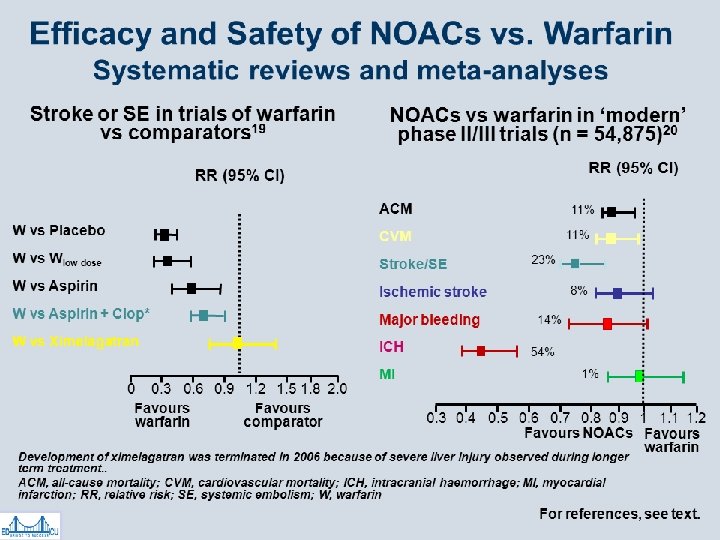
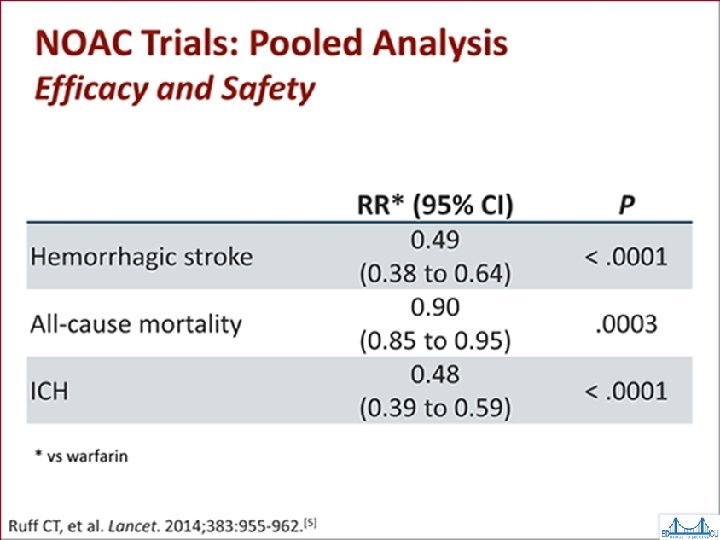
Dabigatran (Pradaxa®) RELY Trial • Dabigatran vs warfarin in AF patients • Mortality & hemorrhagic stroke rates favored dabigatran • Dabigatran slightly more effective than warfarin at a higher dose • Higher MI & GI bleed rates with dabigatran – Drop out trial rate of ~10% • NICE TA 249 Mar 2012 replicated results *Connolly SJ. Dabigatran vs Warfarin in Patients with AF. NEJM 2009; 361: 1139 -1151
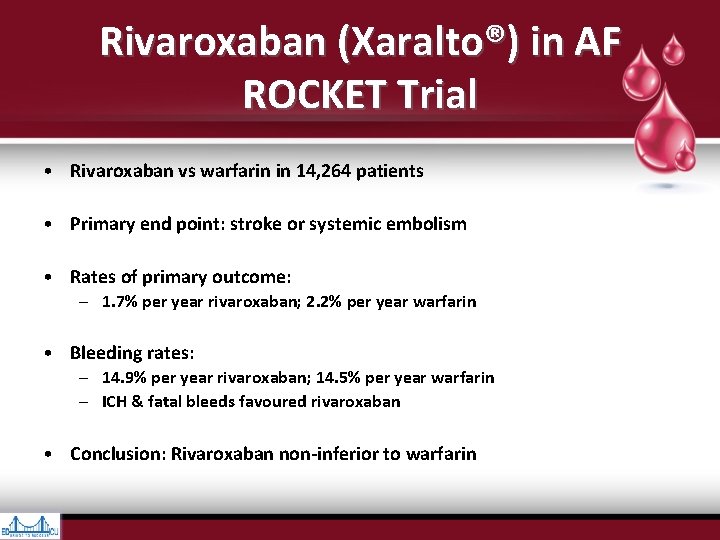
Rivaroxaban (Xaralto®) in AF ROCKET Trial • Rivaroxaban vs warfarin in 14, 264 patients • Primary end point: stroke or systemic embolism • Rates of primary outcome: – 1. 7% per year rivaroxaban; 2. 2% per year warfarin • Bleeding rates: – 14. 9% per year rivaroxaban; 14. 5% per year warfarin – ICH & fatal bleeds favoured rivaroxaban • Conclusion: Rivaroxaban non-inferior to warfarin
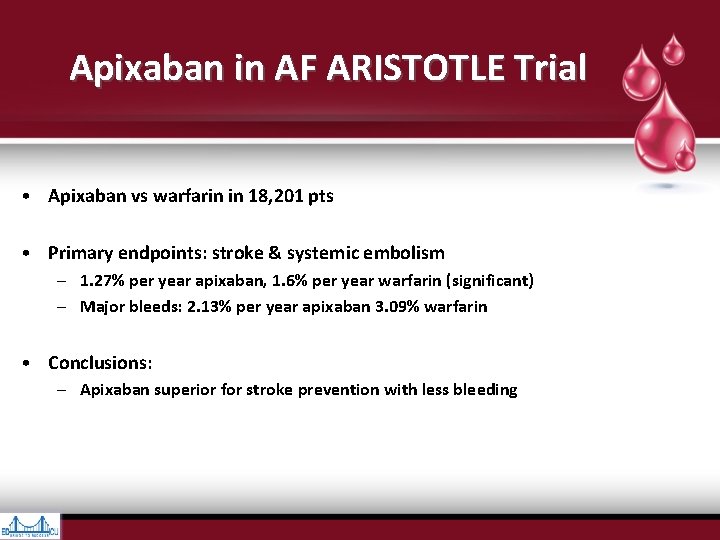
Apixaban in AF ARISTOTLE Trial • Apixaban vs warfarin in 18, 201 pts • Primary endpoints: stroke & systemic embolism – 1. 27% per year apixaban, 1. 6% per year warfarin (significant) – Major bleeds: 2. 13% per year apixaban 3. 09% warfarin • Conclusions: – Apixaban superior for stroke prevention with less bleeding
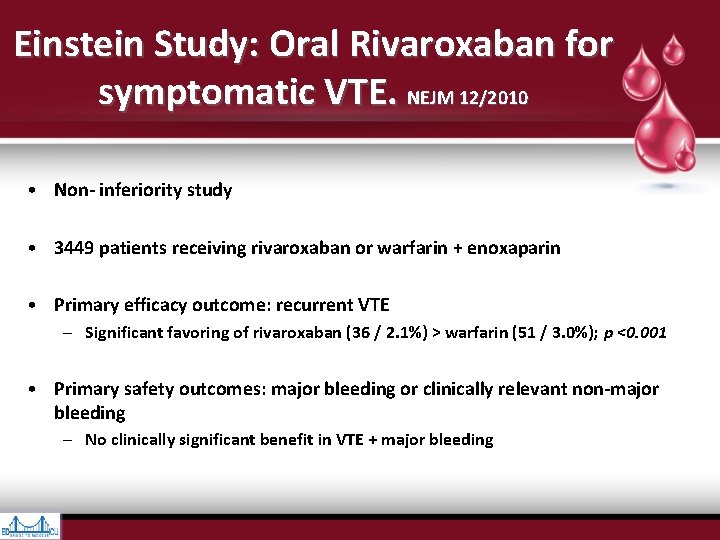
Einstein Study: Oral Rivaroxaban for symptomatic VTE. NEJM 12/2010 • Non- inferiority study • 3449 patients receiving rivaroxaban or warfarin + enoxaparin • Primary efficacy outcome: recurrent VTE – Significant favoring of rivaroxaban (36 / 2. 1%) > warfarin (51 / 3. 0%); p <0. 001 • Primary safety outcomes: major bleeding or clinically relevant non-major bleeding – No clinically significant benefit in VTE + major bleeding
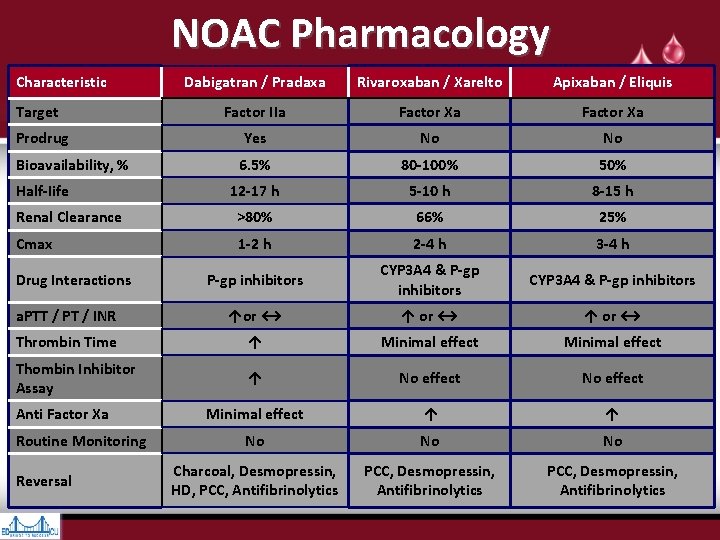
NOAC Pharmacology Characteristic Dabigatran / Pradaxa Rivaroxaban / Xarelto Apixaban / Eliquis Factor IIa Factor Xa Prodrug Yes No No Bioavailability, % 6. 5% 80 -100% 50% 12 -17 h 5 -10 h 8 -15 h Renal Clearance >80% 66% 25% Cmax 1 -2 h 2 -4 h 3 -4 h P-gp inhibitors CYP 3 A 4 & P-gp inhibitors a. PTT / PT / INR ↑or ↔ ↑ or ↔ Thrombin Time ↑ Minimal effect Thombin Inhibitor Assay ↑ No effect Minimal effect ↑ ↑ No No No Charcoal, Desmopressin, HD, PCC, Antifibrinolytics PCC, Desmopressin, Antifibrinolytics Target Half-Iife Drug Interactions Anti Factor Xa Routine Monitoring Reversal
Coagulation Testing • Managing patients requiring emergency surgery is a challenge – Non-specific tests of coagulation: PT, a. PTT, thrombin time – Specific tests: Hemoclot, specific anti-Xa assays, specific drug level • Benefits of emergency surgery vs risk of major hemorrhage
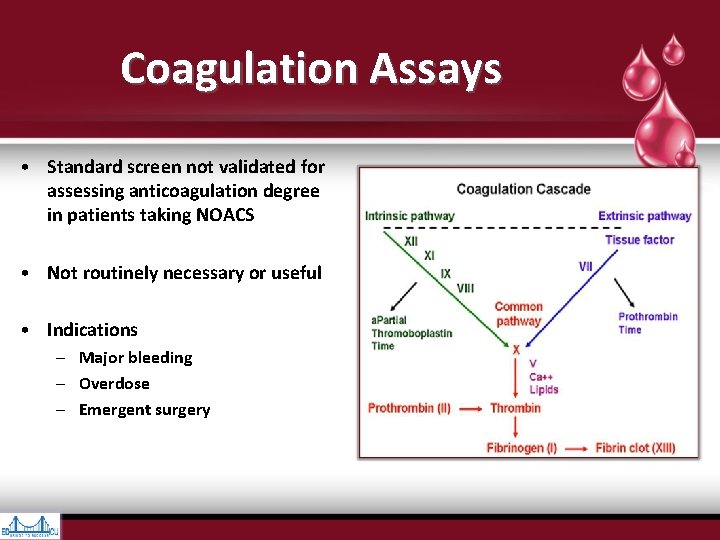
Coagulation Assays • Standard screen not validated for assessing anticoagulation degree in patients taking NOACS • Not routinely necessary or useful • Indications – Major bleeding – Overdose – Emergent surgery
Effect of NOACs on Coagulation Tests* NOAC PT a. PTT TT ECT Dabigatran / Pradaxa® Rivaroxaban / Xarelto® Apixaban / Eliquis® Anti-Xa Activity N/A Rivaroxaban Apixaban N/A N/A ran t a g i Dab Siegal DM, et al. Blood 2014
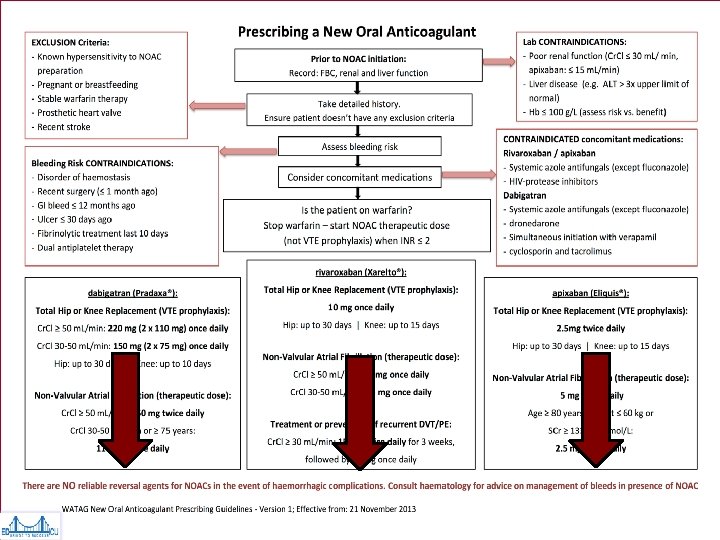
Dabigatran (Pradaxa®) • Indications: – FDA Approved: Stroke prevention in non-valvular afib* – Not FDA Approved: VTE prophylaxis in hip or knee replacement • Relative Contraindications: * – – Cr. Cl<30 ml/min >80 years of age <50 kg Gastritis, esophagitis or GERD • Benefits: – Stroke prevention: 150 mg bid; Renal insufficiency 75 mg bid – DVT prophylaxis: 220 mg qd *Dose decreased, or drug stopped
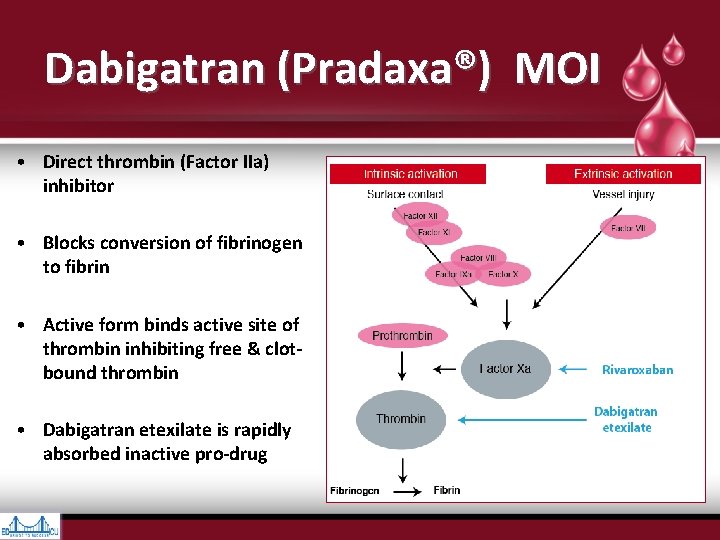
Dabigatran (Pradaxa®) MOI • Direct thrombin (Factor IIa) inhibitor • Blocks conversion of fibrinogen to fibrin • Active form binds active site of thrombin inhibiting free & clotbound thrombin • Dabigatran etexilate is rapidly absorbed inactive pro-drug
Dabigatran (Pradaxa®) Pharmacology • Predictable • Rapid onset – Peak plasma level at 2 hours – Half-life 14 -17 hours • No food interactions, few drug interactions • Fixed dosing • No routine monitoring
Dabigatran (Pradaxa®) Metabolism • 85% renally excreted – Caution with CRI / AKI • Low protein binding • Eliminated by HD & charcoal hemofiltration • Not CYp 450 metabolized • Substrate of efflux transporter P-glycoprotein • Decreased effect with P-g. P inducers – Rifampin – St John’s Wart • Increased effect with P-g. P inhibitors – – – Ketoconazole, Itraconazole Verapamil Amiodarone Quinidine Clarithromycin
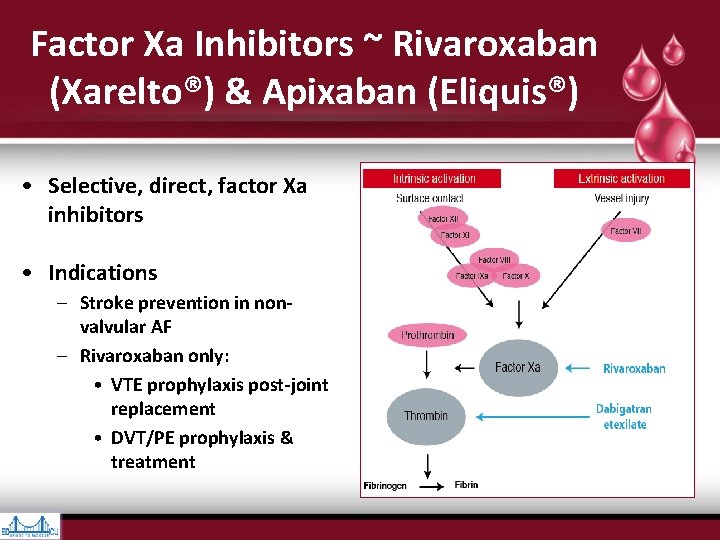
Factor Xa Inhibitors ~ Rivaroxaban (Xarelto®) & Apixaban (Eliquis®) • Selective, direct, factor Xa inhibitors • Indications – Stroke prevention in nonvalvular AF – Rivaroxaban only: • VTE prophylaxis post-joint replacement • DVT/PE prophylaxis & treatment
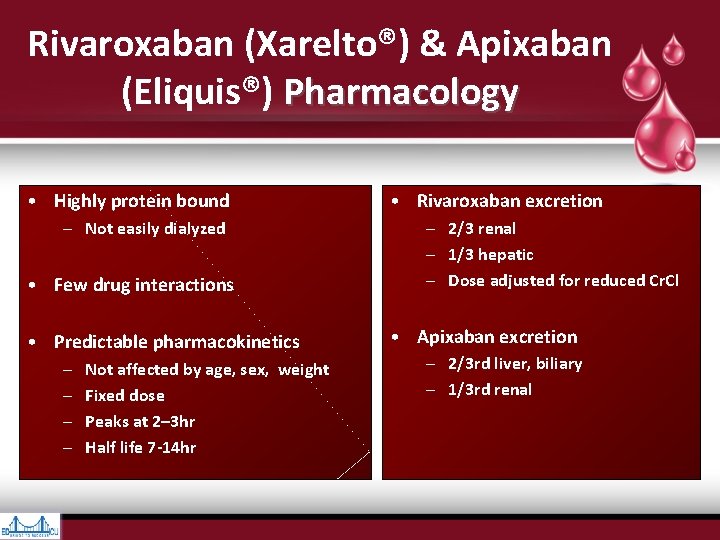
Rivaroxaban (Xarelto®) & Apixaban (Eliquis®) Pharmacology • Highly protein bound – Not easily dialyzed • Few drug interactions • Predictable pharmacokinetics – – Not affected by age, sex, weight Fixed dose Peaks at 2– 3 hr Half life 7 -14 hr • Rivaroxaban excretion – 2/3 renal – 1/3 hepatic – Dose adjusted for reduced Cr. Cl • Apixaban excretion – 2/3 rd liver, biliary – 1/3 rd renal
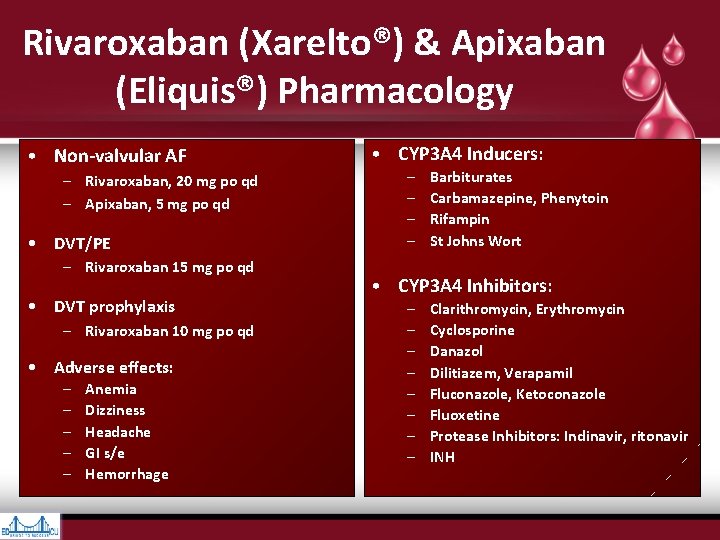
Rivaroxaban (Xarelto®) & Apixaban (Eliquis®) Pharmacology • Non-valvular AF – Rivaroxaban, 20 mg po qd – Apixaban, 5 mg po qd • DVT/PE – Rivaroxaban 15 mg po qd • DVT prophylaxis – Rivaroxaban 10 mg po qd • Adverse effects: – – – Anemia Dizziness Headache GI s/e Hemorrhage • CYP 3 A 4 Inducers: – – Barbiturates Carbamazepine, Phenytoin Rifampin St Johns Wort • CYP 3 A 4 Inhibitors: – – – – Clarithromycin, Erythromycin Cyclosporine Danazol Dilitiazem, Verapamil Fluconazole, Ketoconazole Fluoxetine Protease Inhibitors: Indinavir, ritonavir INH

Emergency Reversal
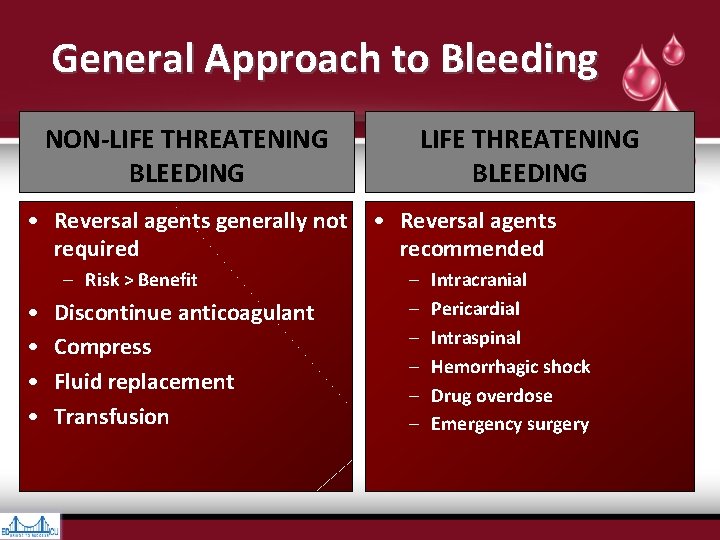
General Approach to Bleeding NON-LIFE THREATENING BLEEDING • Reversal agents generally not • Reversal agents required recommended – Risk > Benefit • • Discontinue anticoagulant Compress Fluid replacement Transfusion – – – Intracranial Pericardial Intraspinal Hemorrhagic shock Drug overdose Emergency surgery
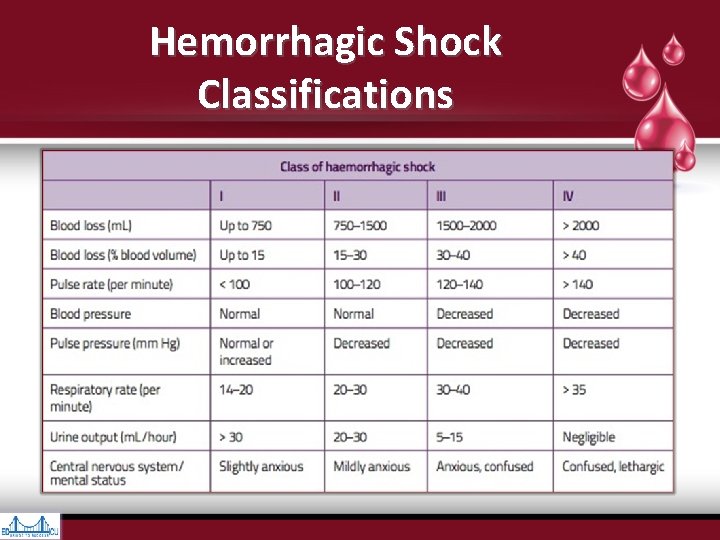
Hemorrhagic Shock Classifications
Reversal Options • Oral charcoal • Charcoal Hemofiltration • Hemodialysis • 3 or 4 Factor Prothombin Complex Concentrate (PCC) • Activated 4 Factor PCC • Recombinant Factor VIIa (r. FVIIa) • Fresh Frozen Plasma (FFP) • Desmopressin • Transexemic Acid
NOAC Reversal: NOAC Oral Activated Charcoal • MOI is adsorption – Toxin attaches to the surface of the charcoal prior to GIT absorbtion – As charcoal not digested, it stays in the GIT & toxin eliminated with stool • “Activated“ = fine particle size increasing surface area & adsorptive capacity – 50 -gram dose has surface area of 10 football fields • Beneficial within 1 -3 hours after intake – Dabigatran 1 -2 hours; Apixaban 1 -3 hours • NOACs bound to charcoal undergo entero-enteric recirculation involving multiple rounds of intestinal absorption – In vitro dabigatran study demonstrated 99. 9% absorption
NOAC Reversal: Charcoal NOAC Hemofiltration / Sorbent Hemoperfusion • Extracorporeal blood filtration to remove a toxin • Blood travels from patient to machine, filtered, travels back to patient via venous access • Adsorbent material removes toxins & normal substances from blood flowing through it – Adsorbent materials activated-carbon or resins coated or immobilized to prevent fine particles entering blood • Device used in treatment of poisonings, hepatic coma, or metabolic disturbances
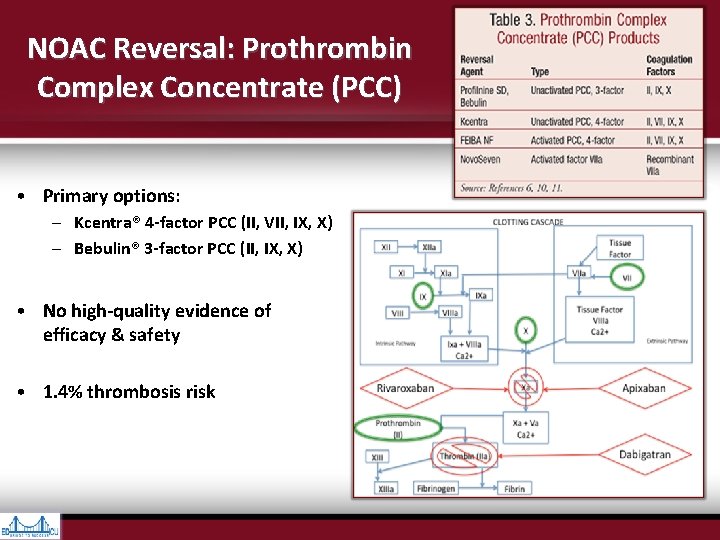
NOAC Reversal: Prothrombin Complex Concentrate (PCC) • Primary options: – Kcentra® 4 -factor PCC (II, VII, IX, X) – Bebulin® 3 -factor PCC (II, IX, X) • No high-quality evidence of efficacy & safety • 1. 4% thrombosis risk
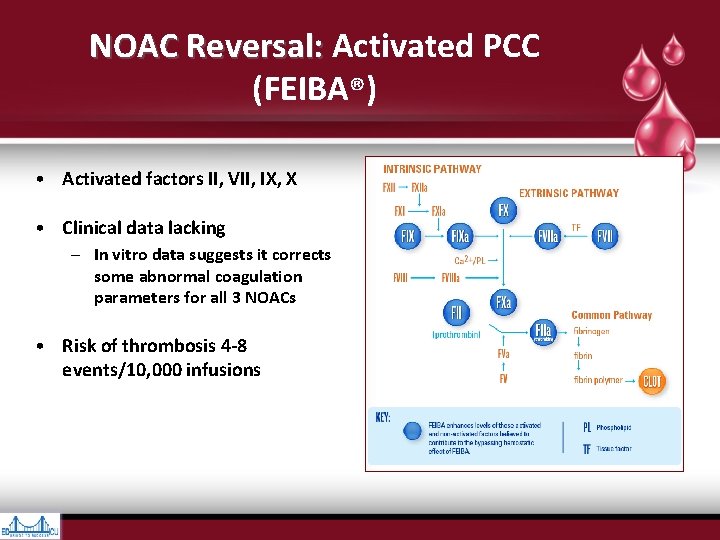
NOAC Reversal: Activated PCC NOAC Reversal: (FEIBA®) • Activated factors II, VII, IX, X • Clinical data lacking – In vitro data suggests it corrects some abnormal coagulation parameters for all 3 NOACs • Risk of thrombosis 4 -8 events/10, 000 infusions
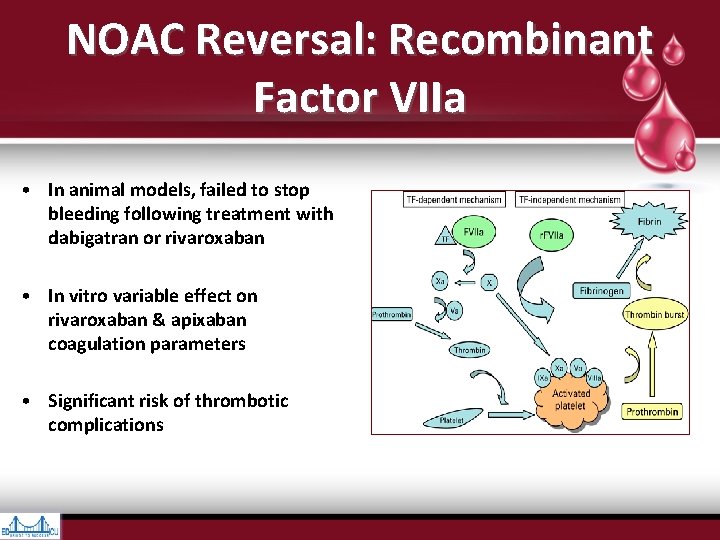
NOAC Reversal: Recombinant Factor VIIa • In animal models, failed to stop bleeding following treatment with dabigatran or rivaroxaban • In vitro variable effect on rivaroxaban & apixaban coagulation parameters • Significant risk of thrombotic complications
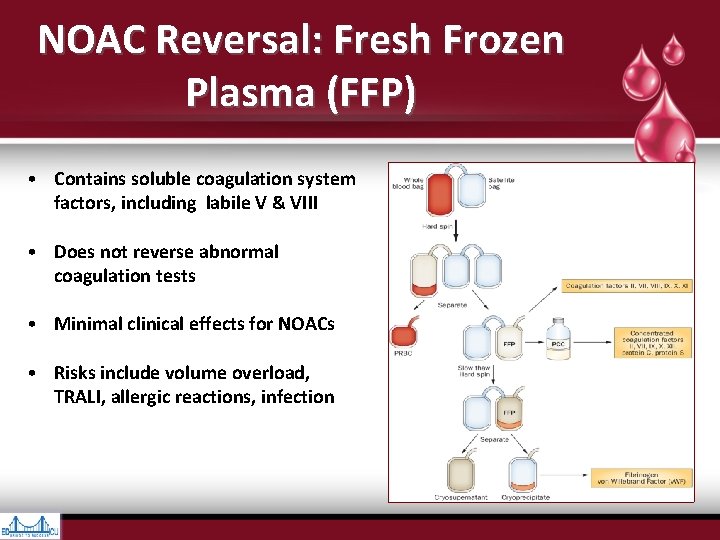
NOAC Reversal: Fresh Frozen Plasma (FFP) • Contains soluble coagulation system factors, including labile V & VIII • Does not reverse abnormal coagulation tests • Minimal clinical effects for NOACs • Risks include volume overload, TRALI, allergic reactions, infection
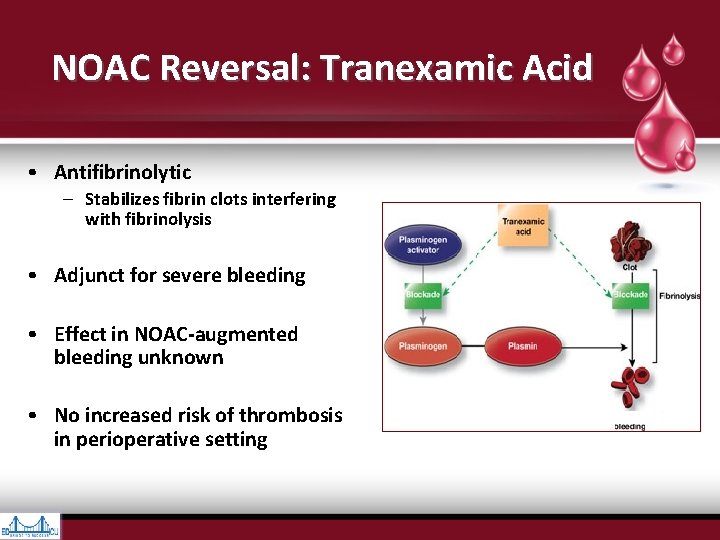
NOAC Reversal: Tranexamic Acid • Antifibrinolytic – Stabilizes fibrin clots interfering with fibrinolysis • Adjunct for severe bleeding • Effect in NOAC-augmented bleeding unknown • No increased risk of thrombosis in perioperative setting
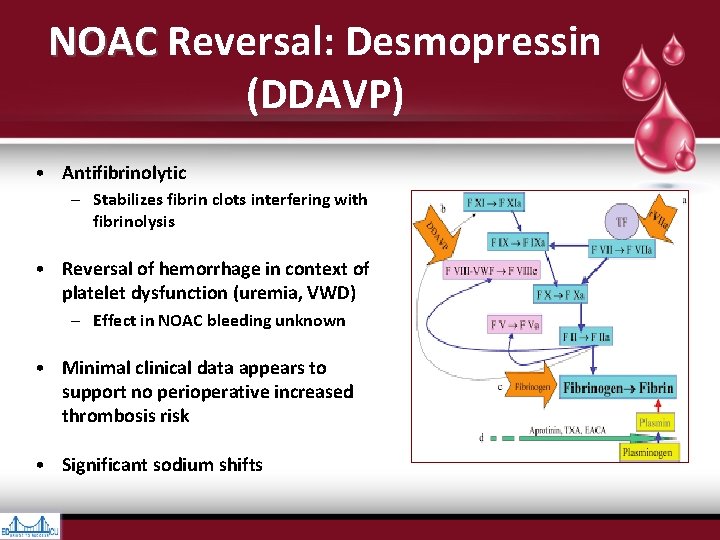
NOAC Reversal: Desmopressin NOAC (DDAVP) • Antifibrinolytic – Stabilizes fibrin clots interfering with fibrinolysis • Reversal of hemorrhage in context of platelet dysfunction (uremia, VWD) – Effect in NOAC bleeding unknown • Minimal clinical data appears to support no perioperative increased thrombosis risk • Significant sodium shifts
NOAC Reversal: Hemodialysis NOAC • Dabigatran dialyzable because of relatively low (≈35%) plasma protein binding • HD accelerates plasma clearance of dabigatran, especially in patients with renal impairment – 62% removed after 2 hours – 68% removed after 4 hours • Rivaroxaban & apixaban highly protein bound limiting HD removal
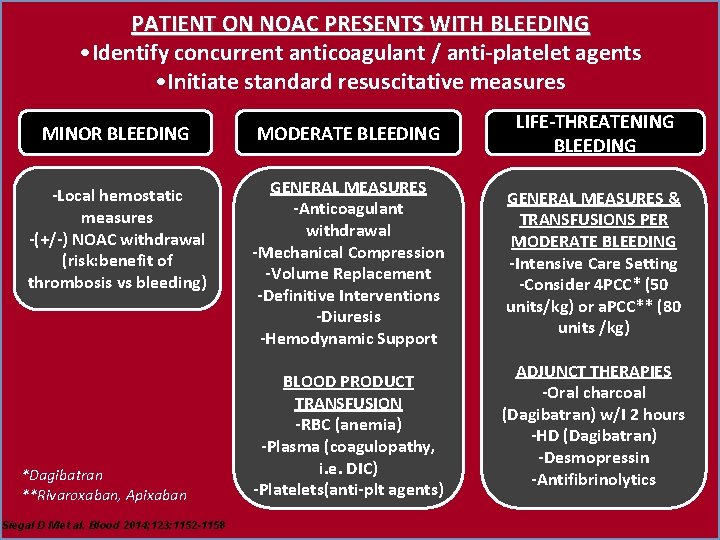
PATIENT ON NOAC PRESENTS WITH BLEEDING • Identify concurrent anticoagulant / anti-platelet agents • Initiate standard resuscitative measures MINOR BLEEDING MODERATE BLEEDING LIFE-THREATENING BLEEDING -Local hemostatic measures -(+/-) NOAC withdrawal (risk: benefit of thrombosis vs bleeding) GENERAL MEASURES -Anticoagulant withdrawal -Mechanical Compression -Volume Replacement -Definitive Interventions -Diuresis -Hemodynamic Support GENERAL MEASURES & TRANSFUSIONS PER MODERATE BLEEDING -Intensive Care Setting -Consider 4 PCC* (50 units/kg) or a. PCC** (80 units /kg) *Dagibatran **Rivaroxaban, Apixaban Siegal D M et al. Blood 2014; 123: 1152 -1158 BLOOD PRODUCT TRANSFUSION -RBC (anemia) -Plasma (coagulopathy, i. e. DIC) -Platelets(anti-plt agents) ADJUNCT THERAPIES -Oral charcoal (Dagibatran) w/I 2 hours -HD (Dagibatran) -Desmopressin -Antifibrinolytics
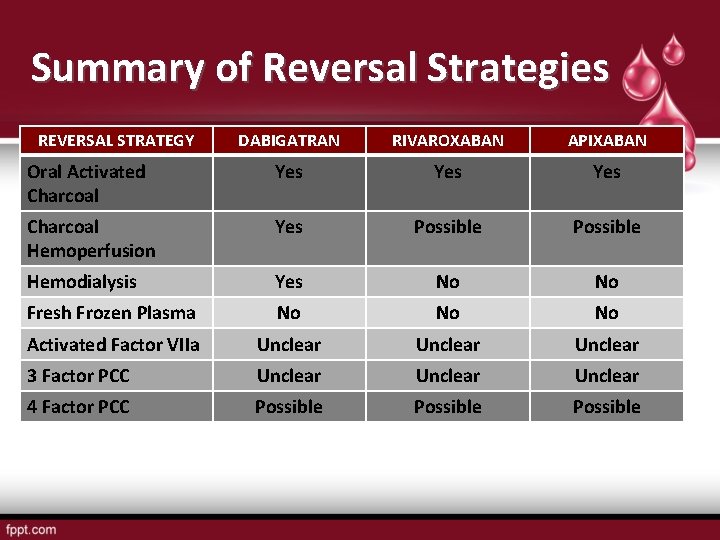
Summary of Reversal Strategies REVERSAL STRATEGY DABIGATRAN RIVAROXABAN APIXABAN Oral Activated Charcoal Yes Yes Charcoal Hemoperfusion Yes Possible Hemodialysis Yes No No Fresh Frozen Plasma No No No Activated Factor VIIa Unclear 3 Factor PCC Unclear 4 Factor PCC Possible

NOACs & Surgery

Perioperative Anticoagulation Goals § Minimize window of “subtherapeutic” anticoagulation § Bridging anticoagulation usually not required with NOACs § Warfarin bridging more complex § Normal hemostasis during surgery § Balance bleeding & thromboembolic risk post-operatively • Perioperative management influenced by: – – Drug pharmacokinetics Renal function Elective vs urgent surgery Bleeding risk of the procedure
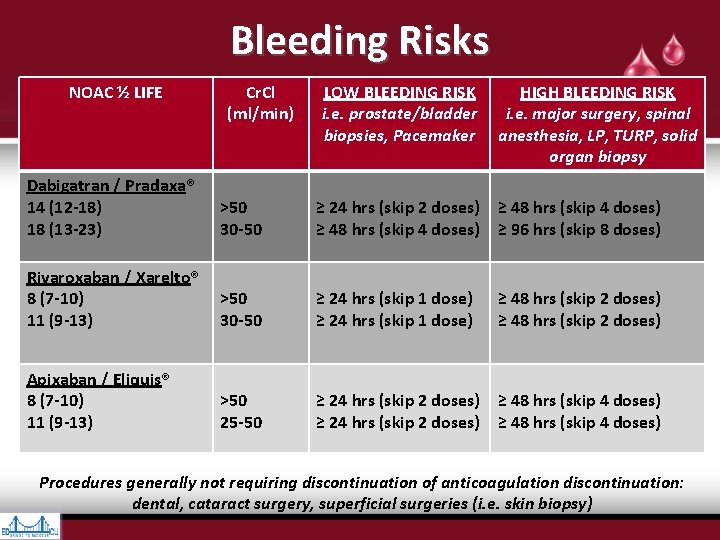
Bleeding Risks NOAC ½ LIFE Cr. Cl (ml/min) LOW BLEEDING RISK i. e. prostate/bladder biopsies, Pacemaker HIGH BLEEDING RISK i. e. major surgery, spinal anesthesia, LP, TURP, solid organ biopsy Dabigatran / Pradaxa® 14 (12 -18) 18 (13 -23) >50 30 -50 ≥ 24 hrs (skip 2 doses) ≥ 48 hrs (skip 4 doses) ≥ 96 hrs (skip 8 doses) Rivaroxaban / Xarelto® 8 (7 -10) 11 (9 -13) >50 30 -50 ≥ 24 hrs (skip 1 dose) Apixaban / Eliquis® 8 (7 -10) 11 (9 -13) >50 25 -50 ≥ 24 hrs (skip 2 doses) ≥ 48 hrs (skip 4 doses) ≥ 48 hrs (skip 2 doses) Procedures generally not requiring discontinuation of anticoagulation discontinuation: dental, cataract surgery, superficial surgeries (i. e. skin biopsy)
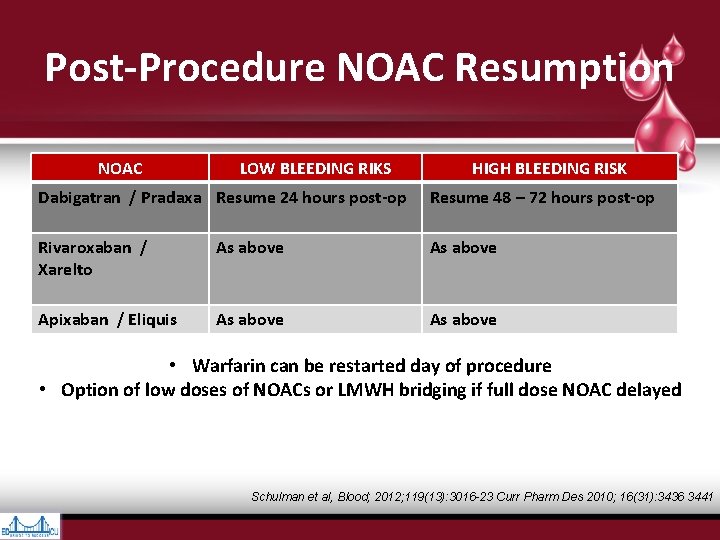
Post-Procedure NOAC Resumption NOAC LOW BLEEDING RIKS HIGH BLEEDING RISK Dabigatran / Pradaxa Resume 24 hours post-op Resume 48 – 72 hours post-op Rivaroxaban / Xarelto As above Apixaban / Eliquis As above • Warfarin can be restarted day of procedure • Option of low doses of NOACs or LMWH bridging if full dose NOAC delayed Schulman et al, Blood; 2012; 119(13): 3016 -23 Curr Pharm Des 2010; 16(31): 3436 3441
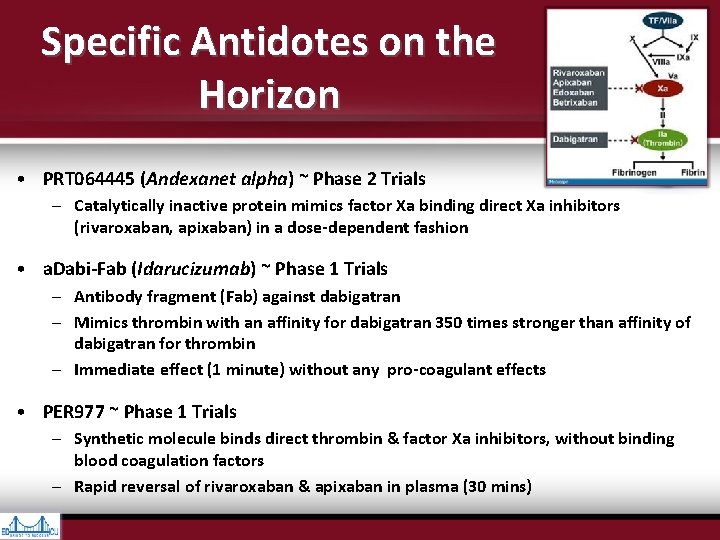
Specific Antidotes on the Horizon • PRT 064445 (Andexanet alpha) ~ Phase 2 Trials – Catalytically inactive protein mimics factor Xa binding direct Xa inhibitors (rivaroxaban, apixaban) in a dose-dependent fashion • a. Dabi-Fab (Idarucizumab) ~ Phase 1 Trials – Antibody fragment (Fab) against dabigatran – Mimics thrombin with an affinity for dabigatran 350 times stronger than affinity of dabigatran for thrombin – Immediate effect (1 minute) without any pro-coagulant effects • PER 977 ~ Phase 1 Trials – Synthetic molecule binds direct thrombin & factor Xa inhibitors, without binding blood coagulation factors – Rapid reversal of rivaroxaban & apixaban in plasma (30 mins)

References • • • • • • Periprocedural Bleeding and Thromboembolic Events with Dabigatran Compared with Warfarin: Results from RE-LY trial. Circulation. 2012; 126: 343 -348 Scott S. The New Oral Anticoagulants: Bleeding, Periprocedureal Management, Lab Evaluation, and Use for VTE Prevention/Treatment. Reviewed Feb 2016 Annelise Gallien, MD FRCPC. Update in the Perioperative and Emergency Management of Novel Oral Anticoagulants. Reviewed Feb 2016 Guidance on the emergent reversal of oral thrombin & factor Xa inhibitors. Katz et. al THNSA meeting proceedings Am J Hematology. 7 March 2012. J Holmes. New oral anticoagulants: an update. Accessed Feb 2016 Emergency Management of bleeding Associated with old and New Oral Anticoagulants. Peacock et. Al Clinical Cardiology May 9 2012. Major Bleeding in Patients With Atrial Fibrillation Receiving Apixaban or Warfarin. The ARISTOTLE Trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation): Predictors, Characteristics, and Clinical Outcomes Management of major bleeding events in patients treated with Rivaroxaban vs. Warfarin: results from the ROCKET AF trial Eckert, Evan. Xalreto. UNM Pharmacy and Theraputics Van Ryn et Al Dabigatran etexilate- a novel reversible oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thrombosis and Haemostasis 103. 6. 2012 Douxfils, J et al. Assessment of impact of rivaroxaban on coagulation assays: Lab recommendations for the monitoring of rivaroxaban and review of the literature Management and Outcomes of Major Bleeding During Treatment with Dabigatran or Warfarin. Circulation. 2013; 128: 2325 -2332 The Einstein Investigators. Oral Rivaroxaban for symptomatic venous thromboembolism. NEJM 12/23/2010. Patel et. Al Rivaroxaban versus warfarin in Non-Valvular Afib NEJM 9/8/2011 Minichiello, T. The new anticoagulants and other updates. Burnet, A. New Oral Anticoagulants: Have we found the Holy Grail? Powerpoint presentation Periprocedural Bleeding and Thromboembolic Events with Dabigatran Compared with Warfarin: Results from RE-LY trial Circulation. 2012; 126: 343 -348 USPharmacist. com europace. oxfordjournals. org; eurheartj. oxfordjournals. org. Pooled analyses review. Queried February 2016 Xarelto® PM, July 18, 2012 ; Pradaxa ® PM November 12, 2012; Eliquis® PM November 27, 2012 Goette Trends Cardiovasc Med. 2013 Drug Class Review: Target Specific Oral Anticoagulants. Dabigatran (Pradaxa), Rivaroxaban (Xarelto), and Apixaban (Eliquis). September 2014. VHA Pharmacy Benefits Management Services, Medical Advisory Panel and VISN Pharmacist Executives
Conclusions • Consider NOACs for DVT/PE treatment in selected patients – Comparable to warfarin in preventing recurrent VTE with a tendency of less bleeding (rivaroxaban & Apixaban), & no mortality difference – Non-inferior to warfarin therapy in prevention of recurrent VTE • Perioperative management & hemorrhage control in patients treated with NOACs is challenging – Despite lack of antidote, outcomes of major bleeding are non-inferior or better compared with warfarin • Treatment for bleeding on NOACs is primarily supportive – Short T½ relative to warfarin – Minimal evidence for reversal agents unless life-threatening bleeding

Questions? prehospitalmd@gmail. com
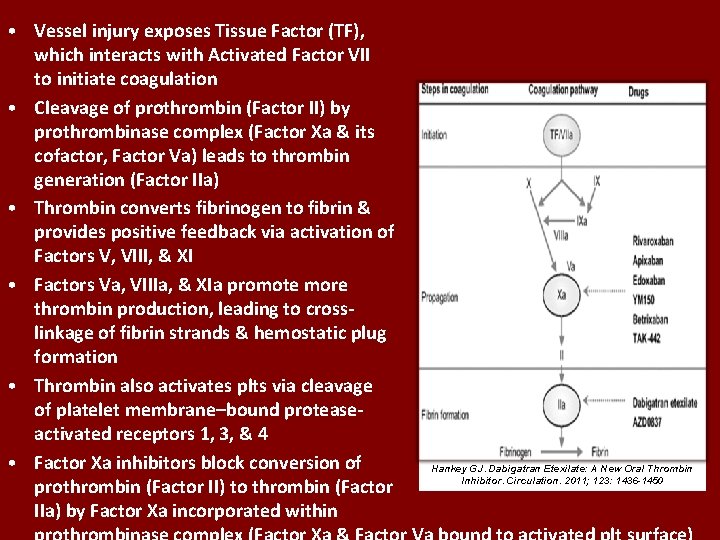
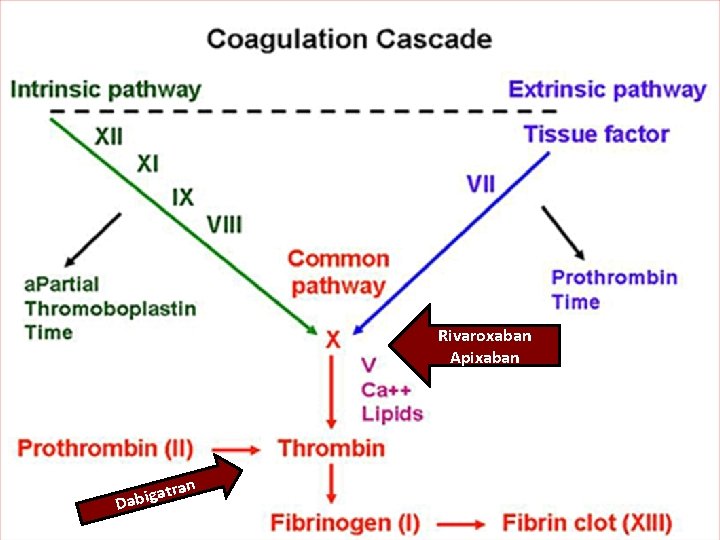
• Vessel injury exposes Tissue Factor (TF), which interacts with Activated Factor VII to initiate coagulation • Cleavage of prothrombin (Factor II) by prothrombinase complex (Factor Xa & its cofactor, Factor Va) leads to thrombin generation (Factor IIa) • Thrombin converts fibrinogen to fibrin & provides positive feedback via activation of Factors V, VIII, & XI • Factors Va, VIIIa, & XIa promote more thrombin production, leading to cross- linkage of fibrin strands & hemostatic plug formation • Thrombin also activates plts via cleavage of platelet membrane–bound protease- activated receptors 1, 3, & 4 • Factor Xa inhibitors block conversion of Hankey GJ. Dabigatran Etexilate: A New Oral Thrombin Inhibitor. Circulation. 2011; 123: 1436 -1450 prothrombin (Factor II) to thrombin (Factor IIa) by Factor Xa incorporated within
- Slides: 55